Published online Jul 6, 2022. doi: 10.12998/wjcc.v10.i19.6736
Peer-review started: February 15, 2022
First decision: March 25, 2022
Revised: March 27, 2022
Accepted: April 22, 2022
Article in press: April 22, 2022
Published online: July 6, 2022
Processing time: 129 Days and 4.3 Hours
Lipemia retinalis (LR) is a rare disease related to hypertriglyceridemia. However, the symptoms of hypertriglyceridemia are insidious and difficult to detect without blood tests. The fundus is the only site where blood vessels can be observed directly. Understanding the specific performance of LR in multimodal imaging fundus examinations can help diagnose more patients with abnormal hyperlipidemia.
A 29-year-old woman with type 2 diabetes presented to our clinic complaining of a six-day loss of visual acuity in the left eye. The fundus color images showed typical LR: Arteries and veins were the same pink-white color. Infrared images showed hyperinfrared reflections of the arteries and veins. Optical coherence tomography (OCT) showed numerous high point-like reflections in the retinal section, corresponding to different calibers of blood vessel sections. Medium reflections were seen in the big vessels of the choroid. Fundus fluorescein angiography (FFA) and optical coherence tomography angiography (OCTA) showed no significant changes. Laboratory examination found a total cholesterol level of 13.98 mmol/L, triglyceride 20.55 mmol/L, which confirmed the diagnosis of LR. After treatment to lower blood lipids and control blood glucose, the fundus imaging showed that the blood lipids in the patient had returned to normal.
LR shows specific changes in fundus color photography, infrared photography, and OCT. FFA and OCTA were not sensitive to LR changes.
Core Tip: Lipemia retinalis (LR) is rarely seen in clinics. It occurs in individuals with extreme hypertriglyceridemia, usually when triglyceride levels reach 2000–2500 mg/dL. However, it is often not recognized by ophthalmologists. Previous examinations have focused on changes in color shown in fundus photography of LR. In this patient, retinal abnormalities were detected by fundus imaging, which were further confirmed by blood tests. We report the characteristics of LR multimodal imaging, which deepened our understanding of the imaging characteristics of LR.
- Citation: Zhang SJ, Yan ZY, Yuan LF, Wang YH, Wang LF. Multimodal imaging study of lipemia retinalis with diabetic retinopathy: A case report. World J Clin Cases 2022; 10(19): 6736-6743
- URL: https://www.wjgnet.com/2307-8960/full/v10/i19/6736.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i19.6736
First described by Heyl[1] in 1880, lipemia retinalis (LR) is a rare disease caused by the disturbance of lipids in the blood. It is characterized by pink-white retinal blood vessels. The common secondary causes of LR include uncontrolled type 1 or type 2 diabetes mellitus, endocrine disorders (e.g., obesity, metabolic syndrome, hypothyroidism, and hypercortisolism), and medications[2,3]. Here, we present the case of an LR patient with diabetes mellitus and provide multimodal images of the fundus.
A 29-year-old woman presented to our clinic complaining of a six-day loss of visual acuity in her left eye.
The patient’s symptoms began a month ago, with a decrease in the visual acuity of the left eye, which had worsened over the last six days. Recently, the patient had eaten a great deal of fried food, and she had poor blood glucose control. No other diabetic complications were observed.
The patient had had type 2 diabetes mellitus for two years almost certainly because of poor diet and lack of exercise. The patient had been treated for diabetic retinopathy and followed up at our hospital.
The patient had diabetes for two years and denied any diagnoses of family history. The patient had normal menstruation.
The physical examination was normal.
The results of the laboratory examinations revealed the following: total cholesterol (CHO) level of 13.98 mmol/L (3.6–6.5); triglyceride (TG) 20.55 mmol/L (0–1.71); high density lipoprotein (HDL) 0.75 mmol/L (0.83–1.96); low density lipoprotein (LDL) 6.91 mmol/L (0–3.36); apolipoprotein-A1 0.64 g/L (1.0–1.6); apolipoprotein-B 0.49 g/L (0.6–1.1); total protein 85.8 g/L (65–85); fasting blood glucose 10.89 mmol/L (3.9–6.1) (Table 1); glycosylated hemoglobin 12% (4.5–6.3); hemoglobin 216 g/L (115–150); mean corpuscular hemoglobin (MCH) 46.2 pg (27–34); MCH concentration 548 g/L (316–354); erythrocyte sedimentation rate 40 mm/h (0–20); and blood uric acid 424 μmol/L (140–340). Normal reference values are shown in parentheses after the results. The results of other blood routine tests for C-reactive protein 4.45 mg/L (0–10), liver function, kidney function, electrolytes, and homocysteine were normal. A B ultrasound of bile, pancreas, and spleen, an electrocardiogram, and a computed tomography of chest showed normal results. The B ultrasound showed that the patient had fatty liver.
| Date and normative values | CHO (mmol/L) | TG (mmol/L) | HDL (mmol/L) | LDL (mmol/L) | Apolipoprotein-A1 (g/L) | Apolipoprotein-B (g/L) | Blood glucose (mmol/L) |
| August 24, 2021 | 13.98 | 20.55 | 0.75 | 6.91 | 0.64 | 0.49 | 10.89 |
| September1, 2021 | 4.46 | 1.84 | 0.79 | 3.06 | 0.72 | 0.94 | 8.47 |
| Normative values | 3.6-6.5 | 0-1.71 | 0.83-1.96 | 0-3.36 | 1.0-1.6 | 0.6-1.1 | 3.9-6.1 |
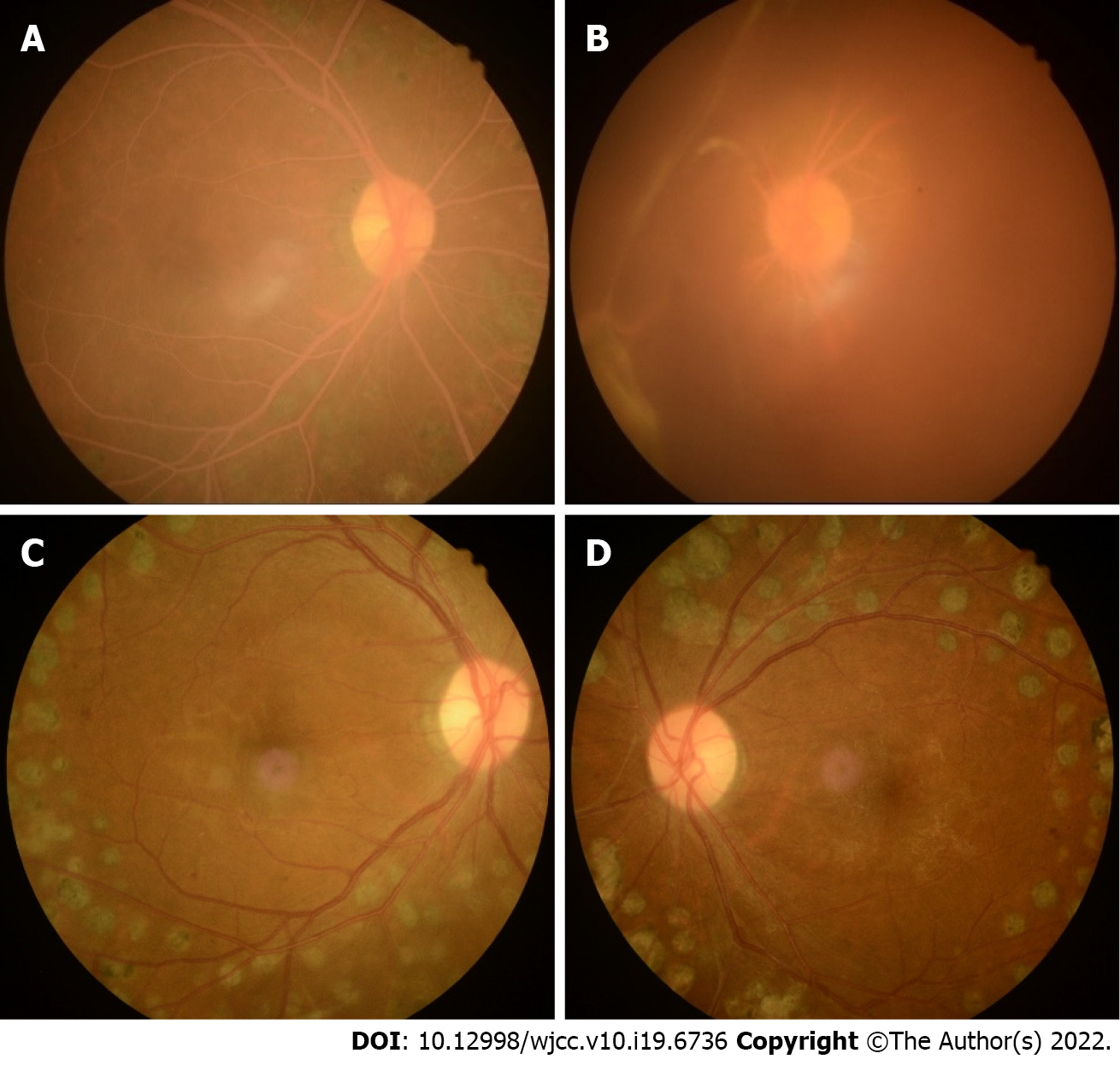
Fundus color images (Kowa, Nonmyd 7, Kowa, Japan) showed a pink–white color of the fundus, arteries, and veins. Arteries and veins could not be distinguished by color, only by the caliber of the vessels (Figure 1A and B). The fundus of the left eye was covered by vitreous blood. Only the optic disk could be seen (Figure 1B).
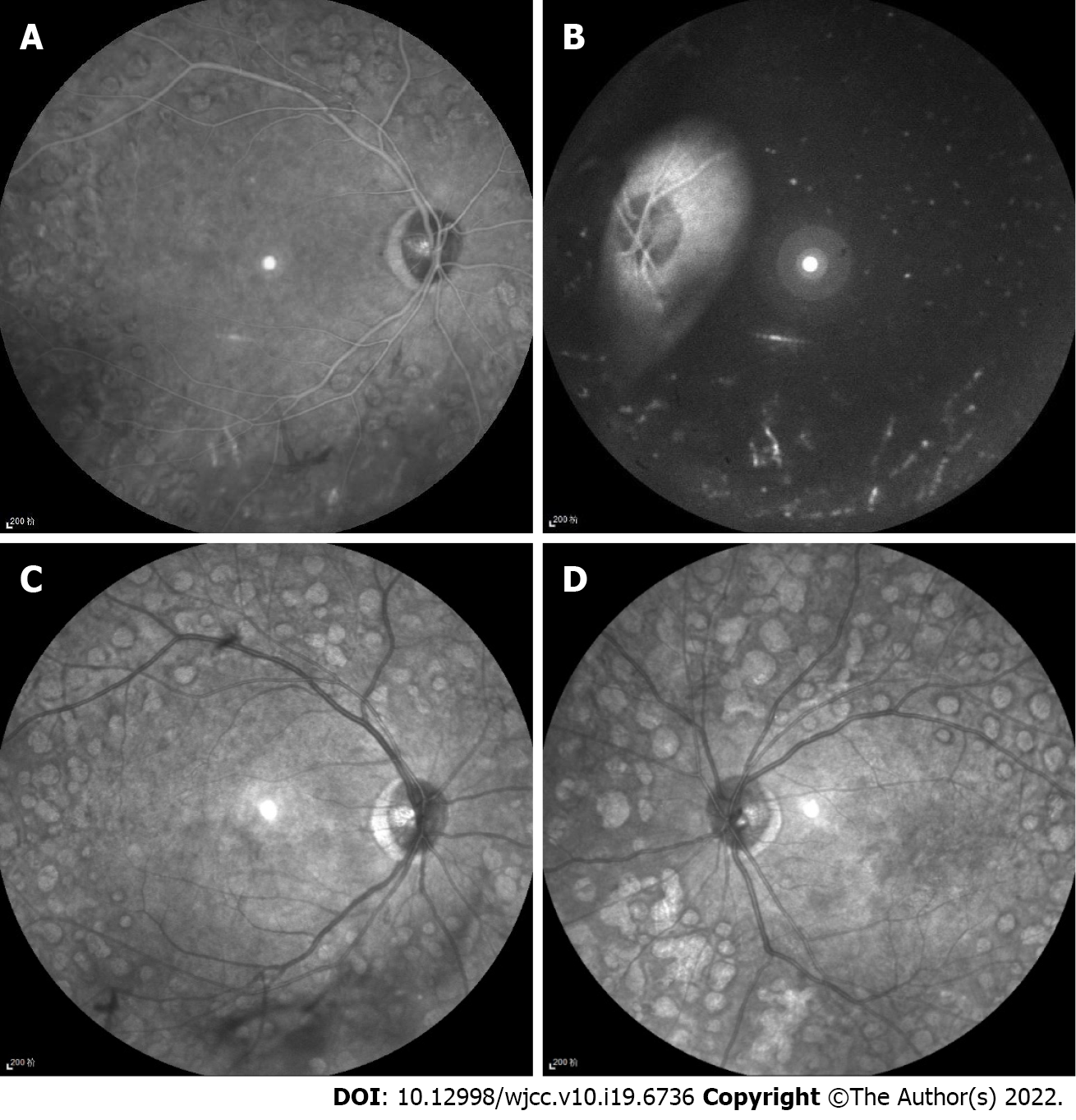
Infrared images (Heidelberg Spectralis, Heidelberg Engineering, Heidelberg, Germany) showed hyperinfrared reflection of arteries and veins, unlike the hypoinfrared reflection of the normal fundus. Arteries and veins could not be distinguished by infrared reflection (Figure 2).
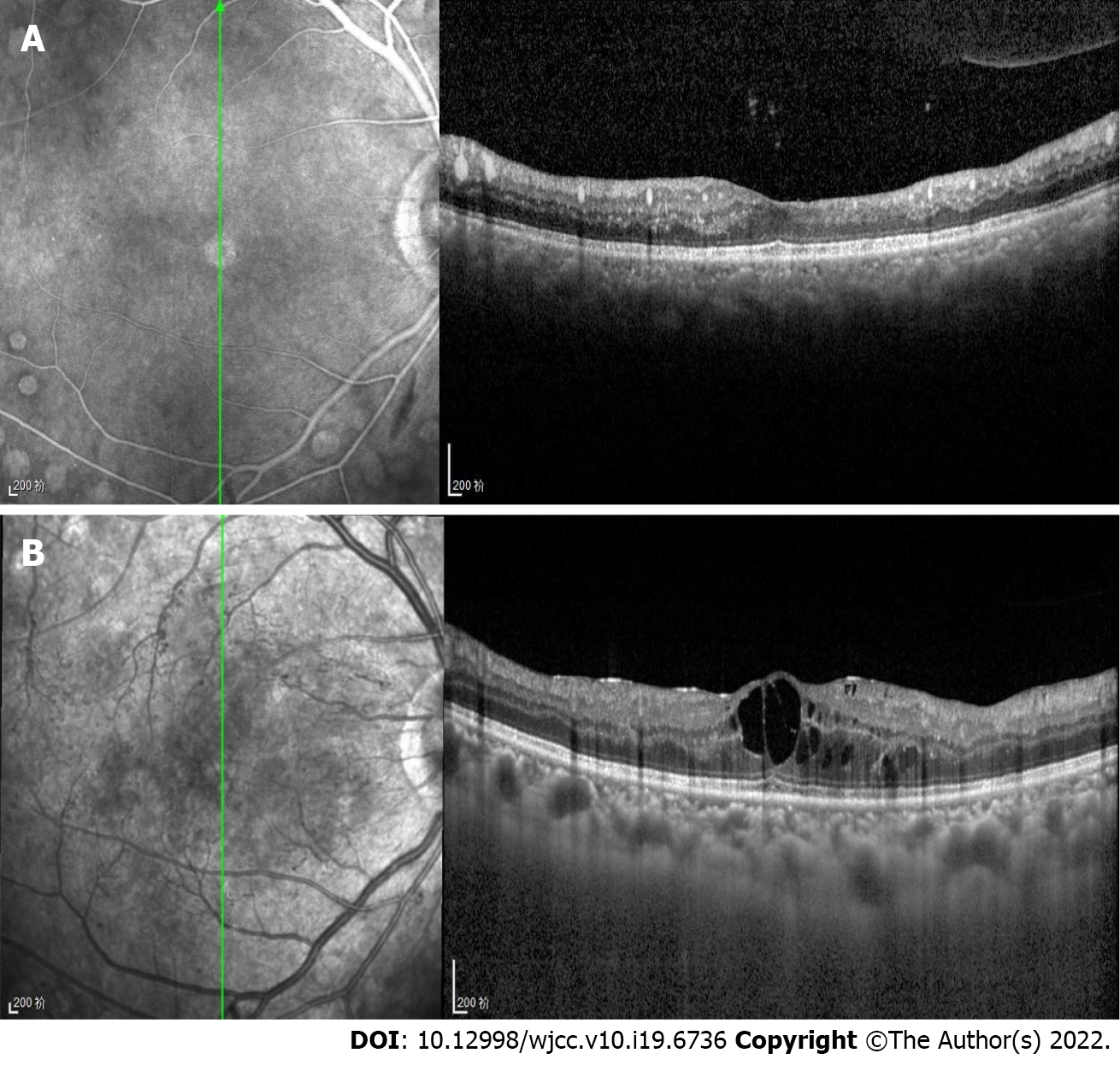
Optical coherence tomography (OCT) (Heidelberg Engineering, Heidelberg, Germany) showed numerous high point-like reflections in the retinal section, which corresponded to different caliber blood vessel sections (Figure 3A). The big vessels in the choroid showed medium reflections, unlike the low reflections of normal choroid vessels. Careful observations were required to detect the great choroidal vessels (Figure 3).
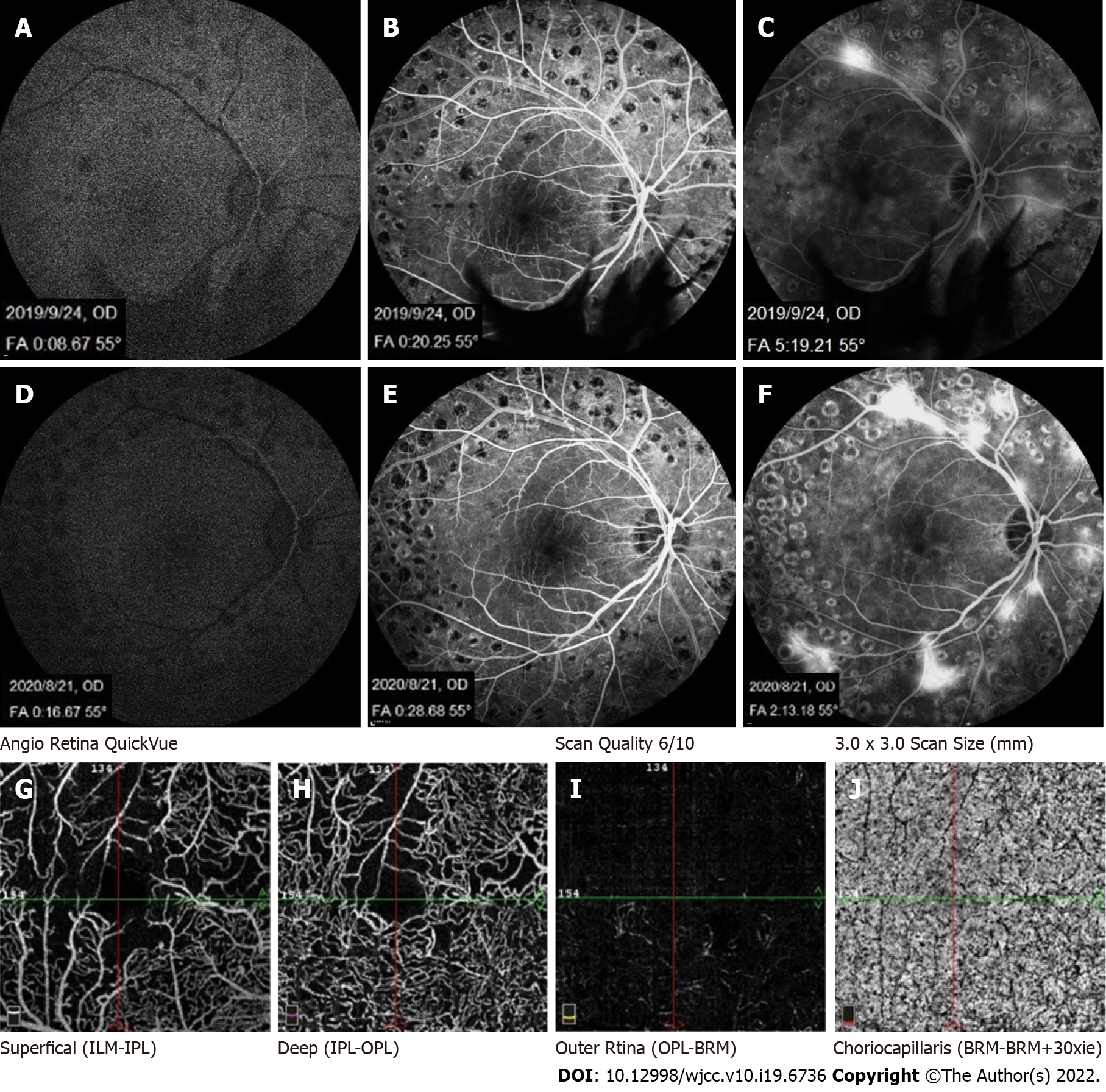
Fundus fluorescein angiography (FFA) (Heidelberg Spectralis, Heidelberg Engineering, Heidelberg, Germany) showed no significant difference in retinal filling time and fundus fluorescence between the patient’s hypertriglyceridemia condition and a normal blood lipid condition (Figure 4).
The patient also underwent optical coherence tomography angiography (OCTA) (Avanti RTVue XR100-2, Optovue Inc, Fremont, CA, United States). The retinal blood flow showed decreased vascular density in the macular area consistent with the fundus fluorescein angiography, which was caused by diabetic retinopathy (Figure 4G).
We made the LR.
The patient was prescribed a low-salt, low-fat diabetic diet. Insulin injections were prescribed to control blood glucose levels. Fenofibrate capsules (0.2 g) were taken once a day with meals. One week later, the blood lipids were close to normal. Laboratory examinations revealed a total CHO level of 6.90 mmol/L, TG 2.74 mmol/L, HDL 0.92 mmol/L, LDL 5.92 mmol/L, apolipoprotein-A1 0.67 g/L, and apolipoprotein-B 1.41 g/L (Table 1).
In the follow-up treatment, the blood lipid and blood glucose levels of the patient were well controlled. At six months follow-up, the vitreous hemorrhage in the left eye had been absorbed. The fundus color and retinal blood vessel color were normal (Figure 1C and D). Diabetic retinopathy was well controlled, and there was no edema or exudation in the macular area.
LR is a rare disease, which has been little reported[4,5]. However, it has been reported that, when TG levels were less than 1500 mg/dL, the peripheral retina became a pink–white color; as TG increased, the pink–white vessels moved toward the macula; when TG was greater than 2500 mg/dL, all vessels were pink–white, and the arteries and veins showed the same color[6]. In the patient case reported here, TG was 20.55 mmol/L, equaling 1820.73 mg/dL, but the entire fundus vessels had already become pink–white. It has been reported that not all hypertriglyceridemia cases present LR, and other factors should be considered, such as changes in hematocrit and vessel translucency[7]. In the present case, TG was low and the fundus was seriously affected, which suggests that the levels in the previous standard were not strict but relative. The occurrence of LR can be affected by other factors that should be determined in further research.
There are only a few imaging studies on LR. It is usually diagnosed based on color fundus photographs[8]. A few previous studies have observed multicolor scanning laser imaging and OCT in LR[9-12]. In the present case, infrared imaging showed hyperinfrared reflections of retinal large vessels, which differed from normal hypoinfrared reflections. To our knowledge, this phenomenon has not been previously reported. Infrared imaging can be performed simultaneously with FFA or OCT. In the OCT, which employed Spectralis OCT (Spectralis HRA + OCT, Heidelberg Co.), infrared imaging was used to indicate the location of B-scan OCT. Each B-scan OCT corresponded to an infrared photograph, and the site of the B-scan OCT in the fundus is present as a line in the infrared image. At present, OCT is commonly applied, which suggests that medical practitioners should pay more attention to infrared images during OCT examinations to better detect hypertriglyceridemia in patients.
In the present case, the OCT examination revealed high reflections in retinal blood vessels and medium reflections in the choroid great vessel. According to Özturk et al[10], high point-like reflections in the retina are caused by lipid extravasation from retinal vessels. However, in the present case, by comparing the OCT B-scan layer with the corresponding infrared image, we found that the high reflection points of the retina in the B-scan corresponded to cross sections of retinal blood vessels of different diameters. The reflection in the choroid vessel was higher than normal, but lower than the reflection in the retinal vessels. These findings indicate that the increase in lipids in choroid vessels caused higher reflection. However, the retinal pigment epithelium blocked the reflection of the choroid, so the reflection in the choroid vessels was lower than the reflection in the retinal vessels. The change in vessels in the retina and choroid indicated the abnormal blood composition of the body vessels, which required treatment to reduce other complications, such as pancreatitis and gastrointestinal hemorrhage[13].
It is well known that hypertriglyceridemia causes slow blood flow. However, in this case, the filling time was normal by FFA, which we considered the reason that the proportion of lipid components in the blood did not significantly slow the retinal blood flow speed. We observed the fundus of a patient with LR using OCTA for the first time. OCTA can display blood flow signals within a limited speed range only, and the slowest detectable blood flow depends on the time interval between two consecutive OCT B-scan sequences. If the blood flow in the lesion is slower than the slowest detectable blood flow, it cannot be displayed in OCTA. In the present case, the OCTA results were consistent with the FFA results, suggesting that the sensitivity of FFA and OCTA to hypertriglyceridemia was not high. The application of artificial intelligence (AI) has become increasingly extensive. Relevant previous studies have linked fundus photography to the condition of the human body, such as blood lipids, blood pressure, and blood sugar[14]. The combination of various non-invasive fundus imaging examinations and AI has the potential to contribute to human health in the future.
LR shows specific changes in fundus color photography, infrared photography, and OCT, which can help detect changes in hypertriglyceridemia. In the case reported here, FFA and OCTA were not sensitive to changes in LR. The diagnosis and treatment of hypertriglyceridemia could benefit from an improved understanding of the manifestations of LR in diverse imaging results.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Ophthalmology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kharlamov AN, Netherlands; Mansour AM, Lebanon S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Heyl AG. Intra-Ocular Lipæmia. Trans Am Ophthalmol Soc. 1880;3:54-66. [PubMed] |
| 2. | Leaf DA. Chylomicronemia and the chylomicronemia syndrome: a practical approach to management. Am J Med. 2008;121:10-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Gayathri K, Ramalingam PK, Santhakumar R, Manjunath BV, Karuppuswamy N, Vetriveran B, Selvamani S, Vishnuram P, Muruganathan A, Natarajan K. Lipemia Retinalis due to Secondary Hyperlipidemia in Type 1 Diabetes Mellitus. J Assoc Physicians India. 2016;64:83-84. [PubMed] |
| 4. | Kempegowda P, Chen W, Melson E, Leong A, Amrelia P, Syed A. Incidental finding of lipaemia retinalis on diabetic retinal screening. Endocrinol Diabetes Metab Case Rep. 2021;2021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Ortiz de Salido-Menchaca J, Tazón-Varela MA, de la Hera-Vegas D, Herreras-Martínez R, Álvarez-Agudelo SA, Arencibia-Hernández N. Retinal lipemia as expression of hyperchylomicronemia syndrome. Colomb Med (Cali). 2021;52:e7024059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Park YH, Lee YC. Images in clinical medicine. Lipemia retinalis associated with secondary hyperlipidemia. N Engl J Med. 2007;357:e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Vinger PF, Sachs BA. Ocular manifestations of hyperlipoproteinemia. Am J Ophthalmol. 1970;70:563-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Wetzel B, Mylonas G, Puntus T, Prager F, Bernhart C, Amon M. Lipemia Retinalis During Chemotherapy with Adjunctive Glucocorticoid Treatment in a Patient with Colon Carcinoma. Retin Cases Brief Rep. 2021;15:450-452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Rivera-De La Parra D, Perez-Peralta L, Toldi J, Levine J, Fikhman M, Graue-Hernandez EO. Multicolor Scanning Laser Imaging in Lipemia Retinalis. Retin Cases Brief Rep. 2017;11 Suppl 1:S132-S135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Özturk BT, Bozkurt B, Meşen A, Gonul S, İpekci SH. Spectral-Domain Optical Coherence Tomography Findings in Lipemia Retinalis. Ophthalmic Surg Lasers Imaging Retina. 2016;47:589-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Teoh CS, Yuen YS. Spectral-Domain OCT Imaging of Lipemia Retinalis. Ophthalmol Retina. 2021;5:1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Tanaka M, Azuma T, Shinohara K. Lipemia Retinalis: Hyperreflective Vascular Changes Detected by Optical Coherence Tomography. Retina. 2021;41:e10-e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Rosenson RS, Shott S, Tangney CC. Hypertriglyceridemia is associated with an elevated blood viscosity Rosenson: triglycerides and blood viscosity. Atherosclerosis. 2002;161:433-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Zhang L, Yuan M, An Z, Zhao X, Wu H, Li H, Wang Y, Sun B, Ding S, Zeng X, Chao L, Li P, Wu W. Prediction of hypertension, hyperglycemia and dyslipidemia from retinal fundus photographs via deep learning: A cross-sectional study of chronic diseases in central China. PLoS One. 2020;15:e0233166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |