Published online Jul 6, 2022. doi: 10.12998/wjcc.v10.i19.6744
Peer-review started: February 16, 2022
First decision: March 10, 2022
Revised: March 18, 2022
Accepted: April 22, 2022
Article in press: April 22, 2022
Published online: July 6, 2022
Processing time: 127 Days and 22.5 Hours
Squamous cell carcinoma (SCC) of the liver is rare, and is more commonly found in the skin, rectum, cervical or inguinal lymph nodes.
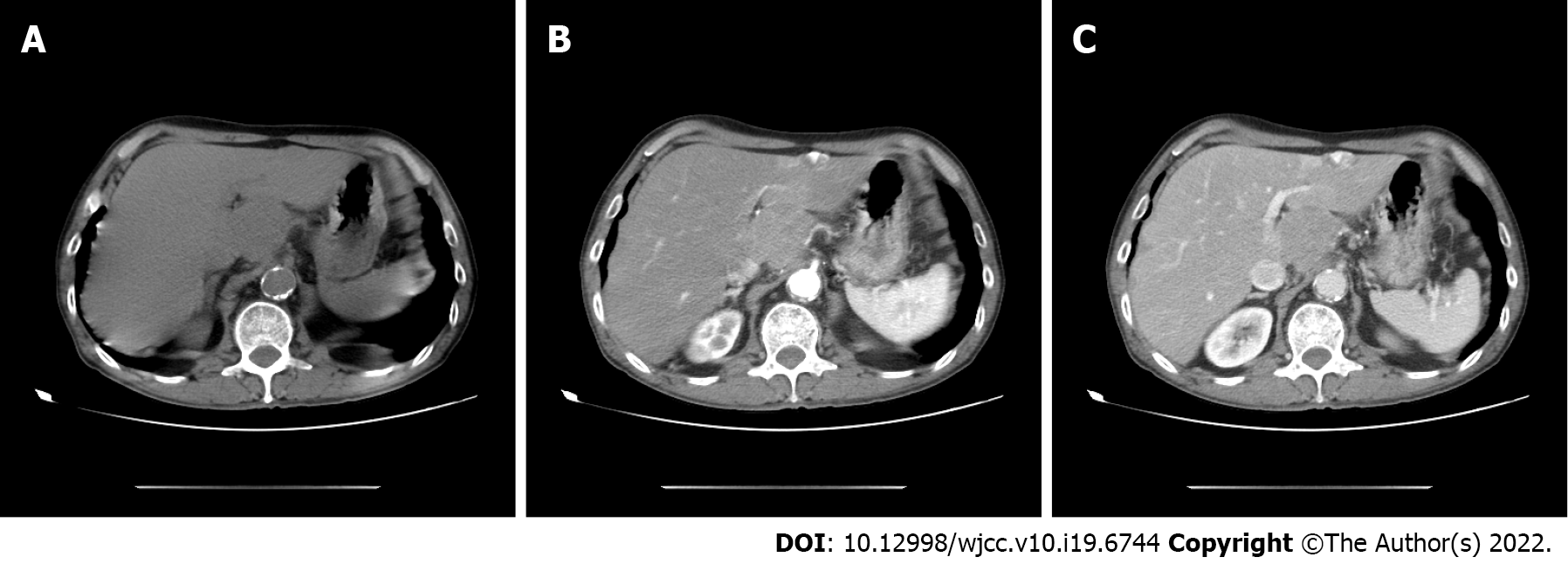
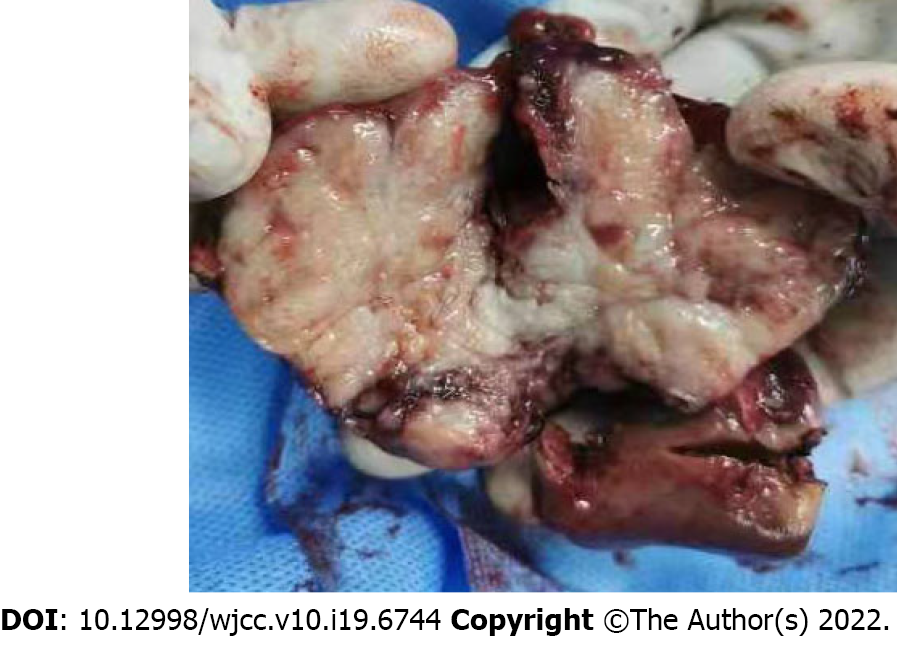
A 73-year-old man had been experiencing right upper quadrant discomfort for some weeks. He had a 50-year history of smoking and drinking. On average, he smoked 20 cigarettes and consumed 200 galcoholdaily. He didn’t have a history of hepatitis or surgery. Fever, vomiting, jaundice, dysuria, chills, and abdominal distention were not observed at the time of admission. Tenderness in the right upper quadrant was found on physical examination, but there was no palpable abdominal mass. No obvious abnormalities in laboratory tests and tumor markers were found. The plasma retention rate of indocyanine green (ICG) at 15 min was 1.35%. Subsequent abdominal ultrasonography showed a mixed echoic mass approximately 3.8 cm diameter in the left caudate lobe of the liver. Abdominal computed tomography confirmed a 3.0 cm × 3.5 cm irregular mass with inhomogeneous density and moderate delayed enhancement in the left caudate lobe of the liver. Laparoscopic left caudate lobectomy was performed to remove the liver mass. Intra-operative findings confirmed a non-cirrhotic liver, with a 3 cm × 3.5 cm white tumor mass in the left caudate lobe with no tumor rupture and no hemoperitoneum. The resection margin was 1.0 cm in width.
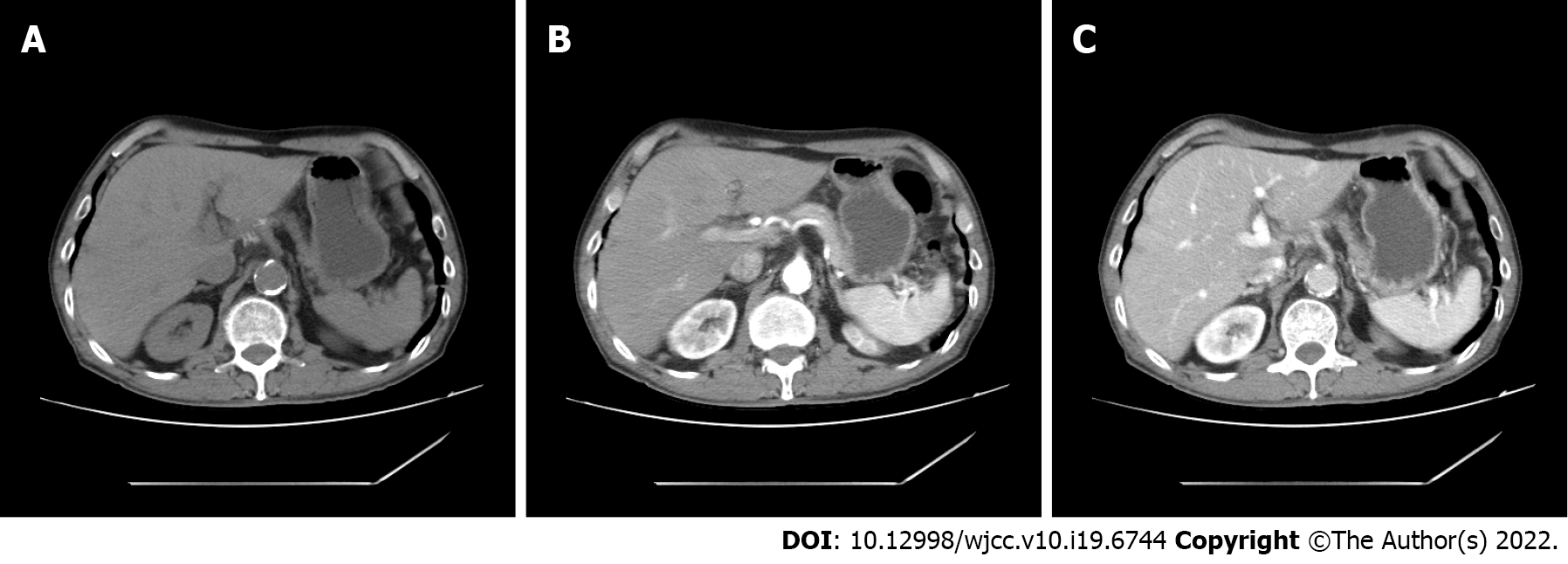
We describe the first case of SCC in the left caudate lobe of the liver, which was successfully treated by surgical resection and postoperative immunotherapy. No tumor recurrence was observed during the 8-mo follow-up.
Core Tip: Primary squamous cell carcinoma (SCC) of the liver is very rare. Here we report the first case of SCC of the left caudate liver lobe successfully treated by laparoscopic hepatectomy. The patient refused to undergo systemic chemotherapy, and received immunotherapy, and the disease-free survival was 8 mo. However, there is no available literature on the effectiveness of immunotherapy in this disease, and this requires further study.
- Citation: Kang LM, Yu DP, Zheng Y, Zhou YH. Primary squamous cell carcinoma of the liver: A case report. World J Clin Cases 2022; 10(19): 6744-6749
- URL: https://www.wjgnet.com/2307-8960/full/v10/i19/6744.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i19.6744
Squamous cell carcinoma (SCC) of the liver is rare, and is more commonly found in the skin, rectum, cervical or inguinal lymph nodes. It accounts for 4%-5% of cancers with unclear primary locations[1]. However, there have been a total of 31 similar occurrences described in the literature[2]. Hepatic teratoma, hepatic cyst, and hepatolithiasis have all been linked to primary SCC of the liver. Poorly differentiated SCC of the liver can be completely cured with systemic chemotherapy and surgery, and it also responds to hepatic arterial injections of low-dose chemotherapeutic agents[3,4]. We present the first case of primary SCC in the left caudate liver lobe which was successfully resected with an 8 mo disease-free survival. To our knowledge, 31 cases of primary SCC of the liver have been reported. Here, we here describe a case of left liver caudate lobe SCC which was treated successfully by surgical resection, with a disease-free survival of 8 mo.
Before his admission in June 2021, the 73-year-old man had been experiencing right upper quadrant discomfort for some weeks.
The patient’s symptoms started some weeks ago with recurrent right upper quadrant discomfort. There was no obvious aggravation of symptoms.
The patient had a 50-year history of smoking and drinking. On average, he smoked 20 cigarettes and consumed 200 g alcohol daily. He didn’t have a history of hepatitis or surgery.
No fever, vomiting, jaundice, dysuria, chills, or abdominal distention were observed at the time of admission. Tenderness in the right upper quadrant was found on physical examination, but no palpable abdominal mass was identified.
Laboratory test results were as follows: hemoglobin 12.8 g/dL; white blood cell count 12100/mm3; platelet count 21000/μL; prothrombin time 10.7/11.2 s; international normalized ratio 1.43; albumin 3.7 g/dL; direct bilirubin 0.32 mg/dL; complete bilirubin 0.57 mg/dL; aspartate aminotransferase 24 IU/L; alanine aminotransferase 3 IU/L; alkaline phosphatase 113 U/L; blood urea nitrogen 12 mg/dL; creatinine 1.1 mg/dL; sodium 136 meq/L; potassium 3.8 meq/L. The serum level of carcinoembryonic antigen (CEA) was < 5 ng/mL, alpha-fetoprotein was < 10 ng/mL, and carbohydrate antigen 19-9 was < 34 U/mL, which had previously been 2.05 ng/mL, < 3 ng/mL, and 4.78 U/mL, respectively. Urine analysis was normal. Electrocardiogram, chest x-ray and arterial blood gas was also normal.
Subsequent abdominal ultrasonography showed a mixed echoic mass approximately 3.8 cm diameter in the left caudate lobe of the liver. Abdominal computed tomography (CT) confirmed an irregular mass 3.0 cm × 3.5 cm in size with inhomogeneous density and moderate delayed enhancement in the left caudate liver lobe (Figure 1). The patient was unable to undergo magnetic resonance examination as he had difficulty holding his breath.
According to the postoperative pathological results, the final diagnosis in this patient was primary SCC of the liver.
In June 2021, the patient underwent laparoscopic left caudate lobectomy to remove the liver mass. Intra-operative findings confirmed a non-cirrhotic liver with a 3 cm × 3.5 cm white tumor mass with no tumor rupture and no hemoperitoneum. The resection margin was 1.0 cm in width (Figure 2).
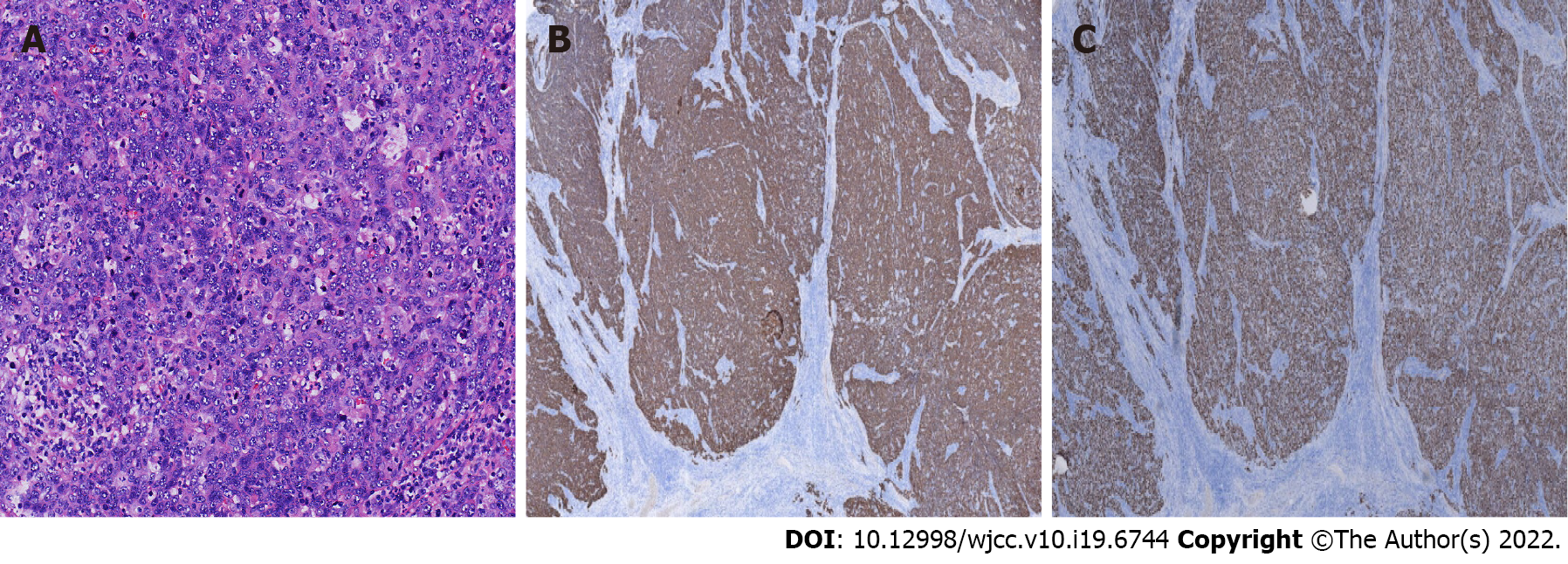
Histopathological examination confirmed a moderately differentiated SCC of the liver composed of non-keratinized squamous cells (Figure 3A). SCC of the liver had the pathological characteristics of different sized cancer cells, nest-like in appearance, intercellular bridges, large and deep staining nuclei, a mitotic phase, abundant cytoplasm, dichroism, incomplete keratosis of cancer cells, the formation of keratotic beads, and no adenoid carcinoma tissue. According to previous research, immunohistochemistry is often positive for cytokeratin (CK) 10, CK14, CK19 and CEA[5].We used the immunohistochemical Envision two-step method for further examination of liver samples. Immunohistochemistry revealed positivity for CK5/6 (mouse anti-human monoclonal antibody, Fuzhou Maixin Biotech., Co., Ltd), P40 (rabbit anti-human monoclonal antibody, Fuzhou Maixin Biotech., Co., Ltd) (Figure 3B and C), and occasional positivity for Ki-67 (90%) (mouse anti-human monoclonal antibody, Fuzhou Maixin Biotech., Co., Ltd). However negativity for thyroid transcription CD34, Arg-1, CPS1, and Syn indicated a SCC of the liver. Subsequent gastroscopy showed that the esophagus and stomach were normal. The results of postoperative pathological examination showed a primary hepatic SCC. We doubted that this tumor was a metastatic tumor from the skin, nasopharynx, lung or gastrointestinal tract, and the patient underwent further physical examination, CT of the brain, nasopharynx and chest, in addition to gastroscopy and enteroscopy. These tests were negative. Limited by hospital conditions, we were unable to perform a positron emission tomography-CT examination. The patient refused systemic chemotherapy, and was treated witha 3 wk regimen of immunotherapy consisting of 200 mg xindilimab (Daboshu) injections [Xinda Biopharmaceutical (Suzhou) Co., Ltd.]. His postoperative course was uneventful and no tumor recurrence or distant metastasis developed during the 8mo follow-up period (Figure 4).
Primary SCC of the liver is very rare. It has been stated that primary SCC of the liver is caused by chronic inflammation of bile duct epithelium or the formation of a hepatic cyst with subsequent malignant transformation[6-8]; however, the underlying mechanism is still unclear.
Here, we report the first case of SCC in the left caudate lobe of the liver, with a solitary solid tumor without parasitic infection, which was successfully treated by laparoscopic hepatectomy. Pathological examination of the tumor showed moderately differentiated SCC composed of squamous cells without keratinization. Positive staining of acidic CK5/6 indicated basal cells of non-keratinized squamous epithelium and the beginning of cancer cells. Strong positivity of P40 suggested possible lung cancer; however, chest CT examinations were negative. Clinically, panendoscopy, chest CT, and ENT examination revealed negative results in this case. Taken together, these findings indicated primary SCC of the liver.
The prognosis of primary SCC of the liver is dismal with a survival of less than one year, as the tumor is typically recognized late[9]. Complete remission of poorly differentiated SCC after systemic chemotherapy (cisplatin and 5-fluorouracil) and surgery has been reported[10,11]. If tumor recurrence is found in the late stage, reoperation, systemic chemotherapy or hepatic artery infusion chemotherapy are considered treatment options[4]. However, in the present case, as the patient refused systemic chemo
We describe the first case of SCC in the left caudate lobe of the liver, which was successfully managed by surgical resection. The patient was also treated with continuous postoperative immunotherapy and disease-free survival was 8 mo. Further research on the treatment and prognosis of primary SCC of the liver is required.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Chudek JT, Poland; Limaiem F, Tunisia S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Nosaka T, Ohtani M, Namikawa S, Takahashi K, Naito T, Ofuji K, Matsuda H, Hiramatsu K, Imamura Y, Nakamoto Y. Advanced primary adenosquamous carcinoma of the liver with a small cell carcinoma component: an autopsy case report. Clin J Gastroenterol. 2021;14:1496-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Zhao R, Zhu K, Wang R, Gao J, Cui K, Yu F, Zhang B, Li S. Primary squamous cell carcinoma of the liver: A case report and review of the literature. Oncol Lett. 2012;4:1163-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Weimann A, Klempnauer J, Gebel M, Maschek H, Bartels M, Ringe B, Pichlmayr R. Squamous cell carcinoma of the liver originating from a solitary non-parasitic cyst case report and review of the literature. HPB Surg. 1996;10:45-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Zhang XF, Du ZQ, Liu XM, Lv Y. Primary Squamous Cell Carcinoma of Liver: Case Series and Review of Literatures. Medicine (Baltimore). 2015;94:e868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Lee HL, Liu YY, Yeh CN, Chiang KC, Chen TC, Jan YY. Primary squamous cell carcinoma of the liver: a successful surgically treated case. World J Gastroenterol. 2006;12:5419-5421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Xiao J, Ma L, Li J, Yin B, Liang J, Wang J. Primary Squamous Cell Carcinoma of the Liver is Rare but Hostile: Case Series and Comprehensive Review of the Literature. Cancer Manag Res. 2021;13:829-837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Sun Y, Jin G. Primary squamous cell carcinoma of the liver: a case report. J Int Med Res. 2021;49:3000605211021275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Yagi H, Ueda M, Kawachi S, Tanabe M, Aiura K, Wakabayashi G, Shimazu M, Sakamoto M, Kitajima M. Squamous cell carcinoma of the liver originating from non-parasitic cysts after a 15 year follow-up. Eur J Gastroenterol Hepatol. 2004;16:1051-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Wei D, Lu L, Ying C, Qingsong K, Dongbo L, Feibo L. Primary hepatic adenosquamous carcinoma: A rare case report. Indian J Pathol Microbiol. 2021;64:S140-S142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Yoo TK, Kim BI, Han EN, Kim DH, Yoo JH, Lee SJ, Cho YK, Kim HJ. Primary squamous cell carcinoma of the liver: a case report. Clin Mol Hepatol. 2016;22:177-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Zhu KL, Li DY, Jiang CB. Primary squamous cell carcinoma of the liver associated with hepatolithiasis: a case report. World J Gastroenterol. 2012;18:5830-5832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |