Published online Jul 6, 2022. doi: 10.12998/wjcc.v10.i19.6587
Peer-review started: November 23, 2021
First decision: April 8, 2022
Revised: April 16, 2022
Accepted: May 8, 2022
Article in press: May 8, 2022
Published online: July 6, 2022
Processing time: 213 Days and 2.1 Hours
Most cancer patients are accompanied by anemia, which will be more serious when combined with end-stage renal disease (ESRD). At present, cancer-related anemia and renal anemia treatments mainly include erythropoiesis-stimulating agents (ESAs), iron supplementation, and blood transfusion, but their effects are often poor with several safety concerns. We have used roxadustat to treat anemia in a cancer patient with ESRD and achieved a successful outcome for the first time.
A 64-year-old man was diagnosed with right renal cancer (clear cell renal cell carcinoma). He did not receive surgery or radiotherapy before admission. He was treated with oral soltan (sunitinib malate) on April 18, 2017. During oral chemo
Oral roxadustat could achieve good results in treating anemia in cancer patients with ESRD.
Core Tip: Currently, erythropoiesis-stimulating agents (ESAs) are the cornerstones for the treatment of renal anemia or cancer-related anemia. Roxadustat is a newly developed drug for renal anemia treatment, but not for cancer-related anemia, let alone to treat anemia in cancer patients with end-stage renal disease (ESRD). At the beginning, the patient was treated with high-dose ESAs, iron supplementation, and even blood transfusion, but his anemia did not improve. However, after orally taking roxadustat, his hemoglobin gradually increased without significant tumor progression. This is the first case using roxadustat to successfully improve anemia in a cancer patient with ESRD.
- Citation: Zhou QQ, Li J, Liu B, Wang CL. Roxadustat for treatment of anemia in a cancer patient with end-stage renal disease: A case report. World J Clin Cases 2022; 10(19): 6587-6594
- URL: https://www.wjgnet.com/2307-8960/full/v10/i19/6587.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i19.6587
Most cancer patients have anemia symptoms. If they progress to end-stage renal disease (ESRD) and need maintenance hemodialysis treatment, anemia will be more difficult to correct. At present, cancer-related anemia and renal anemia treatments mainly include erythropoiesis-stimulating agents (ESAs), iron supplementation, and blood transfusion. However, ESAs are often used at high dosage and accompanied by thrombosis, hypertension, and other adverse events. Hypoxia-inducible factor prolyl hydroxylase inhibitors (HIF-PHI) are newly developed, oral-based small molecule drugs for the treatment of renal anemia, of which roxadustat (FG-4592, FibroGen, Astellas, AstraZeneca) has been listed in China and Japan[1]. To our best knowledge, it has not been used to treat cancer-related anemia. In this case report, we, for the first time, used roxadustat to treat anemia in a cancer patient with ESRD and achieved a successful outcome.
A 64-year-old man was admitted to our hospital because of general fatigue and tight breath. He had been on maintenance hemodialysis for 1 year.
The patient developed ESRD 2 years ago due to oral chemotherapy for right renal cancer and began hemodialysis on May 23, 2019. At that time, his hemoglobin (Hb) level was 79 g/L. He was given 10000 U of recombinant human erythropoietin (rHuEPO) (weight 50 kg) twice a week via intravenous injection. However, after 2 mo of treatment, his Hb level was still 77 g/L. He was then intravenously injected with 100 mg iron sucrose once a week for 10 wk. His Hb gradually rose to about 100 g/L for a short period and began to decline. During that time, we actively looked for the causes of anemia. The patient had no gastrointestinal bleeding, evident inflammation, hemolytic anemia, erythropoietin (EPO) resistance, and other factors. Therefore, he was given 10000 U rHuEPO three times a week and subjected to adequate hemodialysis three times per week and hemodiafiltration once every 2 wk. In addition, he was prescribed oral ketoacids, intravenous infusion of human albumin, and other nutritional support treatments. However, these measures did not work, and his Hb dropped from 100 g/L to 65 g/L within 5 mo. Meanwhile, he developed discomfort symptoms such as increased heart rate, tight breath, and general fatigue.
The patient had been diagnosed with right renal cancer (clear cell renal carcinoma) in 2017. He did not receive surgery or radiotherapy. He was orally given 50 mg of soltan (sunitinib malate) daily for 14 d since April 18, 2017. He stopped taking it for 7 d and began to retake it. During oral chemotherapy, he had numerous complications, including anemia, hypertension, positive urine protein, thyroid hypofunction, skin pigment loss, and renal function deterioration. At last, he progressed to ESRD.
He took amlodipine besylate tablets 5 mg daily to control blood pressure and levothyroxine sodium tablets 150 mg to treat hypothyroidism.
The patient had no history of smoking or drinking and no family history of similar diseases.
The vital signs of the patient were as follows: Body temperature, 36.5 ℃; blood pressure, 168/82 mmHg; pulse rate, 98 beats/min; and respiratory rate, 26 breaths/min. He was in a semi-recumbent position, and his eyelid conjunctiva was pale. His bilateral thoracic movement weakened, palpation speech fibrillation weakened, percussion sounded dull, and auscultation respiratory sound disappeared. His heart rate was 98 beats/min, his rhythm was neat, and there was no obvious murmur. His abdominal examination showed no obvious abnormalities, and his lower limbs had slight edema.
The blood examination of the patient after admission showed an Hb level of 65 g/L, erythrocyte count of 2.29 × 1012/L, reticulocyte count of 0.079 × 1012/L, serum ferritin level of 345 mg/L, transferrin saturation of 19.9%, EPO level of 28.6 mIU/mL, C-reactive protein level of 15.41 mg/L, albumin level of 24.4 g/L, and intact parathyroid hormone level of 27.3 pg/mL. The direct Coombs test was negative, and anti-EPO antibodies were negative (Table 1).
| Parameter | Recorded value | Reference range |
| White blood cell count (× 109/L) | 5.22 | 4.0-10.0 |
| Red blood cell count (× 1012/L) | 2.29 | 4.0-5.5 |
| Hemoglobin (g/L) | 65 | 120-160 |
| Platelet count (× 109/L) | 146 | 100-300 |
| Hematocrit (%) | 22 | 38.0-50.8 |
| Mean corpuscular volume (fl) | 95.9 | 80-100 |
| Mean corpuscular hemoglobin (pg) | 29.1 | 27-31 |
| Mean corpuscular hemoglobin concentration (g/L) | 304 | 320-360 |
| Reticulocyte count (× 1012/L) | 0.079 | 0.024-0.084 |
| Reticulocyte percentage (%) | 3.45 | 0.5-1.5 |
| Iron (μmol/L) | 7.68 | 10.6-36.7 |
| Serum ferritin (μg/L) | 345 | 30-300 |
| Total iron binding capacity (μmol/L) | 26.51 | 45-75 |
| Transferrin saturation (%) | 19.9 | 20-50 |
| C-reactive protein (mg/L) | 15.41 | < 10 |
| Albumin (g/L) | 24.4 | 40.0-55.0 |
| Calcium (mmol/L) | 2.01 | 2.11-2.52 |
| Phosphonium (mmol/L) | 1.58 | 0.85-1.51 |
| Intact parathyroid hormone (pg/ml) | 27.3 | 150-300 |
| Blood urea nitrogen (mmol/L) | 21.66 | 3.6-9.5 |
| Serum creatinine (μmol/L) | 556 | 57.0-111.0 |
| EPO concentration (mIU/ml) | 28.6 | 4.3-29 |
| Direct Coombs (IgG, C3) tests | Negative | Negative |
| Anti-EPO antibodies | Negative | Negative |
| Fecal occult blood test | Negative | Negative |
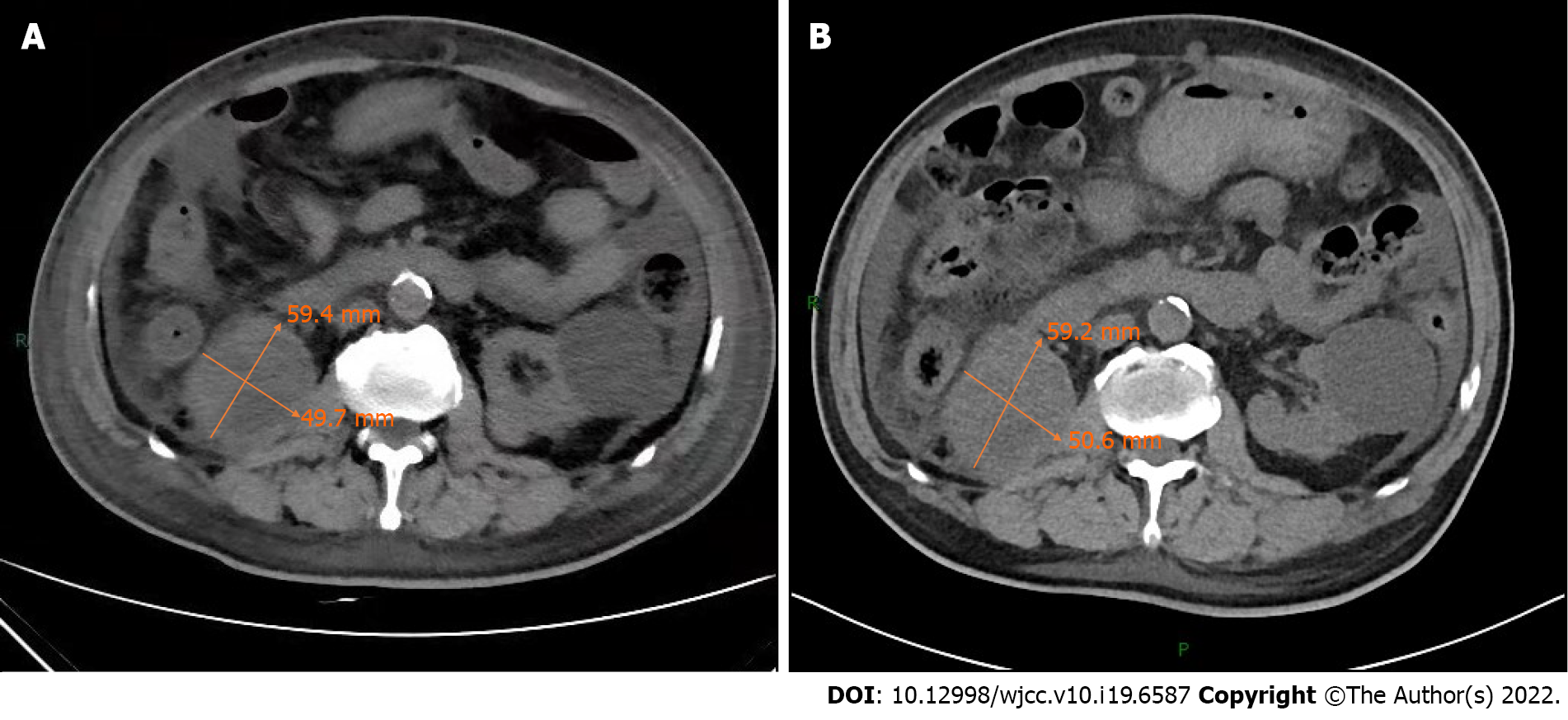
The plain chest computed tomography (CT) scan showed a large amount of effusion in the bilateral thorax, marks on the right, and partial compression atelectasis of both lungs. The plain abdominal CT scan (Figure 1A) showed an enlarged right kidney, irregular soft tissue occupying, unclear boundary, an about 59.4 mm × 49.7 mm larger layer, and thickened perirenal fascia. In addition, nodules with soft tissue density were seen at the lower pole of the left kidney, with a larger layer of about 26.0 mm × 22.1 mm.
The patient was diagnosed with chronic kidney disease (CKD) stage 5, renal anemia, maintenance hemodialysis, right renal cancer, cancer-related anemia, bilateral pleural effusion, very high-risk grade 3 hypertension, and hypoproteinemia.
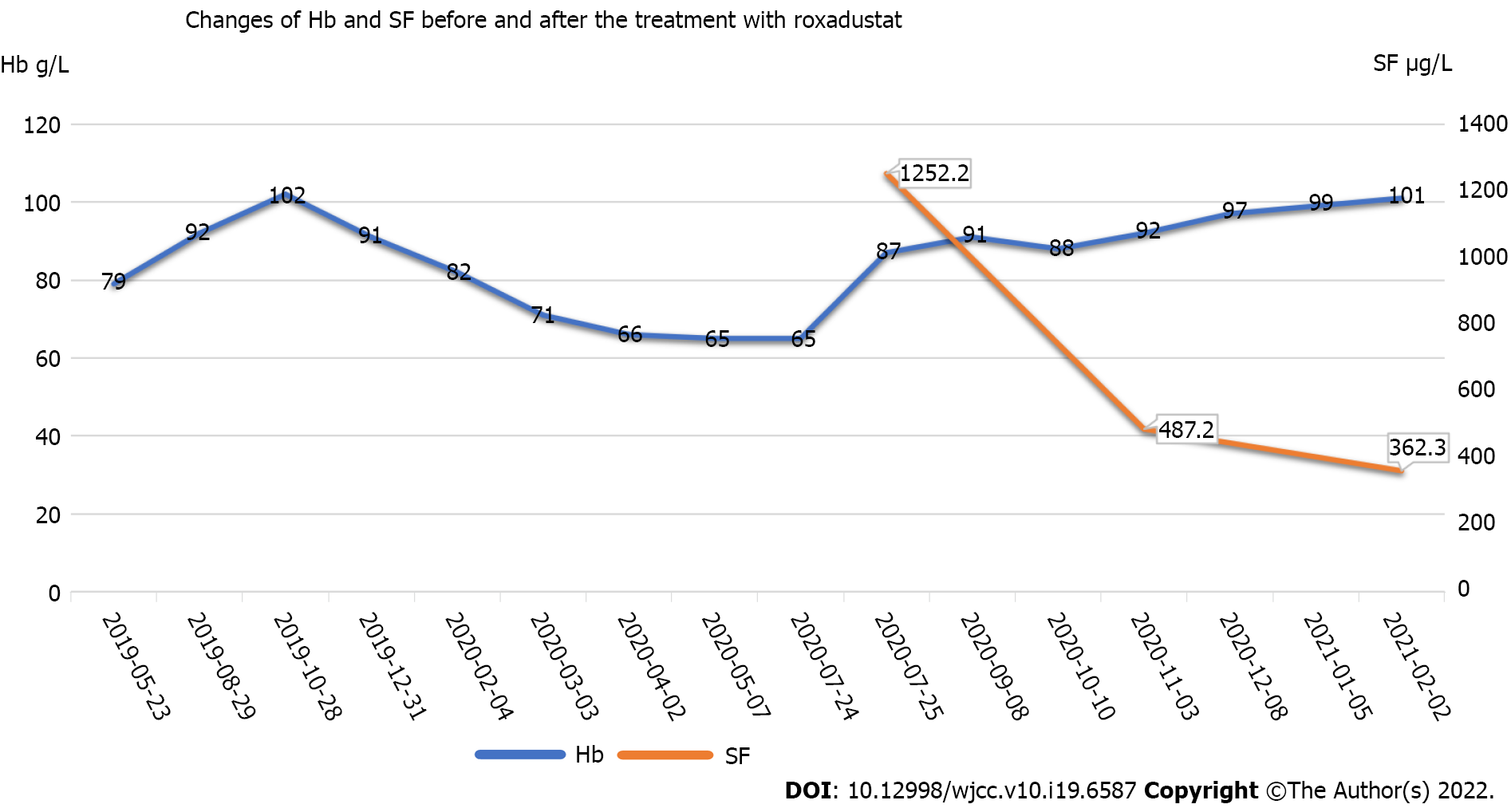
During outpatient dialysis, to correct anemia, we increased the patient's rHuEPO dosage from 20000 U per week to 30000 U per week and gave him a total of 1 g of iron sucrose intravenously. Therefore, he was treated with 1.5 units of red blood cell transfusion after hospitalization. To alleviate the tight breath symptoms, he was subjected to pleural puncture. Examination of the collected chest fluid identified some cancer cells. He was continuously treated with hemodialysis three times a week and hemodiafiltration once every 2 wk. He was also given oral ketoacids, intravenous infusion of human albumin, and other nutritional support treatments. After that, his Hb rose to 87 g/L, but his serum ferritin was as high as 1252.2 mg/L (Figure 2). His general fatigue symptoms were markedly relieved. After a series of symptomatic treatments, the patient's condition improved, and he was discharged from the hospital.
Because the patient was given a very high dosage of rHuEPO, we were worried that further increasing rHuEPO dosage would lead to severe complications. At the same time, the patient had an iron overload. Therefore, we recommended oral roxadustat to improve his anemia. However, the patient was only willing to accept 20 mg rather than the standard starting dose of 100 mg roxadustat treatment three times a week because of the financial burden since August 1, 2020.
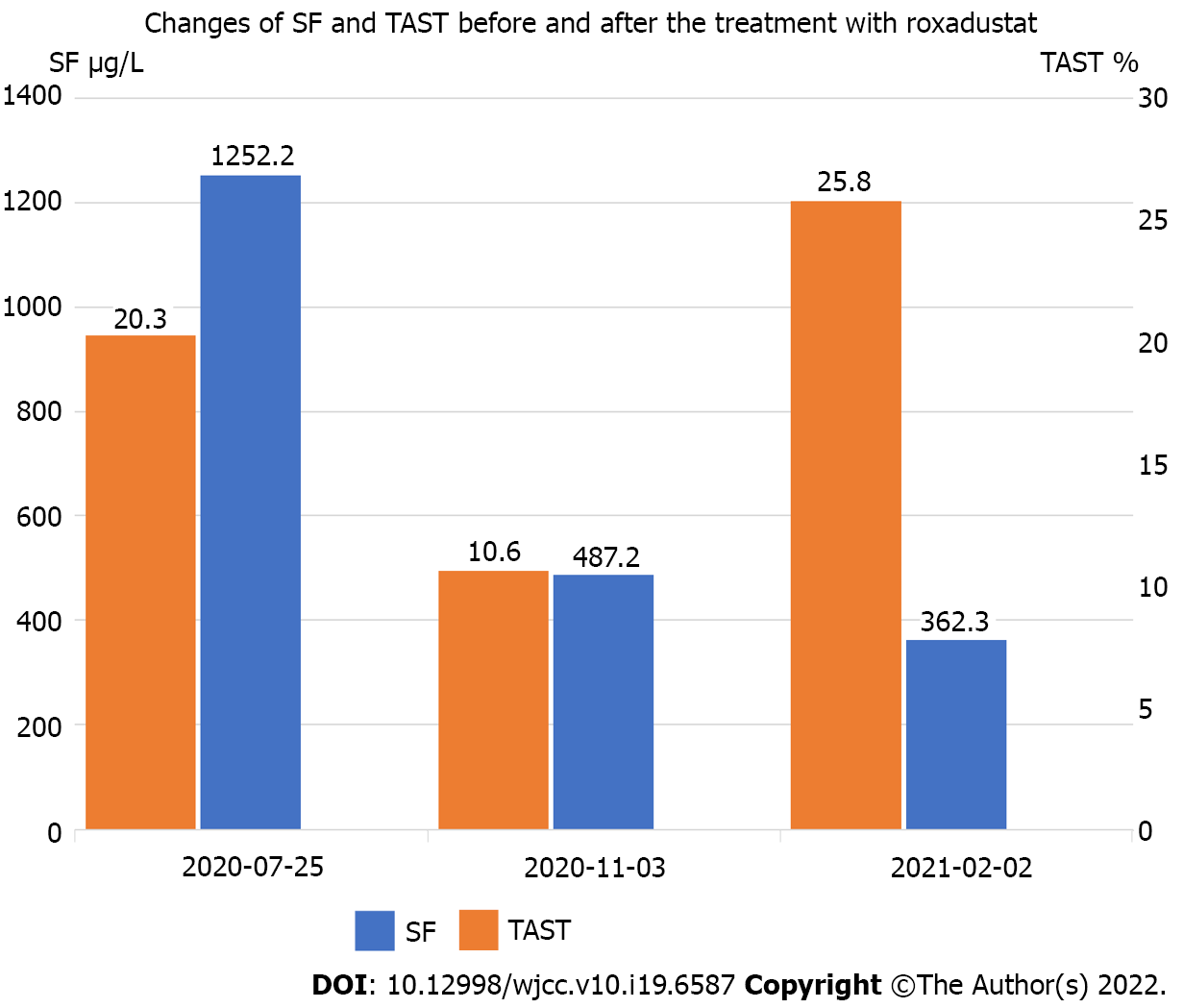
During the 4 mo of follow-up, the patient’s Hb remained stable at about 90 g/L. We repeatedly advised him to increase roxadustat dosage. Finally, he agreed to take 50 mg oral roxadustat three times a week from December 8, 2020. Two months later, his Hb level rose to about 100 g/L (Figure 2). During the course of oral administration, he was regularly monitored for blood parameters and subjected to abdominal CT. His iron index was further improved (Figure 3). The plain abdomen CT scan (Figure 1B) showed an enlarged right kidney, irregular soft tissue occupying, unclear boundary, and an about 59.2 mm × 50.6 mm larger layer. Compared with previous CT, there was no significant change in tumor size. Meanwhile, he did not have obvious discomfort.
To our best knowledge, this is the first case report of roxadustat for the treatment of anemia in a cancer patient with ESRD. Many studies have shown that 30% to 90% of cancer patients have anemia. The incidence and severity of anemia are related to the age, tumor type, tumor stage, disease progression, treatment plan, dosage of chemotherapy drugs, inflammation, and other factors[2,3]. Based on the data from Chinese clinical trials, the prevalence of anemia is significantly higher in patients with CKD than in the general population and increases with the progression of CKD, reaching as high as 91.6%-98.2% in dialysis patients[4-6]. A bidirectional relationship has been observed between kidney disease and cancer. On the one hand, cancer is an important complication noted in kidney disease and a major cause of morbidity and mortality in this group. On the other hand, cancer patients may develop CKD directly or indirectly due to the toxicity of tumors to the kidney itself or treatment-related factors. Currently, ESAs are the cornerstones for the treatment of renal anemia or cancer-related anemia[7] besides iron supplementation and blood transfusion.
ESAs are the analogs of EPO with characteristics of good tolerance and ease to use. They have been used to treat anemia patients with normal physiology and can significantly improve the quality of life of patients. However, ESAs can increase blood pressure, promote tumor growth[8], and increase the risk of stroke and thromboembolism in patients with malignant tumors[9-11]. According to the summary of the American Society of Clinical Oncology/American Society of Hematology Clinical Practice Guideline Update, ESAs should not be offered to patients with chemotherapy-associated anemia whose cancer treatment is curative in intent. Still, it could be provided to patients with chemotherapy-associated anemia whose cancer treatment is not curative in intent and whose Hb is < 10 g/dL[7].
For patients with renal failure caused by tumors or chemotherapy, continuous use of ESAs can lead to functional iron deficiency. At present, the main iron supplements are oral and parenteral[12]. However, due to the close relationship between unstable iron and oxidative stress, bacterial growth, gastro
Transfusion of red blood cells or whole blood can rapidly increase Hb concentration. Thus, it is used for tumor patients with severe anemia, patients with anemia caused by acute hemorrhage, and asymptomatic anemia patients with heart disease, chronic lung disease, and cerebrovascular disease. However, transfusion may lead to a series of risks such as transfusion-related response, blood transfusion-related cycle overload, virus transmission, bacterial infection, iron overload, and red blood cell allogeneic immunity[15].
HIF, a dimer composed of α and β subunits, is the key transcription factor regulating the body’s physiological response to the change of oxygen concentration. There are three subtypes of HIF-α: HIF-1α, HIF-2α, and HIF-3α. HIF-2α is mainly distributed in the kidney and participates in the production of red blood cells. HIF is regulated by the prolyl hydroxylase (PHD) family. PHD mainly regulates HIF by sensing the changes of intracellular oxygen partial pressure. Under normal oxygen conditions, HIF-α is recognized and hydroxylated by PHD and then degraded by proteasome. Under hypoxia, PHD activity decreases, and HIF-α is freed from degradation. HIF-α and HIF-β combine to form a dimer, promoting the upregulation of a series of genes. HIF-PHI drugs weaken the effect of PHD on HIF mainly by replacing the necessary synergistic substrate of PHD or by blocking the interference between enzyme catalytic sites and PHD substrate-α so that HIF can be stably expressed to regulate the transcription and expression of target genes downstream of the HIF signaling pathway[1]. HIF-PHI could promote the expression of iron metabolism-related proteins by promoting EPO production and its receptor expression and reducing hepcidin levels, thereby comprehensively regulating the production of erythrocytes[16,17]. Roxadustat (FG-4592, FibroGen, Astellas, AstraZeneca) is one of HIF-PHI and has been listed in China and Japan. On December 17, 2018, roxadustat was officially approved by the State Drug Administration of China to treat CKD anemia in dialysis patients. But it has not been used to treat cancer-related anemia, let alone to treat anemia in cancer patients with ESRD.
In our case report, the patient had both renal cancer and ESRD and was receiving hemodialysis. His anemia was caused by various factors, including insufficient EPO production, reduced EPO activity, iron deficiency, metabolic disorders, malnutrition, inflammatory state, uremic toxin, and the tumor itself. Because the patient’s cancer treatment was not curative in intent, he was given ESAs to treat anemia at first. Later, to maintain Hb stability, we adjusted ESA dose and supplemented iron to ensure dialysis adequacy and improve nutritional status. However, the patient’s Hb decreased rapidly (Table 1). Although the patient's tumor was incurable, he still hoped to prolong his survival time and improve his quality of life as much as possible. At this time, a large dose of ESAs could not maintain Hb stability. Although blood transfusion temporarily increased Hb, he was still in a state of iron overload and inflammation.
Many studies have shown that roxadustat can effectively treat renal anemia[18,19]. However, it has not been reported to treat cancer-related anemia, cancer complicated with renal anemia, and anemia with a combination of roxadustat and ESAs. Based on the above reasons, we advised the patient to take oral roxadustat on the basis of maintaining the original treatment regimen. Unfortunately, he received 20 mg rather than the initial 100 mg of roxadustat three times a week. He was also given 30000 U of rHuEPO via intravenous injections per week. During the subsequent follow-up months, his Hb remained at about 90 g/L, his BP slightly increased but was controllable by drugs, and his iron index was improved (Figure 3). With our repeated efforts, the patient accepted treatment with 50 mg roxadustat three times a week, and his condition further improved: His Hb gradually rose to about 100 g/L. The patient’s abdominal CT (Figure 1B) revealed no apparent progression of the renal tumor. Over the period of oral roxadustat, the patient had no obvious discomfort symptoms. Therefore, we believe that roxadustat was effective for anemia treatment in cancer patients with ESRD and should be recognized as a routine treatment.
Some studies have shown that increased HIF expression in cancers is associated with a poor prognosis[20]. It is unclear whether HIF is a pro-tumor factor or whether an increased HIF level reflects the hypoxic environment in faster-growing and more aggressive tumors. Currently, its causative relationship with primary tumors has not been clinically proven. Since our report is just an individual case, more clinical trials are needed to observe the therapeutic effect and mechanism of roxadustat for anemia treatment in cancer patients with ESRD.
Oral roxadustat is safe and effective for anemia treatment of cancer patients with ESRD undergoing hemodialysis. A combination of roxadustat and ESAs may be an additional treatment option for these patients. However, more studies are needed to assess the risk and adverse effects of roxadustat.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cabezuelo AS, Spain S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR
| 1. | Working group of guidelines for renal anemia of nephrologist branch of Chinese Medical Association. Clinical practice guidelines for diagnosis and treatment of renal anemia in China. Zhonghua Yixue Zazhi. 2021;101:1463-502. [DOI] [Full Text] |
| 2. | Knight K, Wade S, Balducci L. Prevalence and outcomes of anemia in cancer: a systematic review of the literature. Am J Med. 2004;116 Suppl 7A:11S-26S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 367] [Article Influence: 17.5] [Reference Citation Analysis (1)] |
| 3. | Li Y, Shi H, Wang WM, Peng A, Jiang GR, Zhang JY, Ni ZH, He LQ, Niu JY, Wang NS, Mei CL, Xu XD, Guo ZY, Yuan WJ, Yan HD, Deng YY, Yu C, Cen J, Zhang Y, Chen N. Prevalence, awareness, and treatment of anemia in Chinese patients with nondialysis chronic kidney disease: First multicenter, cross-sectional study. Medicine (Baltimore). 2016;95:e3872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 4. | Wang Y, Wei RB, Su TY, Huang MJ, Li P, Chen XM. Clinical and pathological factors of renal anaemia in patients with IgA nephropathy in Chinese adults: a cross-sectional study. BMJ Open. 2019;9:e023479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Li Y, Zhang W, Ren H, Wang W, Shi H, Li X, Chen X, Shen P, Wu X, Xie J, Chen N. Evaluation of anemia and serum iPTH, calcium, and phosphorus in patients with primary glomerulonephritis. Contrib Nephrol. 2013;181:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Huang Z, Song T, Fu L, Rao Z, Zeng D, Qiu Y, Wang X, Xie L, Wei Q, Wang L, Lin T. Post-renal transplantation anemia at 12 mo: prevalence, risk factors, and impact on clinical outcomes. Int Urol Nephrol. 2015;47:1577-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Latcha S. Anemia management in cancer patients with chronic kidney disease. Semin Dial. 2019;32:513-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 8. | Henke M, Laszig R, Rübe C, Schäfer U, Haase KD, Schilcher B, Mose S, Beer KT, Burger U, Dougherty C, Frommhold H. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trial. Lancet. 2003;362:1255-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 913] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 9. | Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, Zwahlen M, Clarke M, Weingart O, Kluge S, Piper M, Rades D, Steensma DP, Djulbegovic B, Fey MF, Ray-Coquard I, Machtay M, Moebus V, Thomas G, Untch M, Schumacher M, Egger M, Engert A. Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomised trials. Lancet. 2009;373:1532-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 402] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 10. | Wright JR, Ung YC, Julian JA, Pritchard KI, Whelan TJ, Smith C, Szechtman B, Roa W, Mulroy L, Rudinskas L, Gagnon B, Okawara GS, Levine MN. Randomized, double-blind, placebo-controlled trial of erythropoietin in non-small-cell lung cancer with disease-related anemia. J Clin Oncol. 2007;25:1027-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 326] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 11. | Leyland-Jones B, Semiglazov V, Pawlicki M, Pienkowski T, Tjulandin S, Manikhas G, Makhson A, Roth A, Dodwell D, Baselga J, Biakhov M, Valuckas K, Voznyi E, Liu X, Vercammen E. Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: a survival study. J Clin Oncol. 2005;23:5960-5972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 500] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 12. | The society of chemotherapy; Chinese Anti-Cancer Association; Committee of neoplastic supportive-care (CONS); China Anti-Cancer Association. A consensus on the clinical diagnosis, treatment, and prevention of cancer and chemotherapy-related anemia in China (2019 edition). Chin J Clin Oncol. 2019;11:78-85. |
| 13. | Li X, Cole SR, Kshirsagar AV, Fine JP, Stürmer T, Brookhart MA. Safety of Dynamic Intravenous Iron Administration Strategies in Hemodialysis Patients. Clin J Am Soc Nephrol. 2019;14:728-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Bailie GR, Larkina M, Goodkin DA, Li Y, Pisoni RL, Bieber B, Mason N, Tong L, Locatelli F, Marshall MR, Inaba M, Robinson BM. Data from the Dialysis Outcomes and Practice Patterns Study validate an association between high intravenous iron doses and mortality. Kidney Int. 2015;87:162-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 142] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 15. | Spivak JL, Gascón P, Ludwig H. Anemia management in oncology and hematology. Oncologist. 2009;14 Suppl 1:43-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 16. | Locatelli F, Del Vecchio L, De Nicola L, Minutolo R. Are all erythropoiesis-stimulating agents created equal? Nephrol Dial Transplant. 2021;36:1369-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Maxwell PH, Eckardt KU. HIF prolyl hydroxylase inhibitors for the treatment of renal anaemia and beyond. Nat Rev Nephrol. 2016;12:157-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 221] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 18. | Chen N, Qian J, Chen J, Yu X, Mei C, Hao C, Jiang G, Lin H, Zhang X, Zuo L, He Q, Fu P, Li X, Ni D, Hemmerich S, Liu C, Szczech L, Besarab A, Neff TB, Peony Yu KH, Valone FH. Phase 2 studies of oral hypoxia-inducible factor prolyl hydroxylase inhibitor FG-4592 for treatment of anemia in China. Nephrol Dial Transplant. 2017;32:1373-1386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 154] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 19. | Akizawa T, Ueno M, Shiga T, Reusch M. Oral roxadustat three times weekly in ESA-naïve and ESA-converted patients with anemia of chronic kidney disease on hemodialysis: Results from two phase 3 studies. Ther Apher Dial. 2020;24:628-641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 20. | Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1318] [Cited by in RCA: 1361] [Article Influence: 90.7] [Reference Citation Analysis (0)] |