Published online Jul 6, 2022. doi: 10.12998/wjcc.v10.i19.6563
Peer-review started: November 3, 2021
First decision: March 7, 2022
Revised: March 17, 2022
Accepted: May 12, 2022
Article in press: May 12, 2022
Published online: July 6, 2022
Processing time: 233 Days and 5.8 Hours
Relapsing polychondritis (RP) is a rare, long-term, and potentially life-threatening disease characterised by recurrent paroxysmal inflammation that can involve and destroy the cartilage of the external ear, nose, larynx, and trachea.
We here report a case of RP involving solely the tracheobronchial cartilage ring (and not the auricular. nasal or articular cartilage) complicated by Sjögren's syndrome in a 47-year-old female whose delayed diagnosis caused a sharp decline in pulmonary function. After corticosteroid treatment, her pulmonary function improved.
In such cases, our experience suggested that 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) and fiberoptic bronchoscopy should be used to diagnose airway chondritis as relapsing poly
Core Tip: Relapsing polychondritis is a rare immune-mediated systemic disease characterized by recurrent inflammation of cartilage and proteoglycan rich tissues. In approximately 10% of patients with tracheobronchial cartilage ring involvement, characterized by cough and shortness of breath, regular bron
- Citation: Chen JY, Li XY, Zong C. Relapsing polychondritis with isolated tracheobronchial involvement complicated with Sjogren's syndrome: A case report. World J Clin Cases 2022; 10(19): 6563-6570
- URL: https://www.wjgnet.com/2307-8960/full/v10/i19/6563.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i19.6563
Relapsing polychondritis (RP) is a systemic disease that primarily affects cartilaginous structures of the ears, nose, upper and lower airways, and ribs, but can also involve joints, skin, eyes, and the cardiovascular system. RP is considered a rare disease (Orpha code: 728) with an estimated incidence of 3.5/1000000/year[1], although a lower figure has been reported in a recent population-based cohort study in the United Kingdom[2]. Peak incidence is in the fifth decade of life (between 40 and 55 years of age), although the disease has also been described in young children and the older people[3]. Its inconspicuous onset can make early diagnosis very difficult, leading to delayed treatment and consequent increased risk of permanent or life-threatening sequelae. Large upper and/or lower airway tract involvement is a common clinical manifestation, occurring in up to 50% of patients over the course of the disease, and is a major cause of morbidity and mortality[4,5]. Moreover, 10% of patients present with tracheobronchial cartilage involvement as the first manifestation[6]. Thickening of the tracheal wall and destruction of the tracheal cartilaginous rings are characteristic in patients with large airway involvement, whereas tracheomalacia is sometimes observed[7]. Here, we report a case of RP complicated with Sjögren's syndrome.
Cough with expectoration.
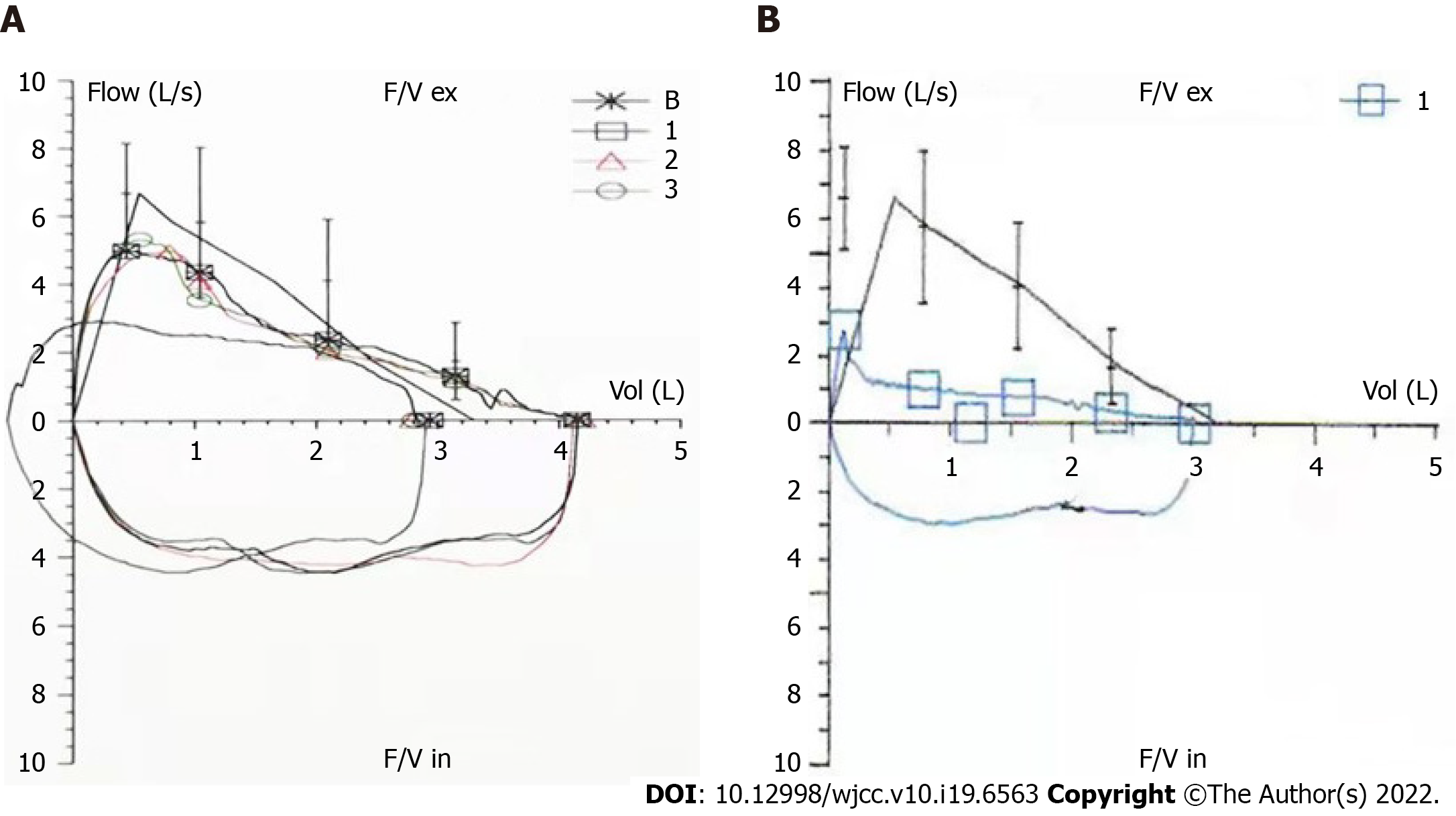
The patient was a 47-year-old Chinese female, had developed a cough with expectoration, producing small volumes of mainly white and sticky phlegm, over the 17 mo prior to diagnosis. Outpatient examination showed a 6-min walk distance within normal range. Pulmonary function tests (Figure 1A) showed an FEV1/FVC of 70.73% (84.48% of the predicted FEV1% value of 103.55%), indicating mild obstructive pulmonary ventilation dysfunction. The patient was given routine treatment. Recently, cough and expectoration gradually become worse and she experienced shortness of breath after an event.
The patient denied any history of past disease, allergy, or exposure to smoke or dust for herself or her parents.
The patient denied any history of past disease, allergy, or exposure to smoke or dust for herself or her parents.
Physical examination only showed rhonchi in both lungs, while other examination parameters were normal.
Maximum white and red blood cell counts were 7.58 × 109/L and 4.28 × 1012/L, respectively; neutrophil count was 58%; lymphocyte count was 33%; maximum platelet count was of 349 × 109/L; erythrocyte sedimentation rate was 43 mm/h; patient was anti-Ro52-positive; and mean IgG4 level was 136 mg/L (normal range: 80-1400 mg/L). Other pertinent blood workups (including renal and hepatic parameters, albumin-to-globulin ratio, and levels of C-reactive protein, creatinine kinase, rheumatoid factor, anti-CCP, anti-ENA (including anti-SSA and anti-SSB), MPO, PR3, p-ANCA, and c-ANCA) reported normal values. Arterial blood pH was 7.415; PO2 was 87.1 mmHg; PCO2 was 32.5 mmHg; and oxygen saturation was 97.3%. Basal salivary flow rate was 0.2 mL/min, reaching 0.5 mL/min after stimulation. The tear film break-up times were 7 s (left) and 10 s (right). Hearing test showed high-frequency hearing loss in the left ear. Upon review of lung function, the forced expiratory volume in 1 s/forced vital capacity (FEV1/FVC) of 38.8% accounted for 47.6% of the expected FEV1% value of 42.6% (Figure 1B). The detailed data of the two lung function tests were compared in Table 1. The airway provocation test was negative, and the bronchial dilation test did not effectively dilate the airway. The Modified Medical Research Council dyspnea scale score was 2.
| Date | FEV1/FVC (%) | FEV1/FVC (%, prediction) | FEV1 (%, prediction) | FVC (%, prediction) |
| December, 2018 | 70.73 | 84.48 | 103.55 | 125.87 |
| May, 2020 | 38.80 | 47.60 | 42.60 | 94.10 |
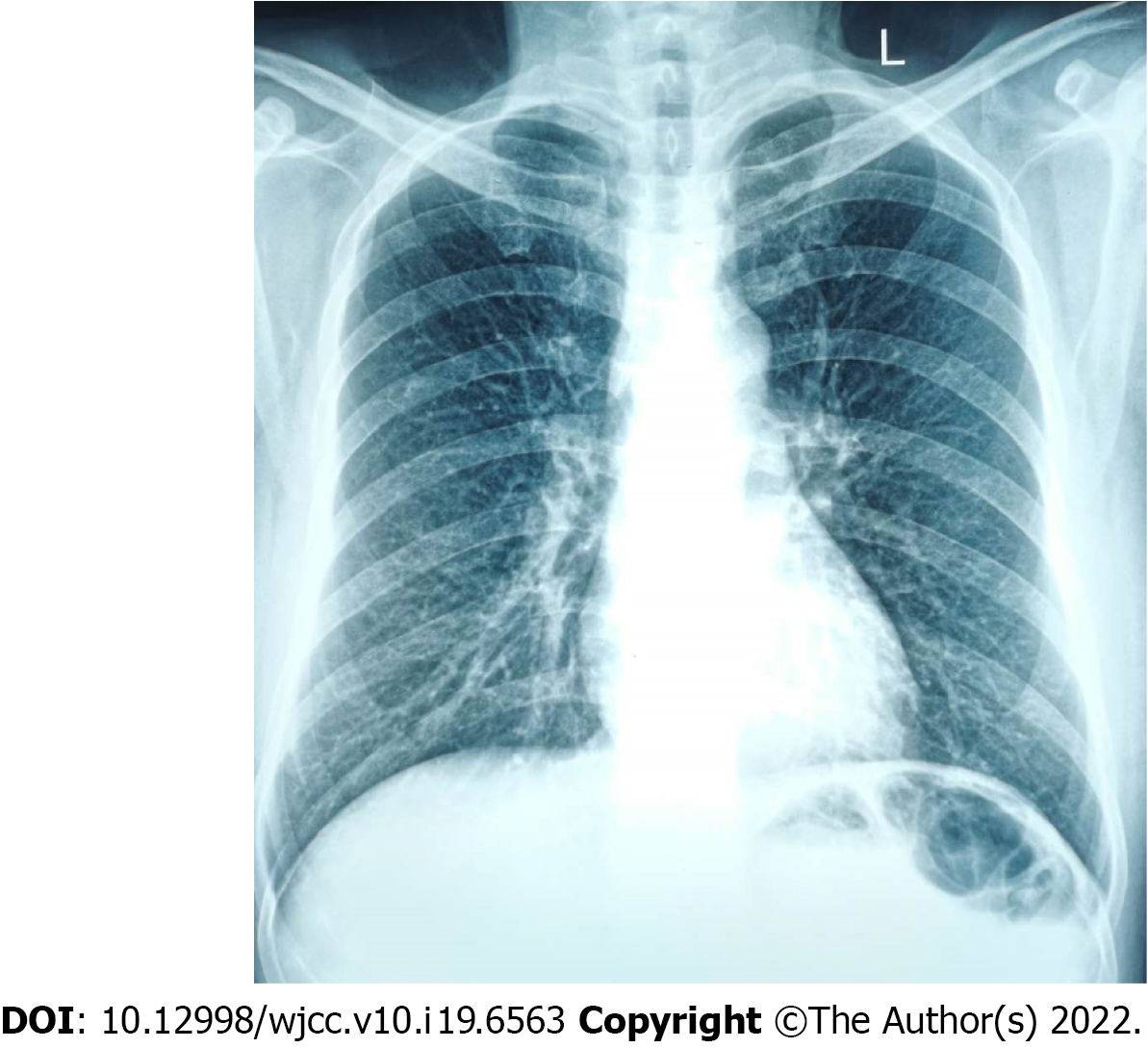
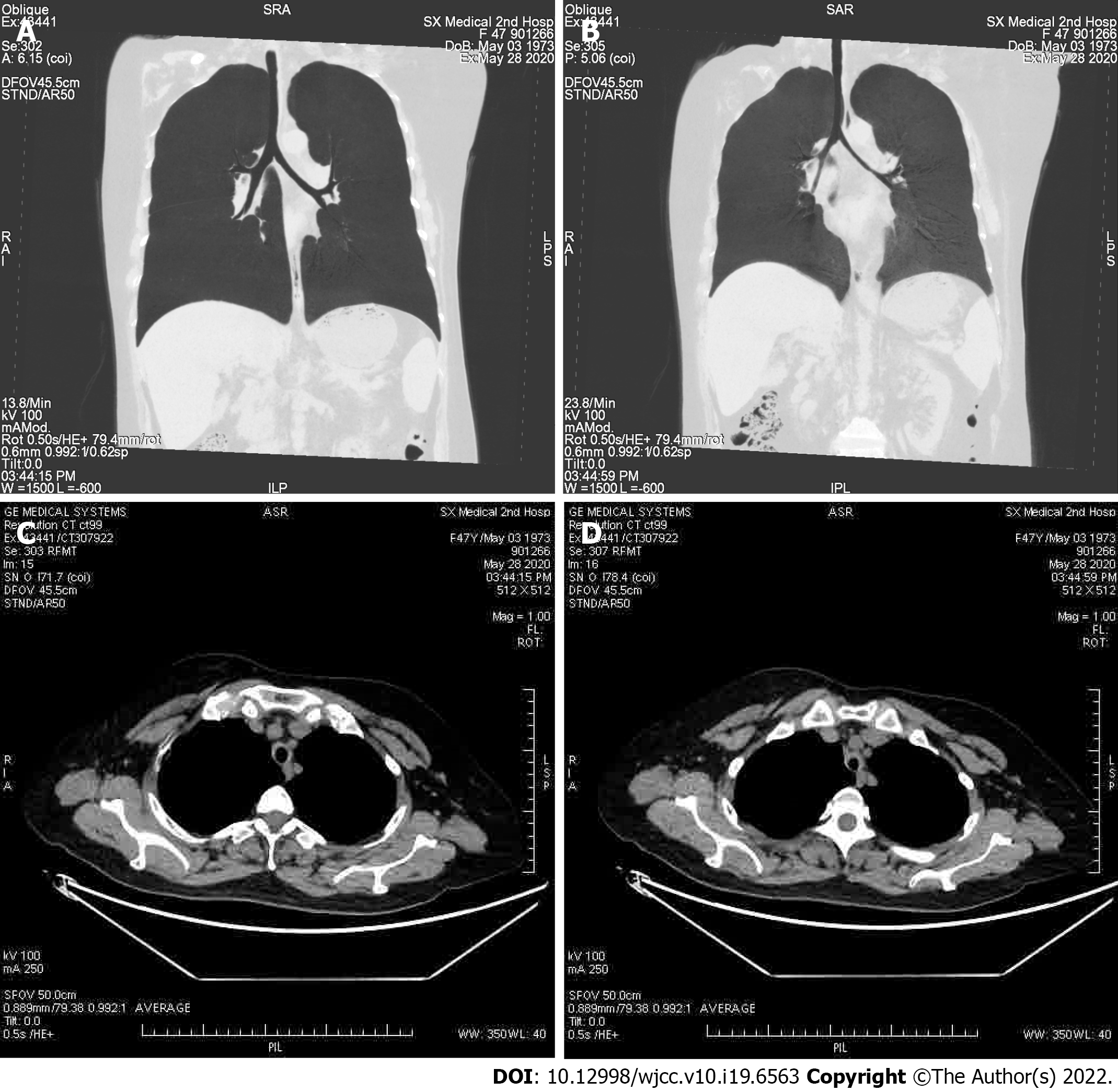
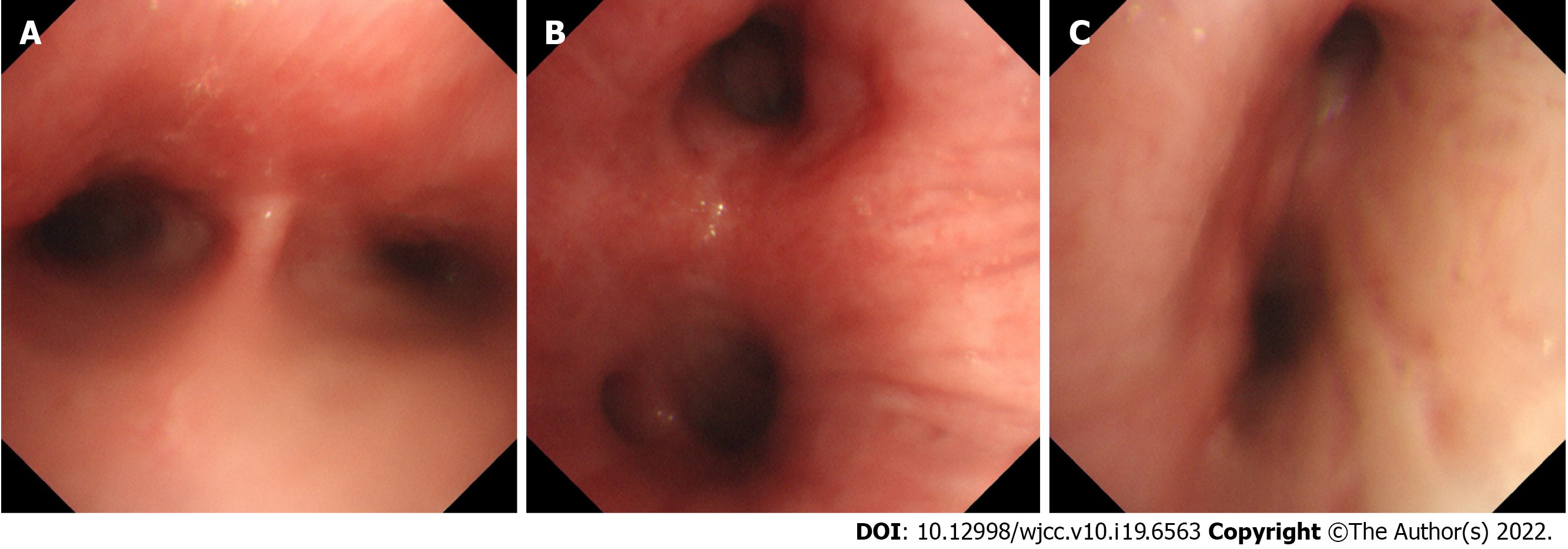
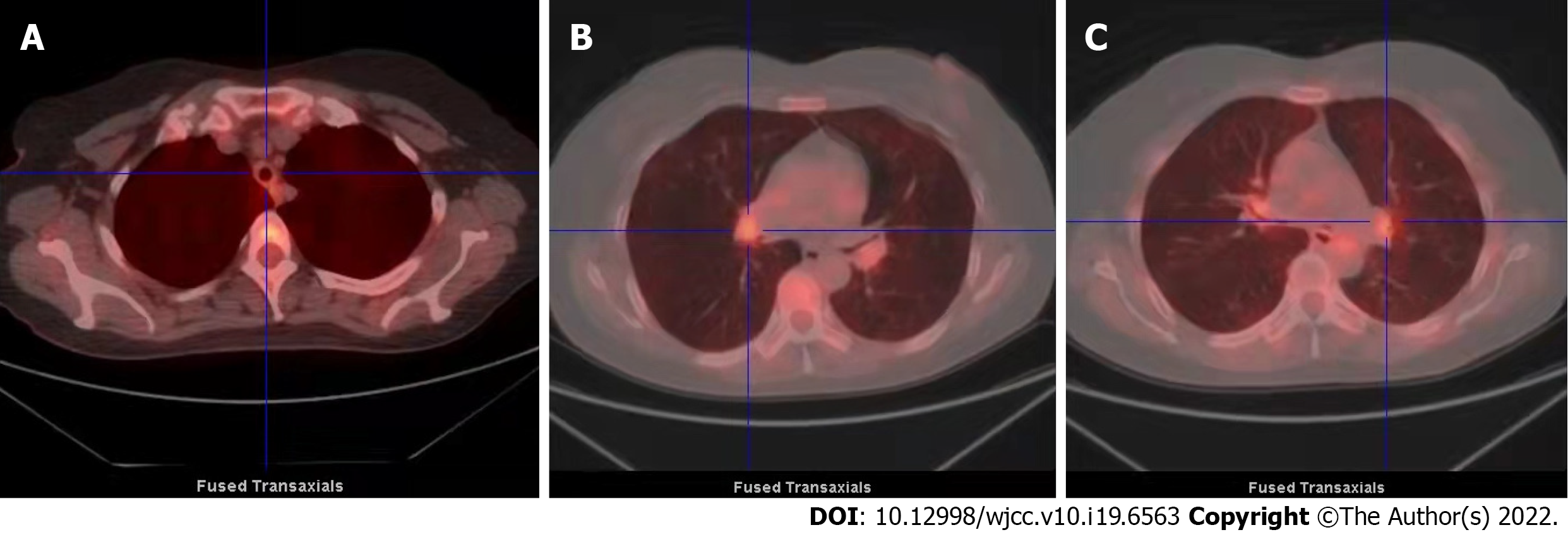
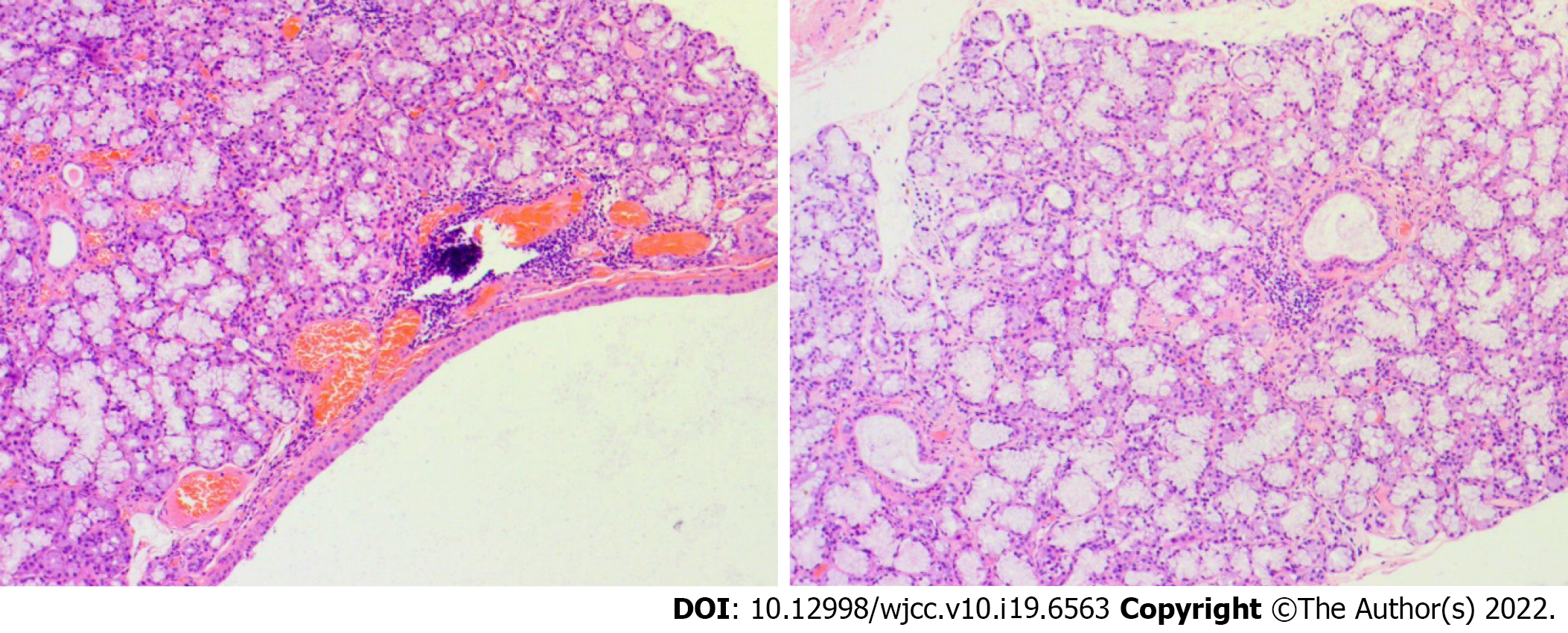
A chest X-ray showed that the texture of both lungs was increased (Figure 2). Chest computed tomography (CT) (Figure 3) revealed thickening of the tracheal wall; bilateral bronchial narrowing in the expiratory phase, compared to the inspiratory phase, was further detected during dynamic breathing. Bronchoscopy (Figure 4) revealed that the tracheal cartilage rings were blurred and the tracheal mucosa was swollen. 18F-fluorodeoxyglucose positron emission tomography/CT (18F-FDG PET/CT) (Figure 5) revealed that the walls of the trachea, the left and right main bronchi, and the lobar and segmental bronchi were evenly thickened and exhibited mild to moderate increases in metabolic activity (SUVmax = 2.91). No abnormal FDG uptake was detected in the paranasal sinuses, nasal cavity, ears, eyes, auricles, or ribs. Biopsy of the lower lip’s salivary gland tissue (Figure 6) exhibited partial ductal dilatation and acinar and periductal infiltration of numerous lymphocytes (> 50 in a focal lesion region).
RP complicated by Sjogren's syndrome.
Prednisone acetate (40 mg/d).
After treatment with prednisone acetate (40 mg/d), the cough was relieved after 2 wk and the respiratory symptoms disappeared at the 4-mo follow-up.
RP is diagnosed principally on the basis of its typical clinical manifestations. In 1976, McAdam et al[8] proposed the first diagnostic criteria for RP on the basis of the clinical presentation observed in 159 patients; those criteria were later modified by Damiani and Levine[9] in 1979, and by Michet et al[10] in 1989. In a 2016 report, Dion et al[11] analysed the clinical characteristics of 142 RP patients and defined three clinical phenotypes, a respiratory phenotype, an hematologic phenotype, and a mild phenotype (with good prognosis) that differed in terms of clinical manifestations, disease progression and treatment, and infection rates. Although diagnostic criteria have traditionally focused on rheumatic diseases, since 1972 several RP cases involving valvular heart disease, myasthenia gravis, and myelodysplastic syndrome have been reported, based on histopathological examination after appearance of severe complications[12-16]. Over the last two decades, the medical literature reported also an increasing number of RP cases associated with bronchopulmonary symptoms[17-20]. One prospective study found that among all RP patients, those with predominant respiratory symptoms were younger and had a higher intensive care unit (ICU) admission rate[21]. This evidence suggests the need to expand awareness and understanding of the respiratory manifestations of the disease among clinicians.
RP is a rare disease, characterised by recurring inflammation of cartilage and proteoglycan-rich tissue triggering progressive anatomical deformation and functional damage[1]. As with our patient, about one third of patients with RP may display associated autoimmune diseases[22]. Infiltration of tissues by different cellular and molecular inflammatory mediators leads to the release of degradative enzymes (such as matrix metalloproteinases) and reactive oxygen metabolites by inflammatory cells and chondrocytes, and ultimately to the destruction of cartilage and other proteoglycan-rich structures[23,24]. Autoantibodies against cartilage, collagen (mostly type II, but also types IX, X, and XI), matrilin-1, and cartilage oligomeric matrix proteins have been consistently detected in RP patients[25]. In turn, cytokines released during the inflammatory process can both amplify the pathologic process and induce constitutional symptoms. Based on positive correlation, detected in a single RP patient, between serum Th1 cytokine (e.g., IFN-γ, IL-12, and IL2) levels and disease activity, it was suggested that RP is a Th1-mediated disease[26].
Feared consequences of tracheobronchial compromise in RP patients include structural malformations such as tracheomalacia and permanent tracheal stenosis[6]. Thus, early diagnosis of RP in patients such as ours, presenting with atypical clinical manifestations, is of great importance but also quite challenging. By relying solely on pulmonary function tests indicating obstructive airflow limitations, a diagnosis of RP can be easily confused with several other conditions in patients presenting only with respiratory symptoms such as cough and dyspnea. In turn, the presence of respiratory symptoms in a patient already diagnosed with RP should raise clinical suspicion of potentially severe airway lesion and prompt further testing and treatment.
Given the non-specific, airway-delimited pathological manifestations of our patient, a diagnosis of RP was initially missed upon applying the aforementioned testing criteria. However, the sharp decline in pulmonary function experienced by our patient (progressing from mild to severe within 18 mo) served as a red flag, and we thus made every effort to perform a correct diagnosis. Expiratory CT abnormalities are present in the majority of RP patients, yet only half of them demonstrate abnormalities on routine inspiratory CT scans[27,28]. In our clinical cases, chest CT objectively reflects airway wall thickening, but does not clearly define the extent of stenosis or obstruction. Pulmonary function tests and chest CT are thus complementary, but the former are more informative. Inflammation of the mucosa and infiltration of the cartilaginous structures are sometimes visible on endoscopy above the thyroid and next to the first tracheal rings. We used FDG-PET/CT to evaluate cartilage metabolism at other sites (as an alternative to biopsy) since this technique proved to be a useful tool for both diagnosis and evaluation of disease activity and has been used for diagnosis of RP[29,30].
In our patient, we detected no abnormal FDG uptake in the nose, ears, or other cartilage-rich areas. Instead, we found that metabolic activities in the trachea and left and right main bronchi were slightly to moderately enhanced, and the cartilage of the tracheal wall was evenly thickened. Tracheobronchial amyloidosis was hence ruled out, because it typically involves inhomogeneous nodular thickening. Tracheal cartilage biopsy is sometimes indicated in patients with RP to visualize lesions and structural anomalies undetected by CT; however, invasive bronchoscopy may aggravate mucosal swelling or cartilage inflammation, triggering airway spasm and even severe or fatal respiratory distress[6]. Considering the patient’s radiographic findings and the marked deterioration in lung function occurring over the preceding 18 mo, a diagnostic biopsy was ruled out. Aided by improved diagnosis and treatment, the prognosis of RP patients has steadily improved, with 10-year survival rates of 55% reported in the 1980’s and 8-year and 10-year survival rates of 94% and 91% reported in 1998 and 2016, respectively[6].
Although patients with severe tracheobronchomalacia can be successfully treated with bronchoscopy-guided intervention therapy[31], we have doubts about the efficacy of the treatment in those patients who have, like ours, pathological laryngopharyngeal reflux. Our patient is currently stable, reports a high quality of life, and is under long-term follow-up to monitor treatment efficacy.
In conclusion, we present a case-report on a patient with RP with airway involvement as the only clinical manifestation and provide an account of the difficulties encountered in establishing the correct diagnosis. We learned from this case that PET/CT and bronchoscopy can help confirm a diagnosis of RP when common disease signs and symptoms are not obvious or absent. Currently, there are no standardized guidelines for RP diagnosis, which is mostly based on clinical manifestations and symptom-driven diagnostic testing[32]. Because RP patients with airway involvement may have higher infection risk and more commonly require admission to an ICU, it is pivotal to recognize and manage the airway morbidities in a timely manner to prevent fatal consequences.
We are thankful to the patient for her kind permission to report the clinical presentations and the laboratory and radiographic data related to her illness.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Respiratory System
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Handa H, Japan S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Borgia F, Giuffrida R, Guarneri F, Cannavò SP. Relapsing Polychondritis: An Updated Review. Biomedicines. 2018;6. [PubMed] [DOI] [Full Text] |
| 2. | Hazra N, Dregan A, Charlton J, Gulliford MC, D'Cruz DP. Incidence and mortality of relapsing polychondritis in the UK: a population-based cohort study. Rheumatology (Oxford). 2015;54:2181-2187. [PubMed] [DOI] [Full Text] |
| 3. | de Montmollin N, Dusser D, Lorut C, Dion J, Costedoat-Chalumeau N, Mouthon L, Chassagnon G, Revel MP, Puéchal X. Tracheobronchial involvement of relapsing polychondritis. Autoimmun Rev. 2019;18:102353. [PubMed] [DOI] [Full Text] |
| 4. | Sharma A, Gnanapandithan K, Sharma K, Sharma S. Relapsing polychondritis: a review. Clin Rheumatol. 2013;32:1575-1583. [PubMed] [DOI] [Full Text] |
| 5. | Behar JV, Choi YW, Hartman TA, Allen NB, McAdams HP. Relapsing polychondritis affecting the lower respiratory tract. AJR Am J Roentgenol. 2002;178:173-177. [PubMed] [DOI] [Full Text] |
| 6. | Puéchal X, Terrier B, Mouthon L, Costedoat-Chalumeau N, Guillevin L, Le Jeunne C. Relapsing polychondritis. Joint Bone Spine. 2014;81:118-124. [PubMed] [DOI] [Full Text] |
| 7. | Ernst A, Rafeq S, Boiselle P, Sung A, Reddy C, Michaud G, Majid A, Herth FJF, Trentham D. Relapsing polychondritis and airway involvement. Chest. 2009;135:1024-1030. [PubMed] [DOI] [Full Text] |
| 8. | McAdam LP, O'Hanlan MA, Bluestone R, Pearson CM. Relapsing polychondritis: prospective study of 23 patients and a review of the literature. Medicine (Baltimore). 1976;55:193-215. [PubMed] |
| 9. | Damiani JM, Levine HL. Relapsing polychondritis--report of ten cases. Laryngoscope. 1979;89:929-946. [PubMed] |
| 10. | Michet CJ Jr, McKenna CH, Luthra HS, O'Fallon WM. Relapsing polychondritis. Survival and predictive role of early disease manifestations. Ann Intern Med. 1986;104:74-78. [PubMed] [DOI] [Full Text] |
| 11. | Dion J, Costedoat-Chalumeau N, Sène D, Cohen-Bittan J, Leroux G, Dion C, Francès C, Piette JC. Relapsing Polychondritis Can Be Characterized by Three Different Clinical Phenotypes: Analysis of a Recent Series of 142 Patients. Arthritis Rheumatol. 2016;68:2992-3001. [PubMed] [DOI] [Full Text] |
| 12. | Conti JA, Colicchio AR, Howard LM. Thymoma, myasthenia gravis, and relapsing polychondritis. Ann Intern Med. 1988;109:163-164. [PubMed] [DOI] [Full Text] |
| 13. | VanDecker W, Panidis IP. Relapsing polychondritis and cardiac valvular involvement. Ann Intern Med. 1988;109:340-341. [PubMed] [DOI] [Full Text] |
| 14. | Hemry DA, Moss AJ, Jacox RF. Relapsing polychondritis, a "floppy" mitral valve, and migratory polytendonitis. Ann Intern Med. 1972;77:576-580. [PubMed] [DOI] [Full Text] |
| 15. | Kamboj AK, Cotter TG, Varghese C. Relapsing Polychondritis with Myelodysplastic Syndrome: A Case Report. Am J Med. 2017;130:e107-e108. [PubMed] [DOI] [Full Text] |
| 16. | Arima Y, Namiki T, Ueno M, Kato K, Tokoro S, Takayama K, Miura K, Yokozeki H. Histiocytoid Sweet syndrome: a novel association with relapsing polychondritis. Br J Dermatol. 2016;174:691-694. [PubMed] [DOI] [Full Text] |
| 17. | Maimon N, Marras T, Hwang D, Paul N, Keshavjee S, Chan CK. A 46-year-old female with dyspnoea, stridor and chronic cough. Eur Respir J. 2006;28:666-669. [PubMed] [DOI] [Full Text] |
| 18. | Braman SS. Diffuse tracheal narrowing with recurrent bronchopulmonary infections. Relapsing polychondritis. Chest. 2003;123:289, 290. [PubMed] [DOI] [Full Text] |
| 19. | Stiller RH, Gadzhiev M, Schachtel AK, Chang OH, Bastawrous S, Hermes Shantz H, Matute-Bello G, Albert TJ. A 68-Year-Old Man With Skin Rash and a Pleural Effusion. Chest. 2020;158:e33-e36. [PubMed] [DOI] [Full Text] |
| 20. | Ji Y, Yu P, Zhao C. A 49-Year-Old Man Presents With Fever of Unknown Origin and Cough. Chest. 2021;159:e25-e28. [PubMed] [DOI] [Full Text] |
| 21. | Ferrada M, Rimland CA, Quinn K, Sikora K, Kim J, Allen C, Sirajuddin A, Goodspeed W, Chen M, Grayson PC. Defining Clinical Subgroups in Relapsing Polychondritis: A Prospective Observational Cohort Study. Arthritis Rheumatol. 2020;72:1396-1402. [PubMed] [DOI] [Full Text] |
| 22. | Cantarini L, Vitale A, Brizi MG, Caso F, Frediani B, Punzi L, Galeazzi M, Rigante D. Diagnosis and classification of relapsing polychondritis. J Autoimmun. 2014;48-49:53-59. [PubMed] [DOI] [Full Text] |
| 23. | Arnaud L, Mathian A, Haroche J, Gorochov G, Amoura Z. Pathogenesis of relapsing polychondritis: a 2013 update. Autoimmun Rev. 2014;13:90-95. [PubMed] [DOI] [Full Text] |
| 24. | Yamashita H, Kubota K, Mimori A. Clinical value of whole-body PET/CT in patients with active rheumatic diseases. Arthritis Res Ther. 2014;16:423. [PubMed] [DOI] [Full Text] |
| 25. | Mathian A, Miyara M, Cohen-Aubart F, Haroche J, Hie M, Pha M, Grenier P, Amoura Z. Relapsing polychondritis: A 2016 update on clinical features, diagnostic tools, treatment and biological drug use. Best Pract Res Clin Rheumatol. 2016;30:316-333. [PubMed] [DOI] [Full Text] |
| 26. | Kraus VB, Stabler T, Le ET, Saltarelli M, Allen NB. Urinary type II collagen neoepitope as an outcome measure for relapsing polychondritis. Arthritis Rheum. 2003;48:2942-2948. [PubMed] [DOI] [Full Text] |
| 27. | Lee KS, Ernst A, Trentham DE, Lunn W, Feller-Kopman DJ, Boiselle PM. Relapsing polychondritis: prevalence of expiratory CT airway abnormalities. Radiology. 2006;240:565-573. [PubMed] [DOI] [Full Text] |
| 28. | Luckey P, Kemper J, Niehues T, Schroten H, Fürst G. Diagnostic role of inspiration and expiration CT in a child with relapsing polychondritis. AJR Am J Roentgenol. 2001;176:61-62. [PubMed] [DOI] [Full Text] |
| 29. | Yamashita H, Takahashi H, Kubota K, Ueda Y, Ozaki T, Yorifuji H, Bannai E, Minamimoto R, Morooka M, Miyata Y, Okasaki M, Takahashi Y, Kaneko H, Kano T, Mimori A. Utility of fluorodeoxyglucose positron emission tomography/computed tomography for early diagnosis and evaluation of disease activity of relapsing polychondritis: a case series and literature review. Rheumatology (Oxford). 2014;53:1482-1490. [PubMed] [DOI] [Full Text] |
| 30. | Baudart P, Aouba A, Beaufrère M, Aide N. FDG PET-CT as a powerful tool for diagnosing and monitoring treatment outcomes of relapsing polychondritis. Eur J Nucl Med Mol Imaging. 2018;45:669-670. [PubMed] [DOI] [Full Text] |
| 31. | Zhou P, Fu B, Zhang C, Chen K, Xia Q, Tang W, Yu W, Huang W. Bronchoscopy-Guided Intervention Therapy With Extracorporeal Membrane Oxygenation Support for Relapsing Polychondritis With Severe Tracheobronchomalacia: A Case Report and Literature Review. Front Med (Lausanne). 2021;8:695505. [PubMed] [DOI] [Full Text] |
| 32. | Rednic S, Damian L, Talarico R, Scirè CA, Tobias A, Costedoat-Chalumeau N, Launay D, Mathian A, Mattews L, Ponte C, Toniati P, Bombardieri S, Frank C, Schneider M, Smith V, Cutolo M, Mosca M, Arnaud L. Relapsing polychondritis: state of the art on clinical practice guidelines. RMD Open. 2018;4:e000788. [PubMed] [DOI] [Full Text] |