Published online Jul 6, 2022. doi: 10.12998/wjcc.v10.i19.6602
Peer-review started: December 1, 2021
First decision: January 12, 2022
Revised: January 20, 2022
Accepted: May 13, 2022
Article in press: May 13, 2022
Published online: July 6, 2022
Processing time: 204 Days and 22.4 Hours
Congenital complete heart block (CCHB) with normal cardiac structure and negativity for anti-Ro/La antibody is rare. Additionally, CCHB is much less frequently diagnosed in adults, and its natural history in adults is less well known.
A 23-year-old woman was admitted to our hospital for frequent syncopal episodes. She had bradycardia at the age of 1 year but had never had impaired exercise capacity or a syncopal episode before admission. The possible diagnosis of acquired complete atrioventricular block was carefully ruled out, and then the diagnosis of CCHB was made. According to existing guidelines, permanent pacemaker implantation was recommended, but the patient declined. With regular follow-up for 28 years, the patient had an unusually good outcome without any invasive intervention or medicine. She had an uneventful pregnancy and led a normally active life without any symptoms of low cardiac output or syncopal recurrence.
This case implies that CCHB in adulthood may have good clinical outcomes and does not always require permanent pacemaker implantation.
Core Tip: Congenital complete heart block (CCHB) is a very rare disorder that is largely diagnosed at the fetal or infant stage. Therefore, it is infrequently diagnosed in adulthood, and the natural history of CCHB in adults is less well known. Despite the controversial literature, permanent pacing is widely recommended for the prevention of sudden death among patients with CCHB. This case illustrated an unexpectedly good course in an adult with CCHB at the onset of syncope who refused permanent pacing but led a normally active life. This suggests that CCHB in adulthood may have good outcomes and does not always require permanent pacing.
- Citation: Su LN, Wu MY, Cui YX, Lee CY, Song JX, Chen H. Unusual course of congenital complete heart block in an adult: A case report. World J Clin Cases 2022; 10(19): 6602-6608
- URL: https://www.wjgnet.com/2307-8960/full/v10/i19/6602.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i19.6602
Congenital complete heart block (CCHB) without intracardiac structural abnormalities is a rare disease that occurs approximately one in every 20000 live births[1]. Considerable evidence has shown that CCHB cases are predominantly due to atrioventricular nodal injury from maternal Ro/La auto
A 23-year-old Chinese woman was referred to our hospital from the emergency department due to persistent palpitations and several episodes of syncope for 8 h in December 1993. She denied chest pain, shortness of breath, and impaired exercise capacity. During the first hour in the emergency room, she experienced three more episodes of syncope. She was unconscious for approximately 10 s and spontaneously recovered to consciousness after each attack.
There was a recent history of a cold with a low fever, stuffy nose, and sore throat in the previous week.
She denied a history of structural heart abnormality, cardiac surgery or drug use. She also did not have a special birth history, and growth and development were normal. Bradycardia was identified when she was 1 year old without further evaluation. The patient has had a regular heart rate of 40-50 beats per minute (bpm) for many years. However, no syncopal episodes had ever occurred before onset.
The patient denied a familial history of syncope, sudden death, and rheumatological disease.
On admission, she was afebrile with stable hemodynamics. There was no cyanosis, edema or bibasilar rales in the lungs. A cardiac murmur, which was strongest at the apex, was a grade III/VI systolic murmur radiating to the neck. She was awake and oriented, with no focal neural deficit.
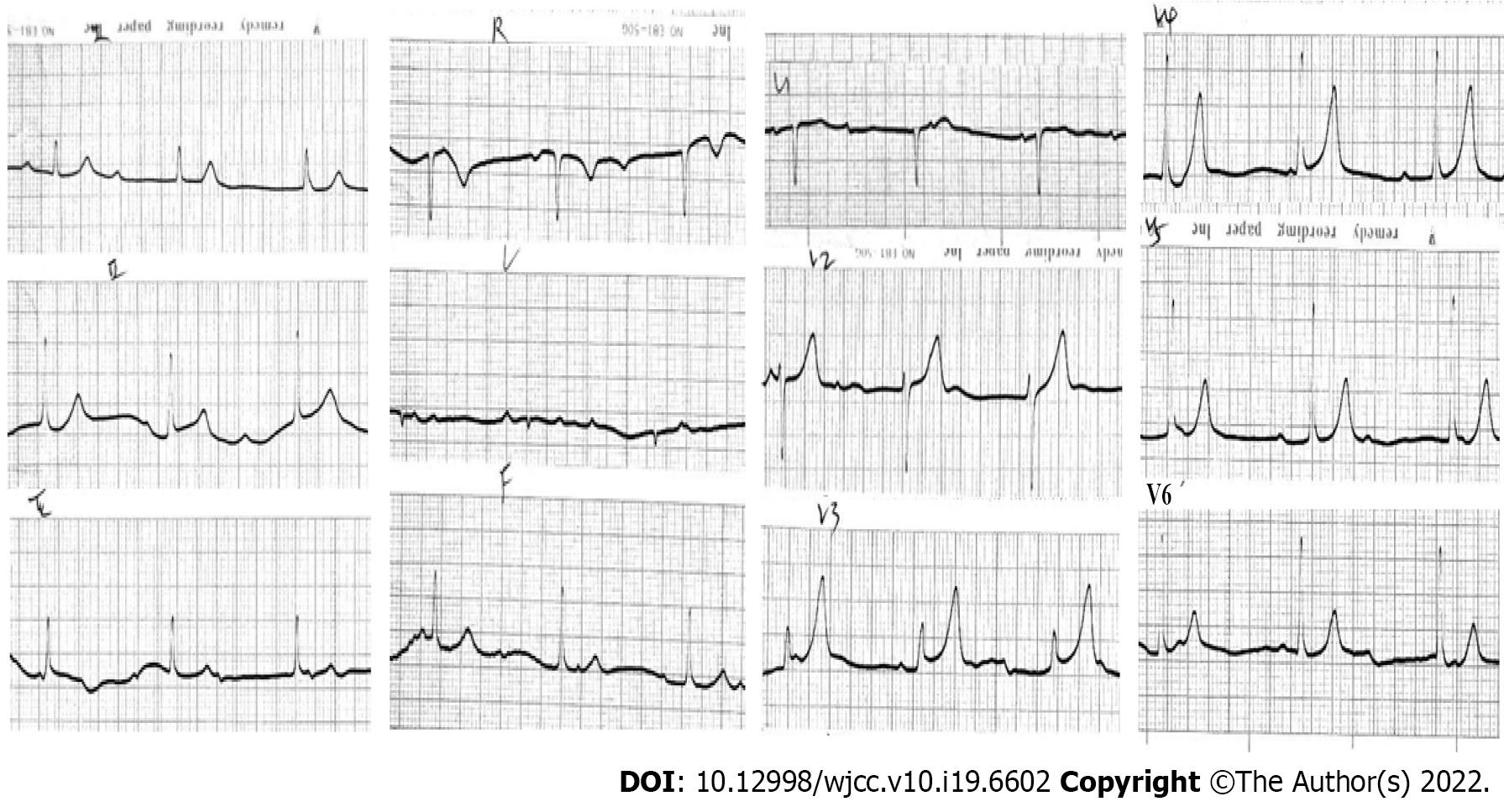
Electrocardiography (ECG) on admission revealed complete atrioventricular block with junctional escape at 48 bpm (Figure 1). Considering the patient’s history of prodromal infection, myocarditis-induced complete atrioventricular block (CAVB) was considered first. Laboratory data showed a white blood cell count of 15.8 × 109/L, neutrophil percentage of 78%, hemoglobin level of 118 g/L, and platelet count of 114 × 109/L. Creatine kinase MB was in the normal range. Antibodies against cytomegalovirus and Epstein–Barr virus were negative. Clearly, the myocarditis-associated laboratory data above did not support the diagnosis of myocarditis. Serum electrolytes, thyroid hormone, erythrocyte sedimentation rate, C-reactive protein, and anti-streptolysin O were all normal, which together ruled out the possibility of electrolyte disturbance, thyroid dysfunction, and rheumatic heart disease. As the majority of CCHB cases were regarded as immune-mediated atrioventricular nodal injury, further investigations including autoantibodies were conducted, which were negative for antinuclear antibodies, anti-Ro, anti-La, anti-ds deoxyribonucleic acid, and rheumatoid factor.
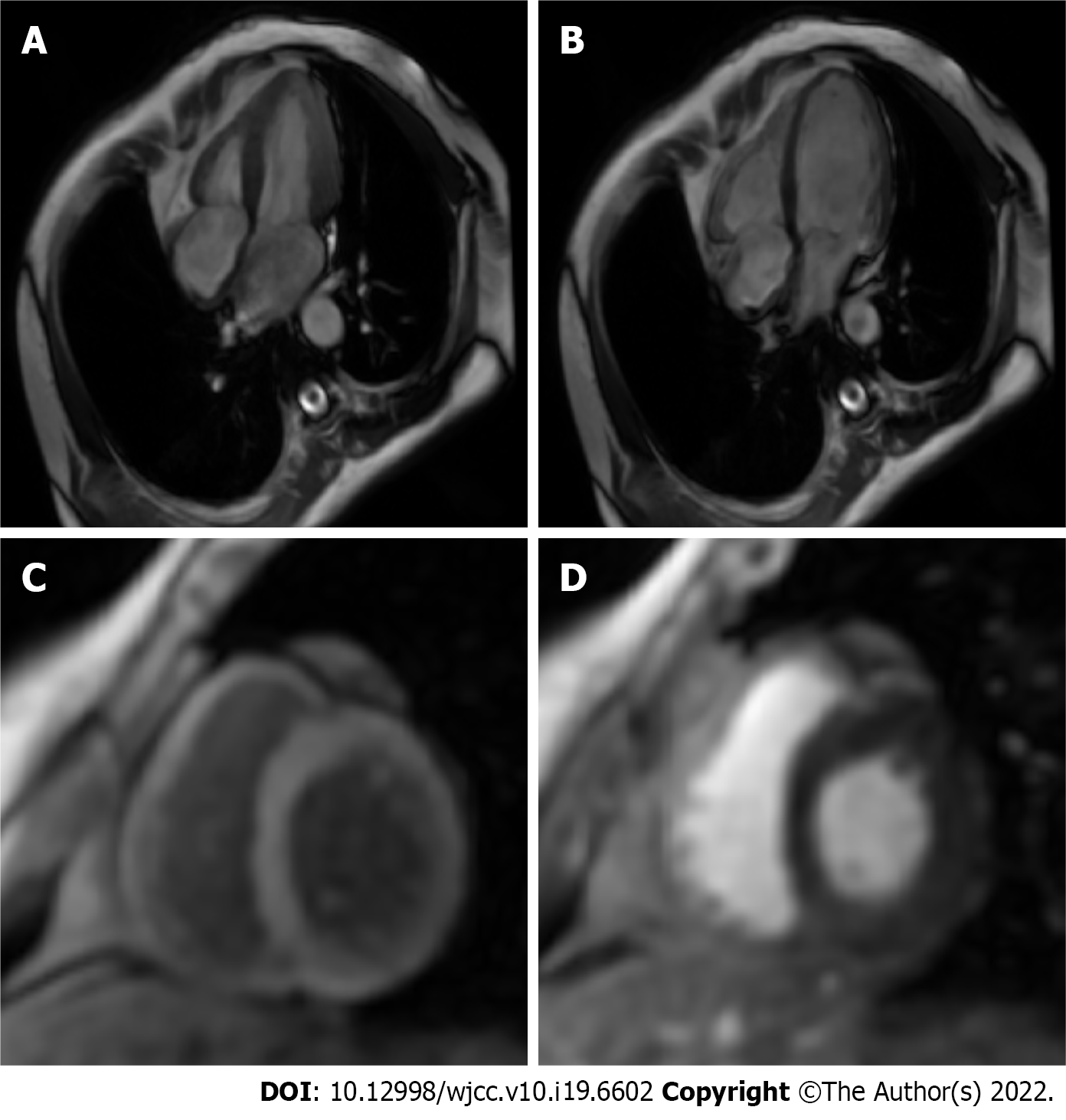
Transthoracic echocardiography and cardiac magnetic resonance imaging (Figure 2) were unre
In contrast to patients with acquired heart block, who frequently have severe cardiac dysfunction largely secondary to structural heart disease and fatal ventricular arrhythmias due to unstable pacemaker site, patients with CCHB often have normal myocardial function and ventricular rate increases with activities seldom complicated by severe ventricular arrhythmias[8]. Furthermore, in CCHB, slow heart rate is often ascertained at an early age in the absence of any infection that might cause heart block; notably, diphtheria, rheumatic fever, chorea, and congenital syphilis[4]. According to the discriminatory features between CCHB and acquired heart block, the final diagnosis of CCHB was made in our case.
The patient experienced frequent syncopal episodes in the emergency department, and simultaneous ECG monitoring exhibited CAVB and intermittent ventricular arrest. Hence, intravenous isoproterenol was immediately initiated, and a temporary transvenous pacemaker was inserted prophylactically. The patient was admitted to the cardiac care unit. During hospitalization, she had no recurrence of syncope or chest discomfort. Continuous ECG monitoring revealed that her heart rate remained steady, ranging from 35 to 71 bpm, with persistent CAVB. Given that Adams-Stokes attack in CCHB is of poor prognostic significance, permanent pacemaker therapy was recommended to prevent sudden death. However, the patient and her family members refused treatment with a permanent pacemaker. Accordingly, the temporary pacemaker was removed on day 14 after onset. On the 33 day, she was discharged in a good and satisfactory condition without medication or pacemaker implantation.
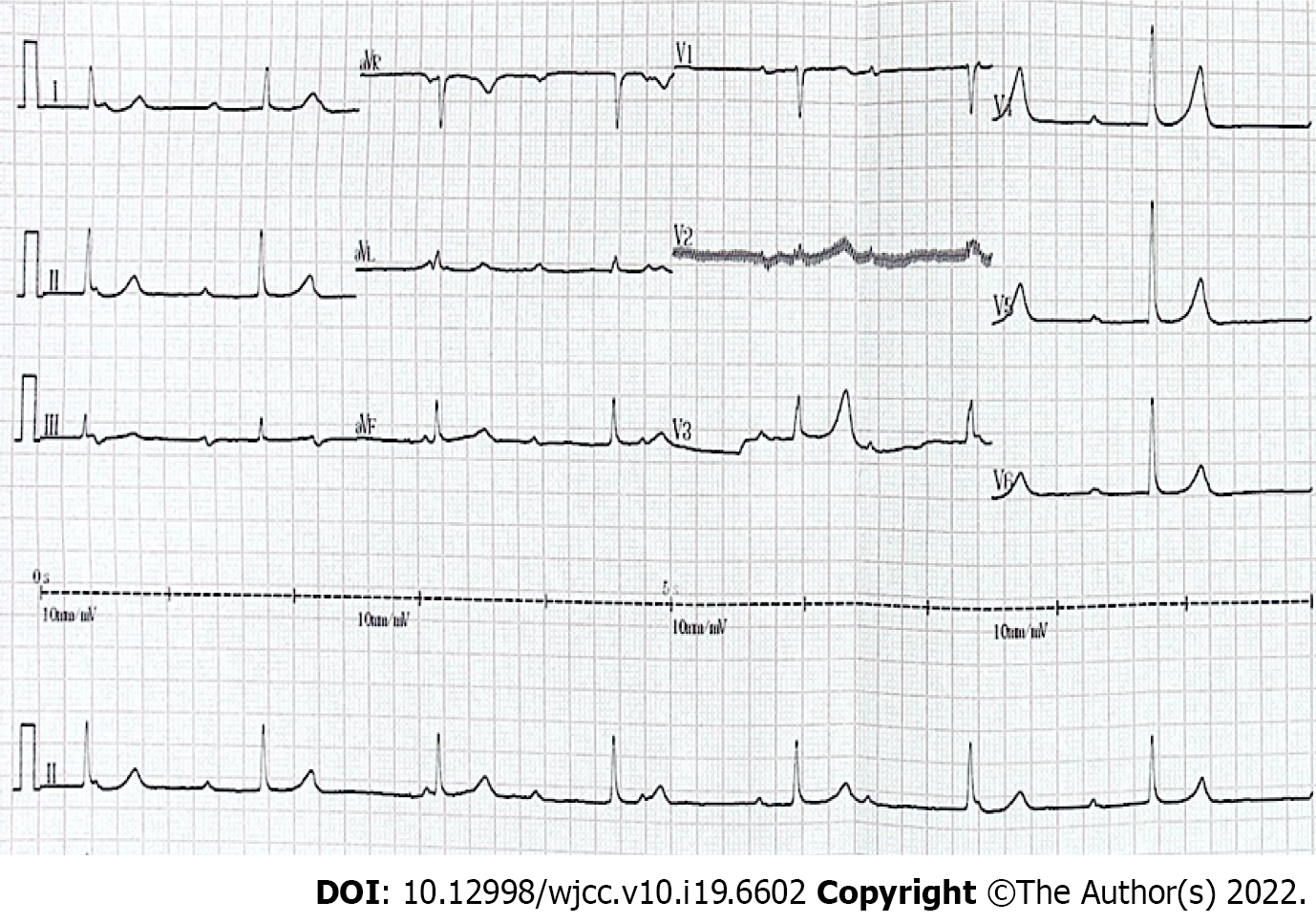
Within the 28-years’ follow-up, the patient was leading an active life without any recurrence of Adams-Stokes attack or low cardiac output symptoms. She could swim often and play badminton for 30 min without any discomfort. Somewhat unexpectedly, she had an uneventful pregnancy at the age of 30 years and developed essential hypertension with a peak blood pressure of 180/100 mmHg at the age of 40 years. At intervals of approximately 5 to 10 years of follow-up, both repeated ECG (Figure 3) and 24-h ambulatory electrocardiogram (Holter) recordings showed CAVB with a steady junctional escape rate ranging from 34 to 70 bpm and a daytime average of 45 bpm, whereas echocardiography revealed no cardiac dilation or mitral regurgitation.
CCHB is defined as an atrioventricular block occurring prenatally, at birth or within the first month of life[3]. A complete heart block is a complete blockade of impulses from the atrium to the ventricle. Thereby, the ventricle beats relatively slowly, depending on a junctional or ventricular rhythm. A slow heart rate could result in fetal hydrops, heart failure, and exercise intolerance[3] while longer pauses may lead to presyncope, syncope, and even death[3]. The estimated prevalence of CCHB in structurally normal hearts is approximately one in 20000 live births[1]. However, the overall mortality for CCHB is estimated to be high and ranges from 14% to 34%[9]. Several publications have suggested that the natural history of patients with CCHB varies dramatically and is determined by the presence of congenital heart disease and the time of diagnosis[7,10,11]. In other words, CCHB seems to be a group of heterogeneous diseases that should not all be managed in the same or only one way.
Based on published data, there are three major etiologies of CCHB, including structural heart defects in the setting of congenital heart disease, antibody-mediated CCHB, and idiopathic CCHB. Importantly, cardiac defects and maternal autoantibodies account for approximately 90% of CCHB cases, whereas the remaining 10% are regarded as idiopathic CCHB[3]. In one previous study, the mortality rate of CCHB without associated structural heart disease was 15% but increased to 42% among patients with congenital heart disease[12]. In addition, immune-mediated CCHB has been proven to have higher mortality and a higher risk of progression to dilated cardiomyopathy than idiopathic CCHB[11]. Accordingly, etiology has a critical impact on the outcome of CCHB, and idiopathic CCHB is perceived to have better clinical outcomes. Given the patient’s normal heart structure and negative autoimmune antibodies, our case should be attributed to idiopathic CCHB. However, the real pathophysiological mechanism of idiopathic CCHB remains unknown.
Of note, CCHB could be easily overlooked if the ventricular rate is not extremely low and may not be diagnosed until adulthood. To date, the existing literature has largely focused on CCHB in the fetal period or childhood, whereas CCHB is rarely diagnosed in adulthood, and its outcome in adults is less well known. A series of studies demonstrated that patients with asymptomatic CCHB could have a normal life and even be able to perform heavy activities without the benefit of pacemakers or medicine[10]. However, the outcome of symptomatic CCHB without any intervention was reported in only one case. In that case, the patient had frequent Adams-Stokes attacks in infancy, but he had rare recurrences for nearly 50 years thereafter[10]. In our case, the patient presented frequent syncopal episodes at onset in adulthood, recovering spontaneously, and she could perform even heavy physical activities without any recurrence over the following 28 years. Overall, our patient had a better clinical course than that of the prior case in the absence of treatment. Additionally, our case had more comprehensive assessments to rule out other cardiovascular diseases that might cause heart block. Accordingly, the excellent outcome of our patient is more supportive of the notion that CCHB in adults even complicated by Adams-Stoke episodes may not always require permanent pacemaker implantation.
Citing the 2018 American Heart Association guidelines, class I pacemaker indication involves symptomatic bradycardia, a wide QRS escape rhythm, a mean daytime heart rate < 50 bpm, complex ventricular ectopy, and ventricular dysfunction[13]. Nonetheless, the supporting literature is not consistent. Some existing studies have suggested that heart rate and the level of block in the conduction system seem to be of limited prognostic significance, other than for symptomatic bradycardia[14-16]. In our case, the patient had syncopal episodes and a mean daytime heart rate below 50 bpm, both of which are class I pacemaker indications. However, the patient lived a normally active life and had an uneventful pregnancy without pacing during the follow-up. In fact, the relatively good clinical outcome of our case confirmed some original studies, in which the prognosis of CCHB was usually said to be good and the first two Adams-Stokes attacks were often not fatal[10,15]. Growing evidence has shown that the population of patients with CCHB represents not a single distinct disease process but several processes with the common manifestation of CAVB[12]. In recent decades, the indication for pacing has widened, and it is estimated that 65%-90% of CCHB patients are treated with a pacemaker[17]. Of chief concern, pacemaker implantation is not a risk-free procedure, and fracture, repeated battery re
This case presented an unexpectedly good outcome of symptomatic CCHB without any intervention. This finding suggests that CCHB in adulthood may have good clinical outcomes and does not always require permanent pacemaker implantation. Consequently, the natural history of CCHB needs further investigation in search of more reliable markers of prognostic significance to determine the timing and indication for pacing.
The authors thank the patient and her family for granting their permission to publish this case report.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Al-Ani RM, Iraq; Shariati MBH, Iran S-Editor: Guo XR L-Editor: Kerr C P-Editor: Guo XR
| 1. | Rein AJ, Mevorach D, Perles Z, Gavri S, Nadjari M, Nir A, Elchalal U. Early diagnosis and treatment of atrioventricular block in the fetus exposed to maternal anti-SSA/Ro-SSB/La antibodies: a prospective, observational, fetal kinetocardiogram-based study. Circulation. 2009;119:1867-1872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 2. | Santos-Pardo I, Villuendas R, Salvador-Corres I, Martínez-Morillo M, Olivé A, Bayes-Genis A. Anti-Ro/SSA antibodies and cardiac rhythm disturbances: Present and future perspectives. Int J Cardiol. 2015;184:244-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Chandler SF, Fynn-Thompson F, Mah DY. Role of cardiac pacing in congenital complete heart block. Expert Rev Cardiovasc Ther. 2017;15:853-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Manolis AA, Manolis TA, Melita H, Manolis AS. Congenital heart block: Pace earlier (Childhood) than later (Adulthood). Trends Cardiovasc Med. 2020;30:275-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Hamilton RM. Editorial commentary: Live better electrically? Trends Cardiovasc Med. 2020;30:287-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Dolara A, Favilli S. Controversies in the therapy of isolated congenital complete heart block. J Cardiovasc Med (Hagerstown). 2010;11:426-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Michaëlsson M, Jonzon A, Riesenfeld T. Isolated congenital complete atrioventricular block in adult life. A prospective study. Circulation. 1995;92:442-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 141] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | McHenry MM, Cayler GG. Congenital complete heart block in newborns, infants, children and adults: recognition and treatment. J Natl Med Assoc. 1969;61:295-302. [PubMed] |
| 9. | DeNoble AE, Kuller JA, Rhee EJ. Controversies in the Management of Isolated Congenital Atrioventricular Block. Obstet Gynecol Surv. 2015;70:518-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Campbell M, Emanuel R. Six cases of congenital complete heart block followed for 34-40 years. Br Heart J. 1967;29:577-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Baruteau AE, Fouchard S, Behaghel A, Mabo P, Villain E, Thambo JB, Marçon F, Gournay V, Rouault F, Chantepie A, Guillaumont S, Godart F, Bonnet C, Fraisse A, Schleich JM, Lusson JR, Dulac Y, Leclercq C, Daubert JC, Schott JJ, Le Marec H, Probst V. Characteristics and long-term outcome of non-immune isolated atrioventricular block diagnosed in utero or early childhood: a multicentre study. Eur Heart J. 2012;33:622-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Kertesz NJ, Fenrich AL, Friedman RA. Congenital complete atrioventricular block. Tex Heart Inst J. 1997;24:301-307. [PubMed] |
| 13. | Writing Committee Members; Kusumoto FM, Schoenfeld MH, Barrett C, Edgerton JR, Ellenbogen KA, Gold MR, Goldschlager NF, Hamilton RM, Joglar JA, Kim RJ, Lee R, Marine JE, McLeod CJ, Oken KR, Patton KK, Pellegrini CN, Selzman KA, Thompson A, Varosy PD. 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2019;16:e128-e226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 14. | Dewey RC, Capeless MA, Levy AM. Use of ambulatory electrocardiographic monitoring to identify high-risk patients with congenital complete heart block. N Engl J Med. 1987;316:835-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 73] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Esscher EB. Congenital complete heart block in adolescence and adult life. A follow-up study. Eur Heart J. 1981;2:281-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Jonzon A. Congenital complete atrioventricular block. A pace in time saves lives (?). Europace. 2002;4:343-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Dandamudi G, Simon J, Cano O, Master V, Koruth JS, Naperkowski A, Kean AC, Schaller R, Ellenbogen KA, Kron J, Vijayaraman P. Permanent His Bundle Pacing in Patients With Congenital Complete Heart Block: A Multicenter Experience. JACC Clin Electrophysiol. 2021;7:522-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Ju YT, Wei YJ, Hsieh ML, Wang JN, Wu JM. Transient Congenital Complete Heart Block: A Case Report. Children (Basel). 2021;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Rangavajla G, Mulukutla S, Thoma F, Kancharla K, Bhonsale A, Estes NAM, Jain SK, Saba S. Ventricular pacing and myocardial function in patient with congenital heart block. J Cardiovasc Electrophysiol. 2021;32:2684-2689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Tsujii N, Miyazaki A, Sakaguchi H, Kagisaki K, Yamamoto T, Matsuoka M, Shima Y, Ichikawa H, Ohuchi H. High Incidence of Dilated Cardiomyopathy After Right Ventricular Inlet Pacing in Patients With Congenital Complete Atrioventricular Block. Circ J. 2016;80:1251-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Song MK, Kim NY, Bae EJ, Kim GB, Kwak JG, Kim WH, Lee JR. Long-term Follow-up of Epicardial Pacing and Left Ventricular Dysfunction in Children With Congenital Heart Block. Ann Thorac Surg. 2020;109:1913-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Blank AC, Hakim S, Strengers JL, Tanke RB, van Veen TA, Vos MA, Takken T. Exercise capacity in children with isolated congenital complete atrioventricular block: does pacing make a difference? Pediatr Cardiol. 2012;33:576-585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |