Published online Jul 6, 2022. doi: 10.12998/wjcc.v10.i19.6609
Peer-review started: December 2, 2021
First decision: February 7, 2022
Revised: February 15, 2022
Accepted: May 7, 2022
Article in press: May 7, 2022
Published online: July 6, 2022
Processing time: 203 Days and 17.6 Hours
Metastasis to the penis is an unusual event, and penile metastasis from rectal carcinoma (PMRC) is extremely rare and associated with a dismal prognosis. Thus far, approximately 80 cases have been reported.
Herein, we report the case of a 49-year-old man with PMRC. The patient presented to the urology clinic with a complaint of penile pain during urination. The patient underwent the Dixon operation for rectal carcinoma 2 mo before the presentation. During hospitalisation, abdominal computed tomography revealed a nodular lesion on the left penis. The postoperative pathological examination revealed a typical intestinal-type adenocarcinoma. Previous cases of PMRC were retrieved from PubMed to characterise the clinicopathological features and identify the prognostic factors of PMRC.
The analysis suggested that approximately 24 mo is the median time to metastasis occurrence and 150 d is the survival time after diagnosis. Furthermore, poor pathological differentiation, lymph node involvement of the primary RC, metastasis time < 6 mo, penile metastatic nodule diameter > 1 cm, and treatment abandonment are negative predictors of survival outcomes. Close follow-up, surgical resection, chemotherapy, and radiotherapy may potentially improve the prognosis of patients.
Core Tip: Rectal carcinoma (RC) is a clinically common malignant tumour. Mainstream treatment methods are chemotherapy and surgery. Clinically, the liver is the most common metastatic site of RC. We report a rare case of penile metastasis from RC following a Dixon operation. Combined with the analysis of the cases indexed in PubMed, urinary discomfort occurring within 6 years after surgery is a concern. Early detection of suspicious lesions is a favourable factor for patient survival. After the discovery of penile metastasis, providing appropriate active treatment has positive effects on the prognosis of patients. The treatment plan should be based on the patient’s response to chemotherapy or radiotherapy, general condition, and willingness to choose the current best treatment. However, clinicians should avoid negative treatment.
- Citation: Sun JJ, Zhang SY, Tian JJ, Jin BY. Penile metastasis from rectal carcinoma: A case report. World J Clin Cases 2022; 10(19): 6609-6616
- URL: https://www.wjgnet.com/2307-8960/full/v10/i19/6609.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i19.6609
Rectal carcinoma (RC) is one of the most common and fatal cancers worldwide, with approximately 1.4 million new diagnoses annually, and its mortality rate reaches approximately 28%[1,2]. As many as 20% of patients with RC develop distant metastasis either at initial presentation or during the natural disease course, of which 70% were located in the liver[3]. However, metastasis to the penis is rarely reported.
Although the penis has abundant and interconnected vasculature, tumours metastasising to the penis are uncommon[4]. The most frequent sites of origin are malignancies arising from the pelvis, typical bladder cancer (32%), and prostate cancer (30%). Penile metastases from lung and liver cancers have also been reported sporadically. However, penile metastasis from RC (PMRC) is extremely rare. Generally, penile metastasis occurs within 2 years after the diagnosis of RC, and the metastatic events are not associated with the adopted management strategy for the primary tumours[5]. Penile metastasis is usually considered a clinical sign of dismal prognosis, with a median survival time (MST) of only approximately 8 mo, presenting as a challenge[6].
Consequently, a better understanding of the characteristics of PMRC is urgently needed. Herein, we present a case of PMRC in a 49-year-old man, and through a literature review of previous cases, we summarise the clinical features and treatment methods and identify potential prognostic factors for PMRC.
A 49-year-old Chinese man presented to the urology clinic with a complaint of penile pain during urination for 2 wk.
Symptoms started 2 wk before presentation with recurrent penile pain during urination.
Three months ago, the patient presented to a local hospital with a complaint of bloody stools for 3 mo. Abdominal computed tomography (CT) revealed an irregular soft tissue density mass in the rectal cavity. A rectal tumour was initially suspected. Colonoscopy revealed a light-red raised cauliflower-like mass located 8 cm from the anal margin, with erosions and necrosis on the surface, accounting for 1/2 of the intestinal cavity. Endoscopic biopsy of the colorectal mass was positive for adenocarcinoma. Combined with the findings of subsequent enhanced pelvic CT, the local hospital considered the patient’s T stage as T3. No distant metastasis was found by positron emission tomography (PET)/CT. Laparoscopic RC excision (Dixon operation) with sigmoid colostomy was performed. Results of the pathological examination of the resected specimen confirmed a moderately to poorly differentiated adenocarcinoma of the rectum (T4N2M0), and the tumour diameter was 4.2 cm × 3.8 cm. Metastatic tumour cells were detected in 13 of 18 resected lymph nodes. Subsequently, adjuvant XELOX chemotherapy regimen (oxaliplatin 240 mg as continuous intravenous infusion over 24 h on day 1 combined with capecitabine 1.5 g on days 1-14) was initiated as one cycle per 3 wk after surgery.
The patient denied any family history of malignant tumours.
On physical examination, the vital signs were as follows: Body temperature, 36.5℃; blood pressure, 117/68 mmHg; heart rate, 82 beats per min; respiratory rate, 19 breaths per min. Furthermore, a painful nodular mass, with a diameter of 1.5 cm, was found on the left penis. There was no obvious redness and swelling. The glans, testis, and epididymis were normal. No secretion was found at the urethral orifice. Digital anal examination was not performed.
Levels of serum tumour markers were normal (carcinoembryonic antigen, 4.3 ng/mL; carbohydrate antigen 19-9, < 2 U/mL; alpha-fetoprotein, 3.1 ng/mL). No abnormality was found in routine blood and urine analyses.
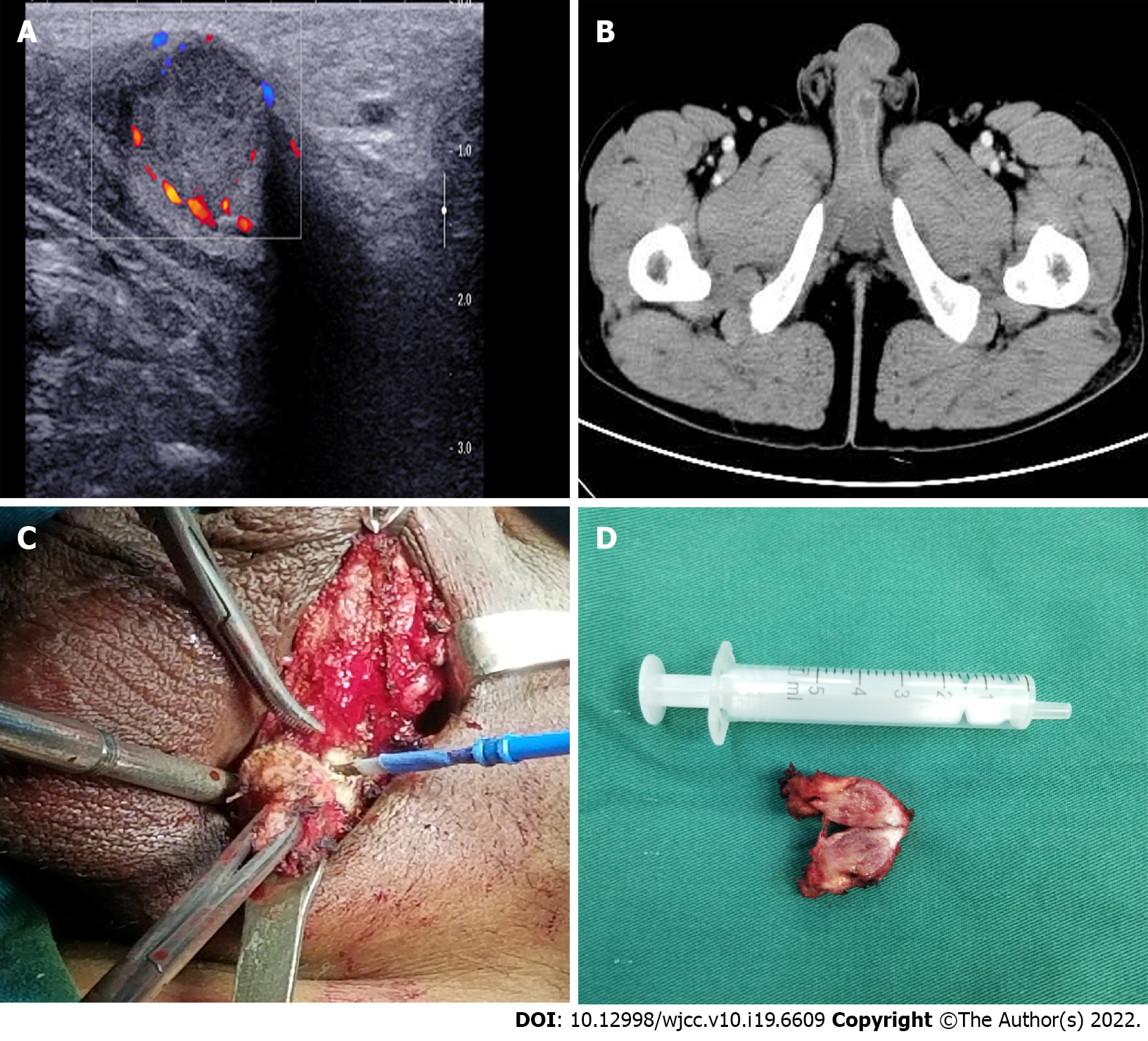
Ultrasonography revealed a hyper-echoic mass, with a diameter of 1.5 cm, on the root of the left penis (Figure 1A). Mass biopsy was recommended; however, the patient refused. Subsequent PET/CT revealed that the penile lesion had a clear edge and high fluorodeoxyglucose uptake, so a tentative diagnosis of malignancy was made. Abdominal contrast-enhanced CT (Figure 1B) revealed a nodular and mildly enhanced mass on the left penis.
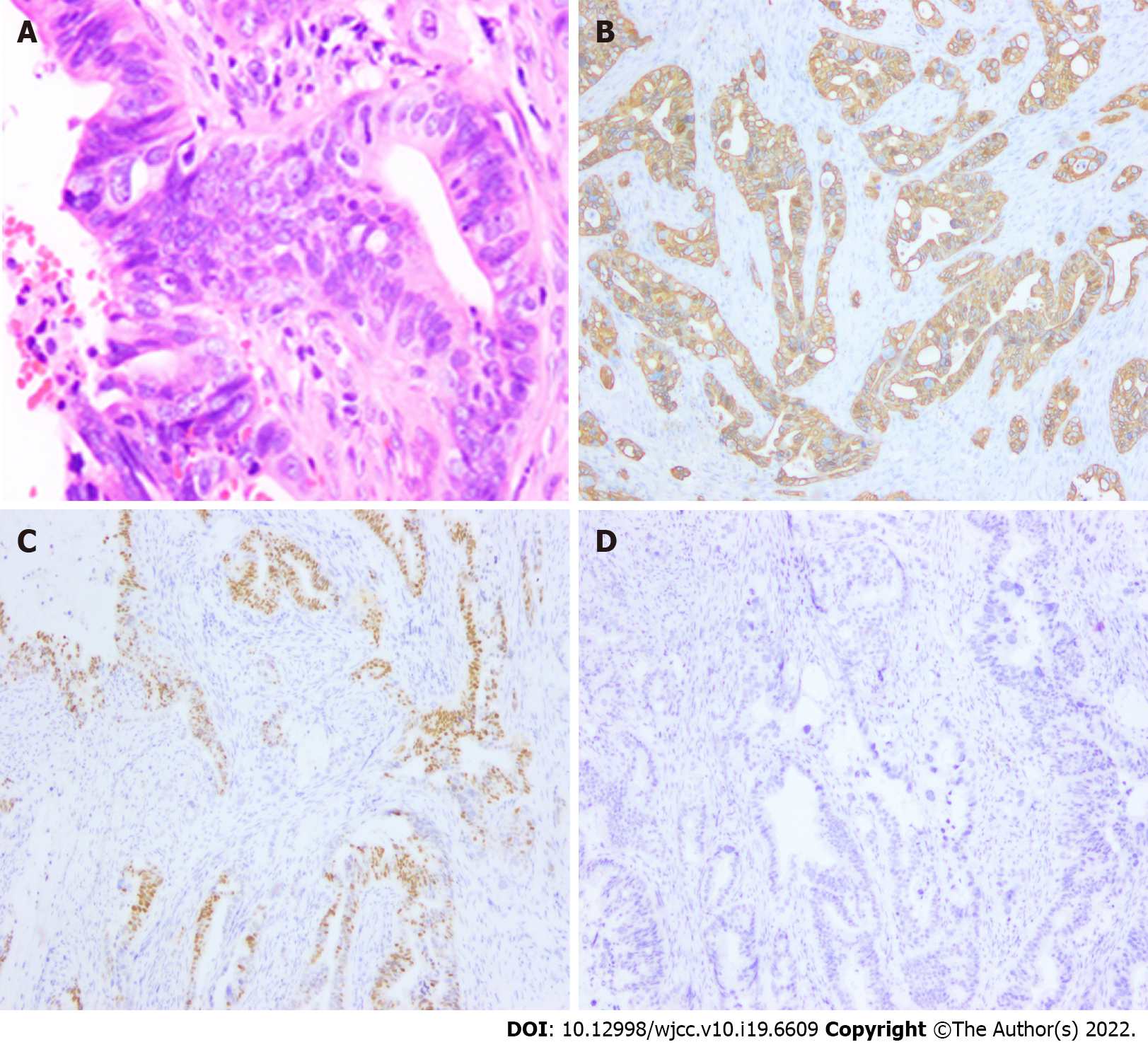
The patient refused biopsy and decisively wanted the mass removed and his penis preserved. Moreover, the penile lesion was preliminarily considered a metastatic tumour, which may be secondary to RC. Subsequently, the penile mass was excised. Intraoperatively, a rigid nodule with distinct margins was found at the distal end of the left spermatic cord (Figure 1C and D). Histopathological examination of the resected specimen (size, 3 cm × 1 cm × 0.8 cm) confirmed that the mass originated from a poorly differentiated adenocarcinoma, the cut edge of the spermatic cord was negative, and nerve invasion was positive (Figure 2A). Immunohistochemical analysis revealed that the tumour was positive for CDX2, Ki67 (70%), and cytokeratin 20 and negative for PAX-8 and prostate-specific antigen (Figure 2B-D).
Combined with the patient’s medical history, the final diagnosis was PMRC.
Postoperatively, the patient recovered well and was discharged on postoperative day 5. At 2 wk postoperatively, the patient continued XELOX chemotherapy at the same dose.
At 5 mo postoperatively, the patient was still alive.
PMRC is a rare disease entity, with < 80 cases reported in the literature. PMRC is mostly detected in older patients, and a penile nodule is the most common clinical manifestation. Approximately 20% of patients experienced pain and discomfort in the penile area, while others may have difficulty urinating with urinary retention[6]. Imaging examination could greatly assist in the clinical diagnosis. CT is valuable to reveal the anatomical relationship between tumour lesions and neighbouring organs, and CT generally depicted an irregular mass, with mild-to-moderate enhancement[7]. Magnetic resonance imaging (MRI), a sensitive tool, can determine the extent of tumour invasion[8]. Cavernosography plays a significant role in evaluating the extent of invasion of secondary penile cancer[9]. However, its invasiveness, which may cause serious complications such as hematoma formation, limits its application value. Histopathological examination remains the gold standard for diagnosis, and the results usually reveal a typical intestinal-type adenocarcinoma that invades the corpus cavernosum. Generally, tumour cells have eosinophilic cytoplasm and an oval nucleus but without regular contours[10].
Given the paucity of reports of secondary penile tumours, the standard treatment has not been established. Common treatment options mainly include surgery, chemotherapy, and local radiotherapy. Instead of curative therapy, palliative management is the main purpose of treatment to relieve and eliminate the symptoms of penile pain and urinary tract obstruction. However, no single treatment can yield superior results, and the prognosis of patients is usually far from satisfactory. In a retrospective study by Chaux et al[11], the mean survival time of 17 patients was only 8 mo.
The metastatic pathway of secondary penile cancer remains controversial. At present, retrograde venous metastasis is favoured by most scholars, and this theory is supported by the anatomical fact that the dorsal vein of the penis enters the prostate and bladder to form a pelvic venous plexus, which is characterised by low pressure, absence of a venous valve, and abundance of anastomotic branches. Once the intra-abdominal pressure increases acutely because of severe cough, defecation, or blockage of the venous return path, primary tumour cells retrogradely enter the dorsal venous system of the penis through the genital vein system, eventually leading to tumour metastasis. Haddad et al[12] found a cancer thrombus in the dorsal vein of the penis, which further supported the retrograde venous metastasis pathway. Additionally, the direct infiltration route appears to be another way by which primary tumour cells infiltrate the bulbous urethra directly along the perineal plane and then cause the formation of a secondary penile tumour[13]. Other hypotheses include nosocomial transmission, such as cystoscopy biopsy and transurethral prostate and bladder resection, and descent of tumour cells into the urethra, causing cancer metastasis. Another hypothesis is that tumour cells may spread to the penis along the nerves[10].
Given the rarity of PMRC, a few studies have investigated its prognostic indicators. We searched PubMed (www.ncbi.nlm.nih.gov/pubmed) for English articles that described PMRC, published between 1966 and 2020. The following medical terms were used: “Secondary penile cancer”, “rectal carcinoma”, and “penile metastasis”. Initially, we collected a total of 80 case reports. After excluding articles with missing data on patient outcomes, we screened out approximately 46 valid articles and summarize them in the table (Supplementary Table 1).
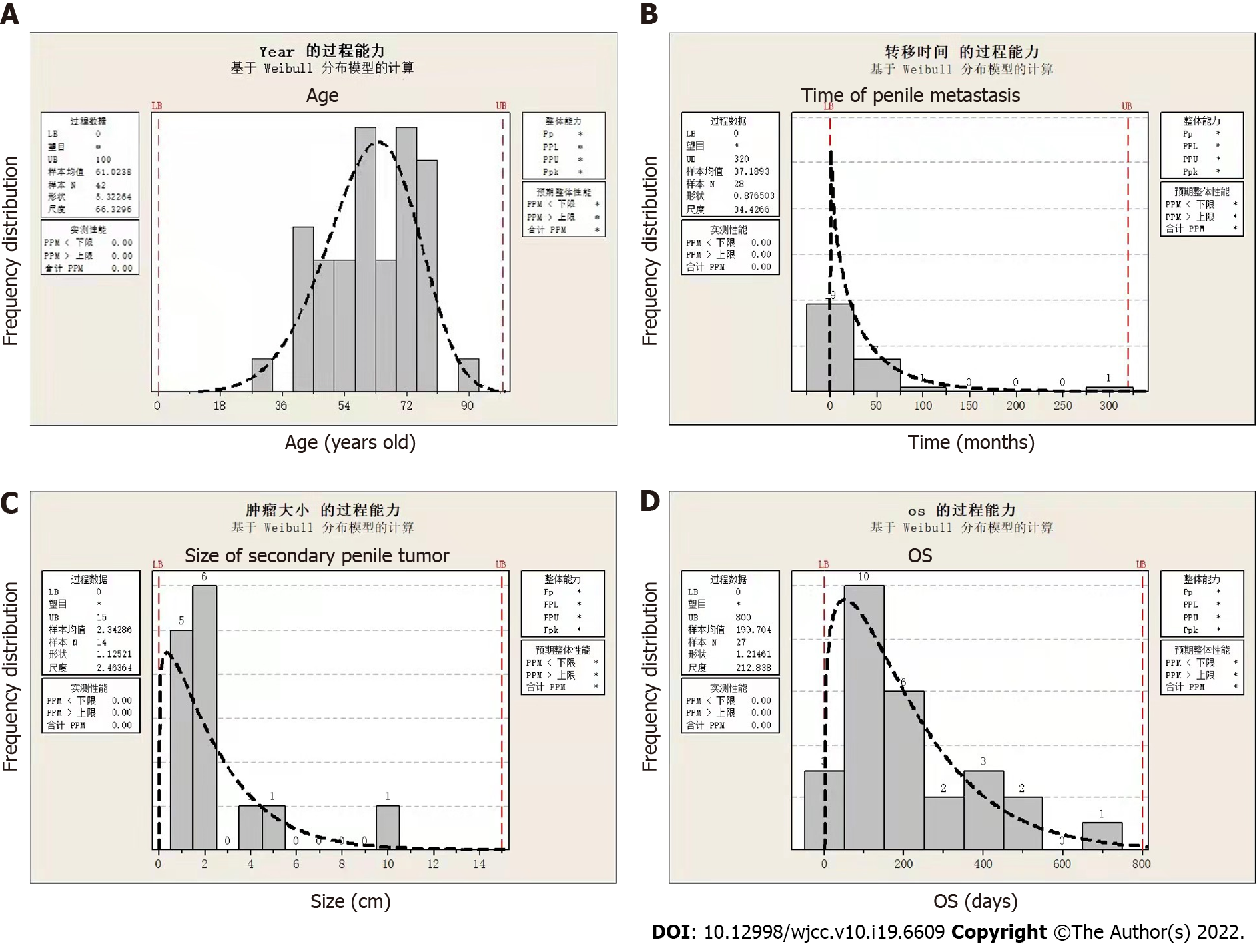
After data collation and analysis, we found that patients with PMRC were 30-88 years old, with a median age of 60 years (Figure 3A). The time to penile metastasis occurrence ranged from 4 to 312 (median, 24) mo; notably, most of the metastases (92.9%) occurred within < 75 mo (Figure 3B). Therefore, within 6 years after surgery, doctors should be suspicious of urethral symptoms and perform penile ultrasound examinations, if necessary. Additionally, the size of secondary penile tumours ranged from 0.5 to 10 cm, and the majority (78.6%) had a size within 0.5-2.5 cm (Figure 3C). For the overall survival time after diagnosis, the median survival time was approximately 150 d, and the majority (59.2%) of cases had a survival time of 50-250 d (Figure 3D). This finding suggests that PMRC is a sign of dismal prognosis.
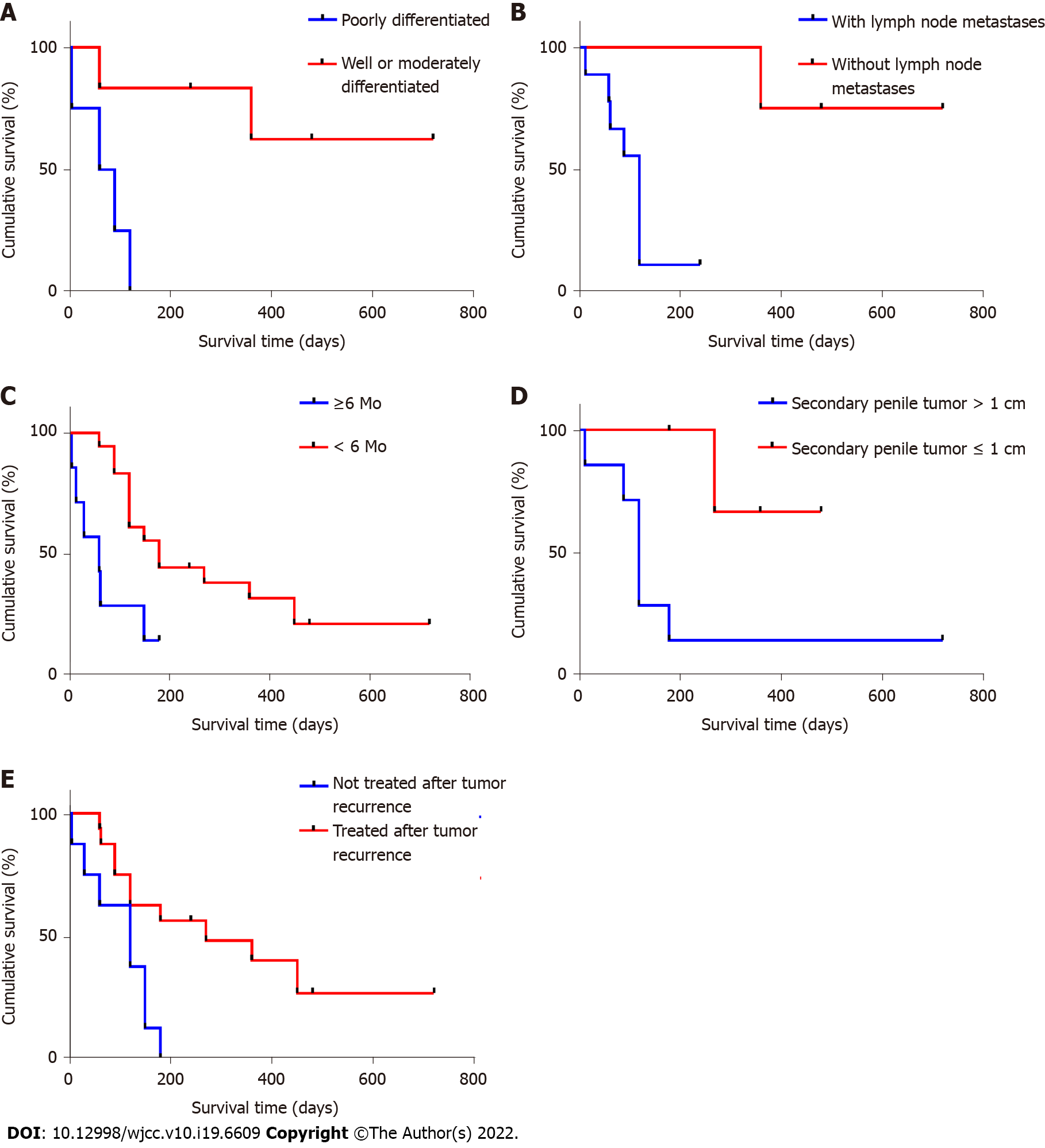
The Kaplan-Meier survival analysis and log-rank test were employed to identify potential prognostic factors (Supplementary Table 1). We analysed several clinical variables that may affect the survival outcomes of patients with PMRC. These variables included patient age, primary tumour differentiation, lymph node metastasis, secondary tumour size, time to metastasis occurrence, and treatment options. Expectedly, the prognosis of patients with poorly differentiated RC was worse than that of patients with well- or moderately differentiated RC (Figure 4A), with an MST of 75 and 360 d, respectively (P = 0.011). Additionally, RC with lymph node metastasis (MST, 120 vs 420 d; P = 0.009; Figure 4B) and time to metastasis occurrence < 6 mo (MST, 63 vs 180 d; P = 0.009; Figure 4C) were identified as negative predictors of survival outcomes. Secondary penile tumour diameter > 1 cm was also associated with worse survival outcomes (MST, 120 vs 225 d; P = 0.036; Figure 4D). Doctors should actively remind patients to pay attention to urethral symptoms and to diagnose and intervene them even in small penile metastases. A significantly shorter survival time and a lower survival rate were observed in patients who abandoned any treatment (MST, 120 vs 210 d; P = 0.007; Figure 4E). Further analysis revealed no significant difference in the overall survival rate among treatment options, including surgical resection, chemotherapy, and radiotherapy (P = 0.739). Based on the above results, we believe that metastasis < 6 mo, secondary penile tumour diameter > 1 cm, and abandonment of treatment are associated with poor survival outcomes. However, with a limited number of cases, multivariable Cox proportional hazards regression analysis was not performed.
Our results are consistent with the findings of previous studies that have investigated penile metastasis from other primary sites. Song et al[14] emphasised that the time to metastasis occurrence was negatively correlated with the prognosis in penile metastasis from oesophageal squamous carcinoma. According to Wen et al[15], lymph node metastasis may have a negative correlation with the prognosis. However, to the best of our knowledge, several prognostic factors such as secondary penile tumour diameter, primary tumour differentiation, and treatment have yet not been reported as effective clinical predictors.
Given the limited reports and the lack of more research data, we cannot provide more accurate prognostic risk factors and clinical recommendations. To determine the prognostic relevance of other clinical variables, further exploration based on adequate data is required. In addition, the heterogeneity caused by different treatment options and management among medical centres may have a certain effect on the interpretation of our findings. To the best of our knowledge, our study may be the first investigation to systematically report on risk factors affecting the prognosis of this disease entity, which may essentially benefit the clinical management.
Due to the rarity of PMRC, its characteristics are still being explored. The above-mentioned risk factors may assist clinicians in the assessment and management of PMRC. For patients with poorly differentiated RC or lymph node metastasis, regular follow-up and close observation are essential for such patients. Once metastasis occurs, corresponding treatments should be recommended. Essentially, the results of our data analysis reveal the lack of evident difference between surgery and chemoradiotherapy alone. However, both lead to significantly better outcomes than non-treatment.
Although PMRC is a very rare entity, combined with our case and cases reported in PubMed, complaints of urinary system discomfort within 6 years after an operation is a cause for concern. Early detection of suspicious lesions is a favourable factor of patient survival. Moreover, ultrasound examination, MRI, and PET/CT, if necessary, are prevailing modalities to detect small penile lesions. After the discovery of penile metastases, the provision of appropriate and active treatment has positive effects on the prognosis of patients. The treatment plan should be based on the patient’s response to chemotherapy or radiotherapy, general condition, and willingness to choose the current and optimal treatment. However, clinicians should avoid making negative treatment. Although PMRC is clinically rare, doctors should make a positive and accurate diagnosis and provide humane care and treatment because patients have experienced both mental and physical problems.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Boopathy Vijayaraghavan KM, India; Gaman MA, Romania; Gul A, Turkey S-Editor: Liu JH L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Martella A, Willett C, Palta M, Czito B. The Selective Use of Radiation Therapy in Rectal Cancer Patients. Curr Oncol Rep. 2018;20:43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Torre LA, Siegel RL, Ward EM, Jemal A. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol Biomarkers Prev. 2016;25:16-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2004] [Cited by in RCA: 2489] [Article Influence: 248.9] [Reference Citation Analysis (0)] |
| 3. | Al Bandar MH, Kim NK. Current status and future perspectives on treatment of liver metastasis in colorectal cancer (Review). Oncol Rep. 2017;37:2553-2564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 4. | Cherian J, Rajan S, Thwaini A, Elmasry Y, Shah T, Puri R. Secondary penile tumours revisited. Int Semin Surg Oncol. 2006;3:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Persec Z, Persec J, Sovic T, Rako D, Savic I, Marinic DK. Penile metastases of rectal adenocarcinoma. J Visc Surg. 2014;151:53-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Berger AP, Rogatsch H, Hoeltl L, Steiner H, Bartsch G, Hobisch A. Late penile metastasis from primary bladder carcinoma. Urology. 2003;62:145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 7. | Singh AK, Saokar A, Hahn PF, Harisinghani MG. Imaging of penile neoplasms. Radiographics. 2005;25:1629-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Gupta S, Rajesh A. Magnetic resonance imaging of penile cancer. Magn Reson Imaging Clin N Am. 2014;22:191-199, vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Gitto S, Vaiani M, Cascella T, Lanocita R. Penile Metastases From Renal Cell Carcinoma: Pre and Postcontrast Sonographic Findings. Ultrasound Q. 2018;34:285-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | 10 Kuliavas J, Dulskas A, Drachneris J, Miseikyte-Kaubriene E, Samalavicius NE. Penile Metastasis from Rectal Carcinoma: Case Report and Review of the Literature. Visc Med. 2018;34:389-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Chaux A, Amin M, Cubilla AL, Young RH. Metastatic tumors to the penis: a report of 17 cases and review of the literature. Int J Surg Pathol. 2011;19:597-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Haddad FS, Manne RK. Involvement of the penis by rectocolic adenocarcinoma. Report of a case and review of the literature. Dis Colon Rectum. 1987;30:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Efared B, Ebang GA, Tahirou S, Tahiri L, Sidibé IS, Erregad F, Sow A, Hammas N, Farih MH, Chbani L, El Fatemi H. Penile metastasis from rectal adenocarcinoma: a case report. BMC Res Notes. 2017;10:564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Song L, Wang Y, Weng G. Metastasis in penile corpus cavernosum from esophageal squamous carcinoma after curative resection: a case report. BMC Cancer. 2019;19:162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Wen S, Ren W, Xue B, Fan Y, Jiang Y, Zeng C, Li Y, Zu X. Prognostic factors in patients with penile cancer after surgical management. World J Urol. 2018;36:435-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |