Published online Jul 6, 2022. doi: 10.12998/wjcc.v10.i19.6626
Peer-review started: December 12, 2021
First decision: January 26, 2022
Revised: February 18, 2022
Accepted: May 16, 2022
Article in press: May 16, 2022
Published online: July 6, 2022
Processing time: 194 Days and 2.9 Hours
Extramedullary hematopoiesis rarely occurs within the liver alone, and is easily misdiagnosed. The radiological literature on this disease is exclusively case reports. There is a paucity of literature on the role of magnetic resonance imaging (MRI). The most common imaging modalities used are computed tomography and ultrasound. This report aims to provide more data on the appearance of extramedullary hematopoiesis using MRI to help radiologists establish the diagnosis.
Three patients (one male and two females) were incidentally found to have a hepatic mass or nodule, without hepatomegaly or splenomegaly. Laboratory tests including liver function, serum hepatic tumor markers, and hepatitis serologic markers were normal. On MRI scans, all lesions showed lower signal intensity on in-phase images than on out-phase images. One case showed changes in signal intensity on T2 weighted images (WI) and diffusion WI, which shifted from hyperintensity to hypointensity with size enlargement between two rounds of imaging examination. These lesions exhibited different enhancement patterns on dynamic contrast enhancement series.
The MRI signal change and in-/out-phase image might provide useful information and help radiologists establish the diagnosis of intrahepatic extramedullary hematopoiesis.
Core Tip: The magnetic resonance imaging signal change, including the signal change on T2 weighted image (WI) and diffusion WI, and the in-/out-phase image might provide useful information and help radiologists establish the diagnosis of extramedullary hematopoiesis. Intrahepatic extramedullary hematopoiesis (IEMH) may exhibit different patterns of enhancement, depending on different stages of the disease. IEMH can also be seen in patients without evidence of hematological disease.
- Citation: Luo M, Chen JW, Xie CM. Magnetic resonance imaging features of intrahepatic extramedullary hematopoiesis: Three case reports. World J Clin Cases 2022; 10(19): 6626-6635
- URL: https://www.wjgnet.com/2307-8960/full/v10/i19/6626.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i19.6626
Extramedullary hematopoiesis seldom occurs within the liver alone[1]. In this rare condition, the lesion can manifest as a mass with no typical radiologic findings, making it difficult to diagnose and differentiate from other hypervascular neoplasms[2]. We present three cases of intrahepatic extramedullary hematopoiesis (IEMH) occurring solely in the liver. These lesions showed lower signal intensity on in-phase images than on out-phase images. In addition, the first case was unique in that the lesion showed changes in magnetic resonance imaging (MRI) signal intensity with size enlargement between two rounds of imaging examination. These manifestations have never been reported before.
Case 1: A 50-year-old woman without any discomfort was admitted to our hospital due to an intrahepatic mass with interval growth.
Case 2: A 30-year-old female with a five-month history of Hodgkin’s lymphoma (nodular sclerosis) was referred to hospital.
Case 3: A 52-year-old male was admitted to our hospital due to the incidental discovery of hepatic nodules. He had no history of alcoholism.
Case 1: Negative.
Case 2: The patient denied alcoholism and had no other symptoms or discomfort.
Case 3: No symptoms or discomfort.
Negative.
Case 1: The patient’s medical history included thyroid carcinoma and lung adenocarcinoma. She had undergone total thyroidectomy in November 2014 and lobectomy of the right lower lung lobe in October 2019, without radiotherapy or adjuvant chemotherapy in the subsequent follow-up.
Case 2: Prior to the patient’s initial treatment, no focal liver lesion was detected by ultrasound or positron emission tomography (PET)/computed tomography (CT). She had undergone 5 cycles of chemotherapy consisting of Brentuximab Vedotin + Adriamycin, Vinblastine (Dacarbazine).
Case 3: The patient had no previous medical and family history.
Case 1: Physical examination was normal.
Case 2: Physical examination was normal. The liver and spleen were impalpable.
Case 3: Physical examination was negative.
Case 1: Laboratory tests, including blood, liver function, and serum tumor marker tests (alpha fetoprotein, carbohydrate antigen 19-9, carbohydrate antigen 125, carcinoembryonic antigen, and protein induced by Vitamin K absence or antagonist-II), were all within the normal range. Hepatitis serologic markers such as hepatitis B surface antigen and hepatitis C virus antibodies were negative, with no history of alcoholism.
Case 2: Platelet, white, and red blood cell counts were normal. The serum tumor markers and hepatitis serologic markers were negative.
Case 3: Laboratory tests revealed that liver function, serum hepatic tumor markers, and hepatitis serologic markers were all normal.
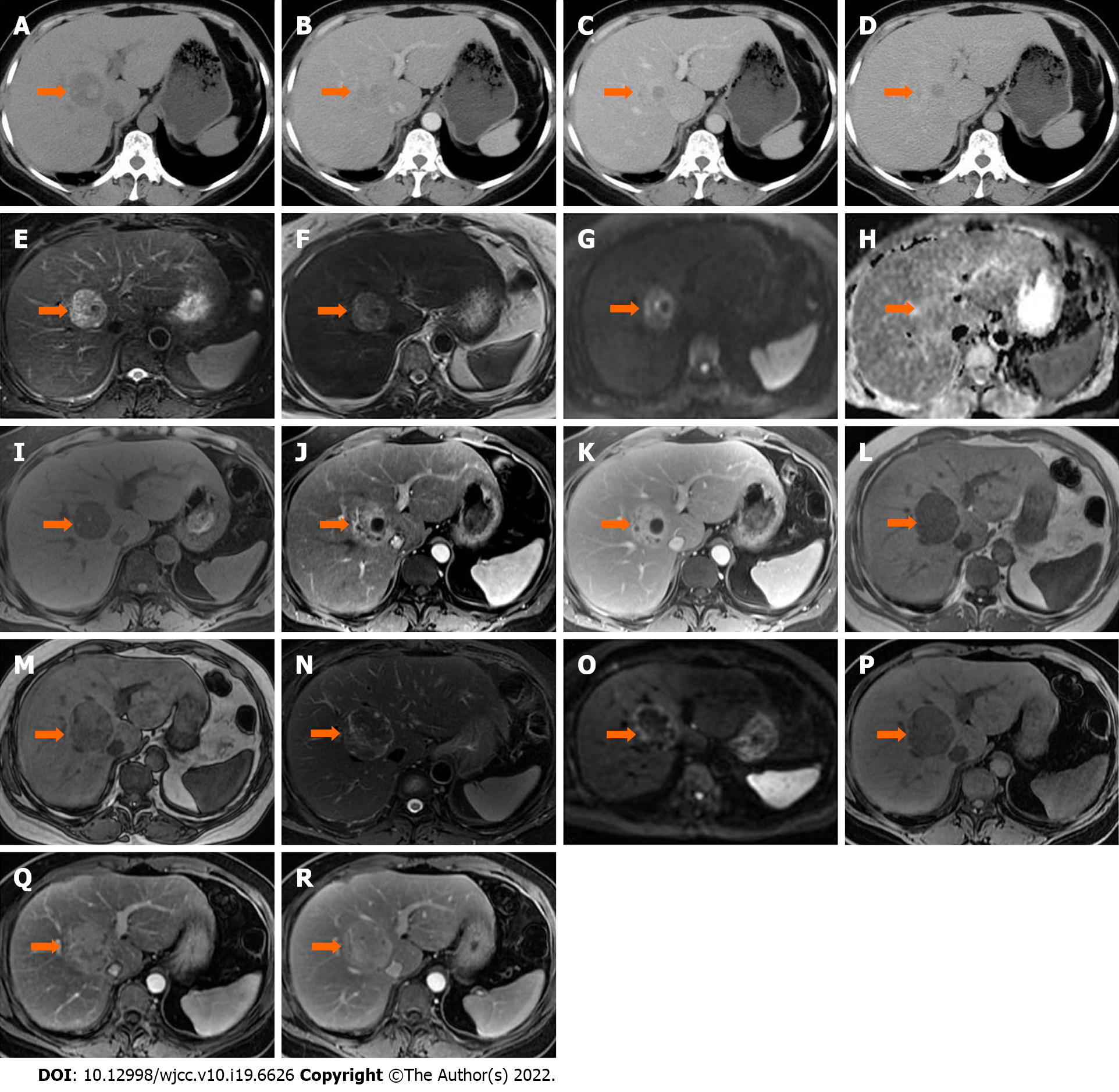
Case 1: In September 2019, pre-operative ultrasound showed a hyperechoic lesion measuring 24 mm × 23 mm in segment VIII. The lesion was diagnosed as possible hemangioma.
In January 2020, during a routine examination, the lesion showed an increase in size on abdominal ultrasound. An abdominal CT scan (Discovery 750 HD, GE Healthcare, Milwaukee, WI, United States) was performed. The lesion appeared heterogeneously hypodense on unenhanced CT (40 HU), and 35 mm × 32 mm × 20 mm in size. The lesion was moderately hyperdense in the arterial phase (81 HU) and markedly hyperdense in the portal venous phase (106 HU), and showed persistent enhancement in the 5-min delayed phase (110 HU) (Figure 1A–D). The following day, liver MRI was performed for further characterization and assessment of the lesion (Trio 3.0 T, Siemens, Erlangen, Germany). The lesion was hypointense relative to surrounding liver tissue on T1 weighted images (WI) with and without fat saturation (FS), and was heterogeneously hyperintense on T2WI or T2WI-FS with clear demarcation. On dynamic contrast enhancement series, the lesion showed considerable enhancement in the arterial phase, remaining hyperintense relative to surrounding liver tissue in both the portal venous and delayed phases (6 min) following the injection of 0.1 mmol/kg of Gd-DTPA (Magnevist, Bayer Healthcare, Berlin, Germany) (Figure 1E–K). The initial diagnosis included atypical angioleiomyolipoma, adenoma, and single metastasis. The patient declined to undergo surgery and opted for observation.
In June 2020, she underwent another abdominal MRI examination. The lesion had enlarged to 50 mm × 46 mm × 43 mm, and it was heterogeneously hypointense on T1WI-FS with scattered foci of hyperintense areas. The signal intensity was significantly lower on the in-phase image than on the out-phase image. The lesion was isointense or mildly hypointense relative to liver tissue on diffusion weighted images (DWI), T2WI, and T2WI-FS. The pattern of enhancement was overt in the arterial phase and remained hyperintense on the delayed phase, without vessel encasement or invasion (Figure 1L–R).
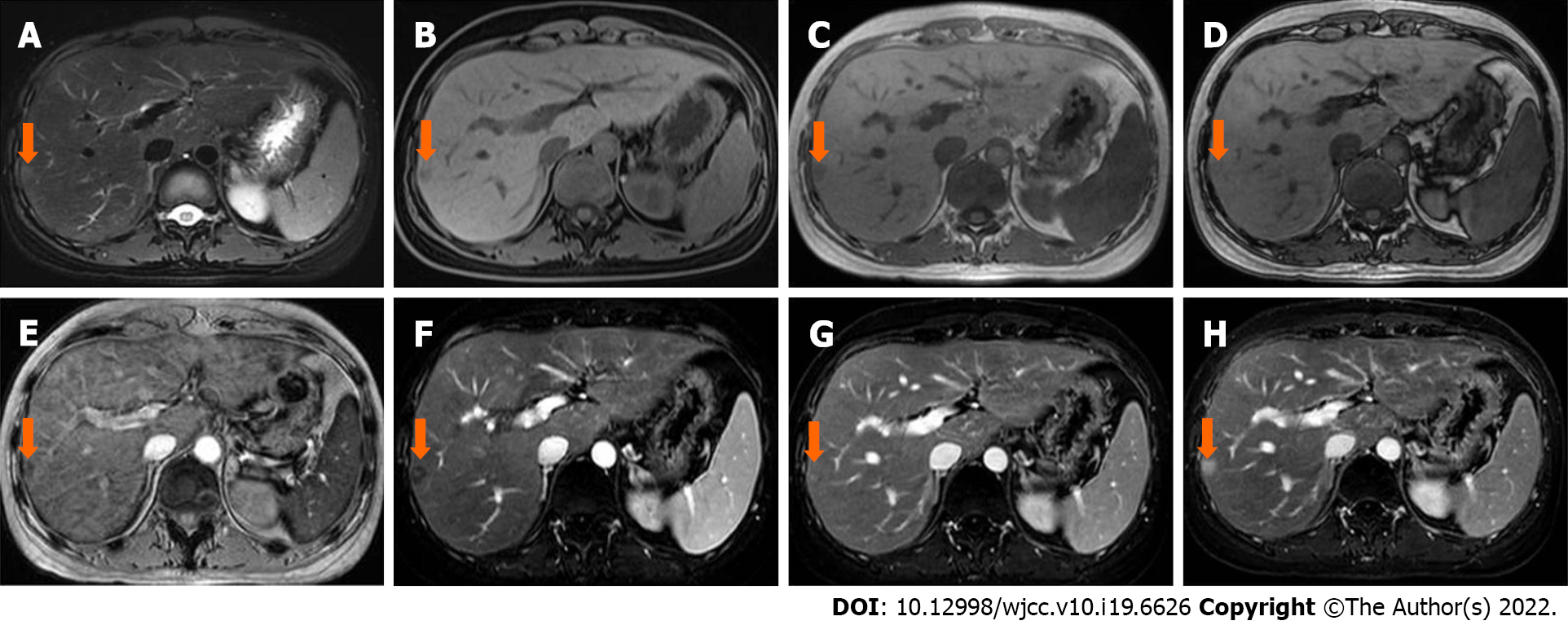
Case 2: In April 2020, abdominal ultrasound was performed during a routine examination and a slightly hyperechoic lesion was found in segment VII. The patient then underwent an MRI scan (Discovery 750W, GE Healthcare, Milwaukee, WI, United States). A 10 mm × 8 mm sized, slightly hyperintense and hypointense lesion was identified on T2WI-FS and T1WI-FS, respectively (Figure 2A and B). The signal intensity was lower on the in-phase image than that on the out-phase image (Figure 2C and D). The signal intensity was lost on susceptibility weighted imaging (SWI) (Figure 2E). After administration of Gd-DTPA, the lesion showed poor enhancement in the arterial phase and enhanced gradually and persistently up to 6 min (Figure 2F-H). The initial diagnosis was lymphoma infiltration.
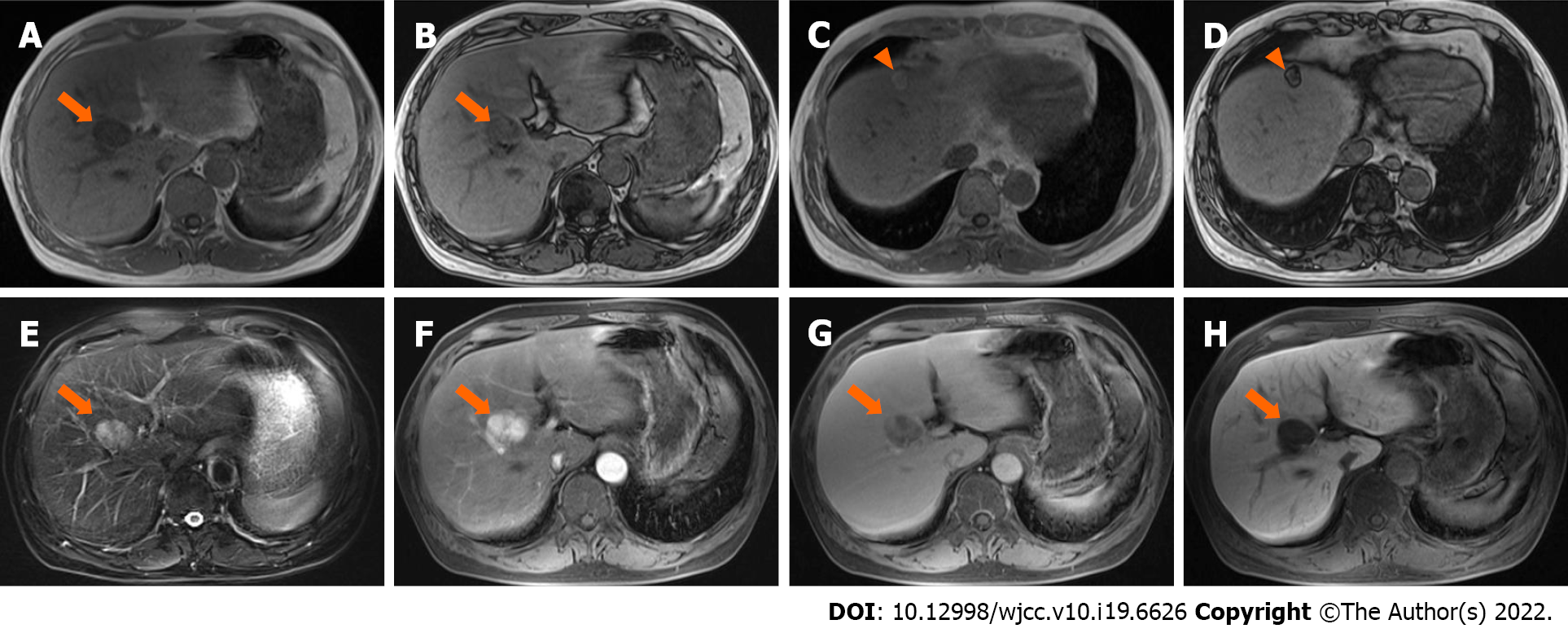
Case 3: MRI (Trio 3.0 T, Siemens, Erlangen, Germany) scans showed a lesion located in the transitional area (34 mm × 27 mm in size) between segment V and VIII, with homogeneously low and high signal intensity on T1WI-FS and T2WI-FS, respectively. The signal intensity on the in-phase image was lower than that on the out-phase image (Figure 3A-E). After administration of Gd-EOB-DTPA, the lesion showed intense enhancement in the arterial phase, with no persistent enhancement in the portal venous and transitional phases, and was hypointense in the hepatobiliary phase (Figure 3F-H). The possible diagnosis at that time included hepatoma, adenoma, and angiomyolipoma.
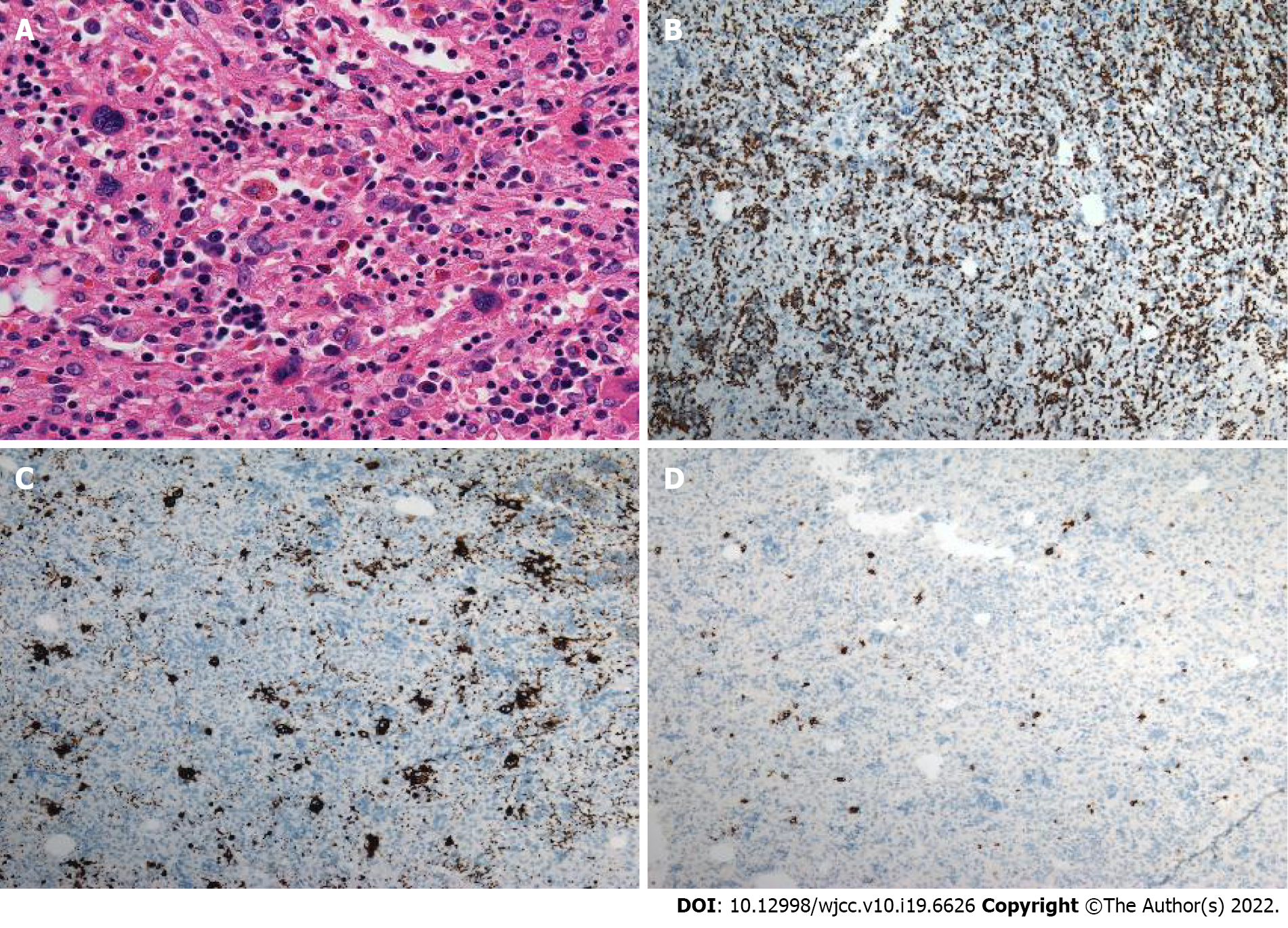
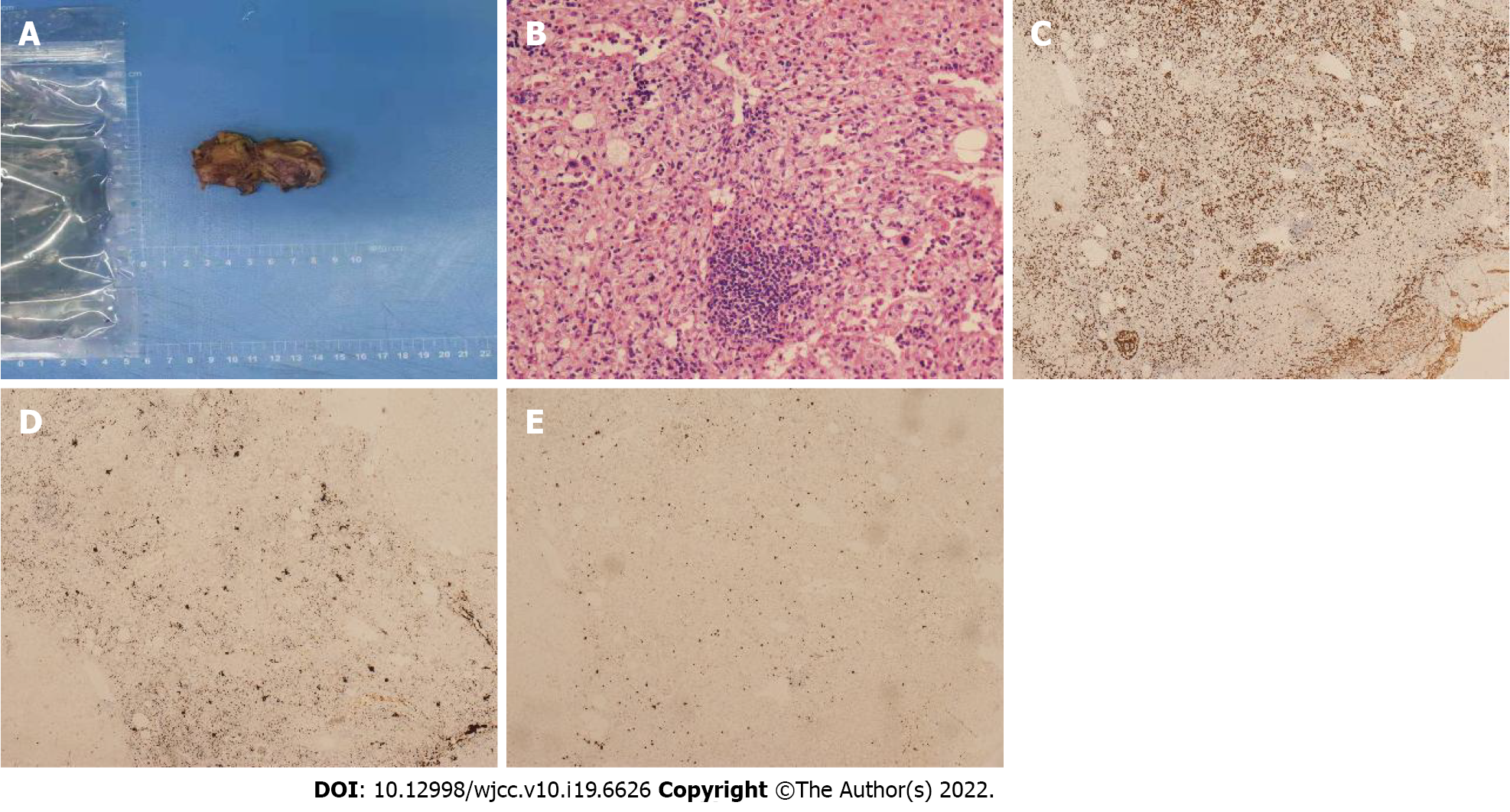
Case 1: Macroscopically, a mass with reddish appearance without necrosis or hemorrhage was seen in the resected specimen. Microscopically, megakaryocytes, and erythroid cells were scattered within the hepatocyte cords (Figure 4). Immunohistochemical staining including CD3, CD20, CD61, CD235, and MPO was performed, and the cells were positive for these markers. These findings were consistent with EMH.
Case 2: Percutaneous fine-needle aspiration biopsy was performed under ultrasound guidance, and cytology showed EMH.
Case 3: The final histopathological diagnosis was EMH (Figure 5).
Case 1: The patient underwent hepatic segmentectomy.
Case 2: After another 4 cycles of chemotherapy, liver MRI (August 2020) showed similar findings, without any increase in the size of the lesion. As the diagnosis was uncertain and the lesion was small, we decided to obtain histopathologic confirmation.
Case 3: As the possibility of malignancy could not be completely excluded, the patient underwent hepatic segmentectomy, although he did not have any predisposing factors for hepatoma.
Case 1: The patient has no recurrent lesions or evidence of new lesions.
Case 2: The patient has no recurrent lesions or evidence of new lesions.
Case 3: The patient has no signs of recurrence.
IEMH is a rare, benign condition of the liver. The radiological literature on this disease is exclusively case reports. All of these cases were misdiagnosed, and IEMH was not considered in the preoperative imaging diagnosis and differentiation. The most common imaging modalities used were CT and ultrasound[3-7]. There is a paucity of literature on the role of MRI, which might be due to the limitations of imaging technology at that time. Moreover, the quality of the images provided in those studies was low. Thus, the radiologic features seemed to be non-specific, and it may have been difficult to make a correct diagnosis preoperatively.
From the pathological aspect, EMH is characterized by numerous hematopoietic cells. Granulocytes, megakaryocytes, and erythrocytes show a mixed distributed within the lesion, in which adipocytes and fibrous tissue can also be seen. Thus, EMH is positive for MPO, CD61, and CD235. Due to the large number of erythrocytes scattered within EMH, CD235 staining is strong and diffusely positive. The presence of iron in the erythrocytes and different amounts of adipose tissue can potentially affect the radiologic appearance.
In recent years, with the development of MRI, there have been sporadic reports regarding the published MRI findings of IEMH. Belay et al[8] provided the first description of T2*WI in their case, considering that this technique might have a potential role in MRI diagnosis. The lesion was hypointense on T2*WI, similar to the signal intensity of the hepatic background on this gradient recalled echo (GRE) sequence, indicating that the lesion had as much iron deposition as the liver parenchyma in the setting of secondary hemochromatosis owing to repeated blood transfusions. Lee et al[2] reported the superparamagnetic iron oxide (SPIO)-enhanced MRI in their case, while Zhang and Zhu[9] applied the chemical shift images in their report. In our three cases, lesions on in-phase images exhibited lower signal intensity than on out-phase images. In Case 2, the lesion was hypointense in SWI. In Case 1, there were signal changes across two time intervals on T1WI, T2WI, and DWI, which shifted from hyperintensity to hypointensity, and without restricted diffusion in two sets of apparent diffusion coefficient (ADC) maps. We speculate that these manifestations and signal changes may be mainly ascribed to iron deposition, depending on iron evolution in the lesion, instead of the increase in cell density or change in intralesional content such as mucin, necrosis, fibrosis, or even calcification. Iron could impact the magnetic field intensity and homogeneity; thus, the signal on DWI could be diverse due to iron content and evolution in IEMH, but not due to diffusion restriction on the ADC map. However, there are limited data regarding EMH on DWI. Rasche et al[10] observed that EMH in the spleen could impact the DWI signal. The GRE sequence was unequivocally sensitive to the presence of small amounts of iron. It should also be noted that, when the echo time was longer, the signal indicating iron deposition in the lesion grew less intense[11]. Such lesions often showed lower signal intensity on SWI and in-phase images relative to the out-phase images.
Some cases report using scintigraphy to diagnose IEMH[3,12,13], and the lesions showed Tc-99m uptake. In addition, PET was applied in the diagnosis of EMH located in the paraspinal region, peritoneum and lung[1,13,14]. High uptake values were observed. However, there are no reports on the PET characteristics of IEMH. We speculate that its property on 18F-FDG might be from mild to intense activity, which may be related to the different stages of the disease. In the initial stage, the synthesis and proliferation of hematopoietic cells were active, thus presenting as hypermetabolism, whereas in the static stage, IEMH could show low uptake.
There are discrepancies regarding the radiologic characteristics in different studies (Table 1). IEMH was described as a fat-containing lesion. For example, in reports published by Gupta et al[15], Navarro et al[6] and Cao and Wang[16], multiple lesions showed fat density. However, the case by Zhang and Zhu[9] showed a solitary lesion without any fat content, as indicated by the lack of fat signal alteration. With regard to enhancement pattern, the lesion presented as a hypervascular mass with heterogeneous enhancement in the report by Wong et al[17] and homogeneous avid enhancement in the report by Zhang and Zhu[9], while mild enhancement was reported by Tamm et al[12]. Elsayes et al[18] considered that an active lesion exhibited iso or hyperintensity on T1WI and T2WI, and was enhanced after injection of contrast material, while older lesions could be hypointense on T1WI and T2WI, and might show no enhancement. Nevertheless, Kumar et al[19] provided a case where the lesion was isointense on T1WI and T2WI, but showed almost no enhancement. Belay et al[8] showed a lesion with low signal intensity on both T1WI and T2WI with marked enhancement. Some authors have indicated that iron deposition and fat infiltration might refer to an old stage in the disease course[20], while other authors thought that iron and fat content were also detectable in the active stage[21]. MRI was found to reflect different stages of hematopoiesis, depending on iron signal evolution, fat content, degree of fibrous organization, and vascular enrichment in the lesion. In this way, the difference in hematopoietic materials, fibrous tissue and fat content could explain the diverse radiologic description and the lack of exclusive imaging patterns.
| Case/Ref. | T1WI (compared to liver) | T2WI (compared to liver) | In/out-phase | Enhancement | Underlying condition |
| Case 1 | Hypointense | Heterogeneous | In-phase low, out-phase high | Heterogeneous and persistent | Thyroid carcinoma and lung adenocarcinoma |
| Case 2 | Slightly hypointense | Slightly hyperintense | In-phase low, out-phase high | Delayed | Hodgkin’s lymphoma |
| Case 3 | Hypointense | Hyperintense | In-phase low, out-phase high | Avid in arterial phase and “washout” in later phases | Unknown |
| Lee et al[2] | Slightly hypointense | Hyperintense | NA | Homogeneous, avid and persistent | Idiopathic myelofibrosis |
| Belay et al[8] | Hypointense | Hypointense1 | NA | Avid and persistent | Myelodysplastic syndrome |
| Zhang and Zhu[9] | Slightly hypointense | Hyperintense | Without signal intensity change | Homogeneous avid in arterial phase and isointense in later phases | Idiopathic myelofibrosis |
| Tamm et al[12] | Hypointense | Hyperintense | NA | Delayed | Gaucher disease |
| Jelali et al[24] | Slightly hyperintense | Slightly hyperintense | NA | Delayed | Sickle cell disease |
| Wong et al[17] | Hyperintense | Heterogeneous | NA | Delayed | β-Thalassemia |
Although most of the patients with IEMH had hematological disease, a few cases had no evidence of this underlying condition (as shown in the Case 1 and Case 3). For example, small cell lung cancer and Noonan syndrome were reported in two cases[4,15], and the cause of IEMH in another two cases remains unknown[22,23].
Two factors differentiate these three cases from those in other reports. First, no hepatomegaly or splenomegaly was found in these patients. Second, a change in MRI signal was observed at two different points in time in Case 1 and it presented more radiologic characteristics over the course of the disease. Interestingly, all our reported lesions were either in segment VII or VIII. IEMH in other segments have been reported in other cases. More cases should be documented in the future.
The differential diagnosis includes benign, primary, and secondary liver malignant lesions. IEMH may mimic these lesions leading to troublesome diagnosis. In the “fat deposition” stage, the characteristic signal intensity on the in-phase image is higher than that on the out-phase image. The differential diagnosis in cirrhotic liver includes fatty metamorphosis in hepatocellular carcinoma (HCC), while that in non-cirrhotic liver includes benign lesions such as focal fatty infiltration (without mass effect), adenoma (hepatocyte nuclear factor 1a–mutated subtype) and lipoma (no enhancement). Angiomyolipoma should be also taken into consideration. In the “iron deposition” stage, the characteristic signal intensity on the in-phase image is lower than that on the out-phase image. The differential diagnosis (intratumoral bleeding) includes benign lesions such as adenoma (inflammatory subtype) and hemangioma. Malignant lesions include hemorrhagic HCC and metastasis. When IEMH demonstrates strong and persistent enhancement, focal nodular hyperplasia, adenoma and hypervascular metastasis need to be considered. An appropriate clinical setting and the application of Gd-EOB-DTPA or SPIO are helpful in the diagnosis. When IEMH shows mild enhancement or avid enhancement with “washout”, atypical metastasis, HCC, or even fibrolamellar carcinoma in young patients should be considered in the differential list. Lymphoma is homogenous isointense with moderate enhancement. Fat and bleeding content is seldom seen in lymphoma.
IEMH has a variable radiologic appearance and is easily misdiagnosed. Given its rarity and the lack of pathognomonic imaging findings, awareness of its presentations might help radiologists establish the diagnosis.
The authors thank Chao Zhang, MD, PhD, and Jing-Ping Yun, MD, PhD, for their contributions to the pathological figure and analysis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Ali H, United States; Chen S, Japan; Maslennikov R, Russia; Shariati MBH, Iran A-Editor: Wang JL S-Editor: Fan JR L-Editor: Webster JR P-Editor: Fan JR
| 1. | Roberts AS, Shetty AS, Mellnick VM, Pickhardt PJ, Bhalla S, Menias CO. Extramedullary haematopoiesis: radiological imaging features. Clin Radiol. 2016;71:807-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 2. | Lee IJ, Kim SH, Kim DS, Lee JM, Han JK, Choi BI. Intrahepatic extramedullary hematopoiesis mimicking a hypervascular hepatic neoplasm on dynamic- and SPIO-enhanced MRI. Korean J Radiol. 2008;9 Suppl:S34-S38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Abbitt PL, Teates CD. The sonographic appearance of extramedullary hematopoiesis in the liver. J Clin Ultrasound. 1989;17:280-282. [PubMed] [DOI] [Full Text] |
| 4. | Bradley MJ, Metreweli C. Ultrasound appearances of extramedullary haematopoiesis in the liver and spleen. Br J Radiol. 1990;63:816-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Aytaç S, Fitoz S, Akyar S, Atasoy C, Erekul S. Focal intrahepatic extramedullary hematopoiesis: color Doppler US and CT findings. Abdom Imaging. 1999;24:366-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Navarro M, Crespo C, Pérez L, Martínez C, Galant J, González I. Massive intrahepatic extramedullary hematopoiesis in myelofibrosis. Abdom Imaging. 2000;25:184-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Cardoso FS, Pires JV, Miranda JS, Araújo JM. Hepatic nodule: a case of primary myelofibrosis. BMJ Case Rep. 2011;2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Belay AA, Bellizzi AM, Stolpen AH. The role of T2*-weighted gradient echo in the diagnosis of tumefactive intrahepatic extramedullary hematopoiesis in myelodysplastic syndrome and diffuse hepatic iron overload: a case report and review of the literature. J Med Case Rep. 2018;12:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Zhang L, Zhu H. Extramedullary hematopoiesis mimicking an intrahepatic neoplasm in a patient with idiopathic myelofibrosis. Dig Liver Dis. 2020;52:1205-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Rasche L, Kumar M, Gershner G, Samant R, Van Hemert R, Heidemeier A, Lapa C, Bley T, Buck A, McDonald J, Hillengass J, Epstein J, Thanendrarajan S, Schinke C, van Rhee F, Zangari M, Barlogie B, Davies FE, Morgan GJ, Weinhold N. Lack of Spleen Signal on Diffusion Weighted MRI is associated with High Tumor Burden and Poor Prognosis in Multiple Myeloma: A Link to Extramedullary Hematopoiesis? Theranostics. 2019;9:4756-4763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Curvo-Semedo L, Brito JB, Seco MF, Costa JF, Marques CB, Caseiro-Alves F. The hypointense liver lesion on T2-weighted MR images and what it means. Radiographics. 2010;30:e38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Tamm EP, Rabushka LS, Fishman EK, Hruban RH, Diehl AM, Klein A. Intrahepatic, extramedullary hematopoiesis mimicking hemangioma on technetium-99m red blood cell SPECT examination. Clin Imaging. 1995;19:88-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Yang M, Roarke M. Diffuse pulmonary extramedullary hematopoiesis in myelofibrosis diagnosed with technetium-99m sulfur colloid bone marrow scintigraphy and single photon emission computerized tomography/CT. Am J Hematol. 2017;92:323-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Agool A, Dierckx RA, de Wolf JT, Vellenga E, Slart RH. Extramedullary haematopoiesis imaging with 18F-FLT PET. Eur J Nucl Med Mol Imaging. 2010;37:1620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Gupta P, Naran A, Auh YH, Chung JS. Focal intrahepatic extramedullary hematopoiesis presenting as fatty lesions. AJR Am J Roentgenol. 2004;182:1031-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Cao DB, Wang YS. Gastrointestinal: Intrahepatic periportal masses with uncommon computed tomography patterns: Hepatic extramedullary hematopoiesis of primary mylofibrosis. J Gastroenterol Hepatol. 2020;35:1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Wong Y, Chen F, Tai KS, Yip LK, Tsang KW, Chan FL, Ooi GC. Imaging features of focal intrahepatic extramedullary haematopoiesis. Br J Radiol. 1999;72:906-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Elsayes KM, Narra VR, Mukundan G, Lewis JS Jr, Menias CO, Heiken JP. MR imaging of the spleen: spectrum of abnormalities. Radiographics. 2005;25:967-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 135] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 19. | Kumar A, Aggarwal S, de Tilly LN. Case of the season. Thalassemia major with extramedullary hematopoiesis in the liver. Semin Roentgenol. 1995;30:99-101. [PubMed] |
| 20. | Granjo E, Bauerle R, Sampaio R, Manata P, Torres N, Quintanilha A. Extramedullary hematopoiesis in hereditary spherocytosis deficient in ankyrin: a case report. Int J Hematol. 2002;76:153-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Tsitouridis J, Stamos S, Hassapopoulou E, Tsitouridis K, Nikolopoulos P. Extramedullary paraspinal hematopoiesis in thalassemia: CT and MRI evaluation. Eur J Radiol. 1999;30:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Warshauer DM, Schiebler ML. Intrahepatic extramedullary hematopoiesis: MR, CT, and sonographic appearance. J Comput Assist Tomogr. 1991;15:683-685. [PubMed] |
| 23. | Du E, Overstreet K, Zhou W, Baird G, Baird S, Bouvet M, Haghighi P. Fine needle aspiration of splenic extramedullary hematopoiesis presenting as a solitary mass. A case report. Acta Cytol. 2002;46:1138-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Jelali MA, Luciani A, Kobeiter H, Zafrani S, Anglade MC, Zegai B, Bachir D, Rahmouni A. MRI features of intrahepatic extramedullary haematopoiesis in sickle cell anaemia. Cancer Imaging. 2006;6:182-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |