Published online Jul 6, 2022. doi: 10.12998/wjcc.v10.i19.6555
Peer-review started: October 31, 2021
First decision: March 7, 2022
Revised: April 10, 2022
Accepted: May 12, 2022
Article in press: May 12, 2022
Published online: July 6, 2022
Processing time: 236 Days and 5.1 Hours
Diffuse large B-cell lymphoma (DLBCL) is curable with first-line chemoimmunotherapy but patients with relapsed/refractory (R/R) DLBCL still face a poor prognosis. For patients with R/R DLBCL, the complete response rate to traditional next-line therapy is only 7% and the median overall survival is 6.3 mo. Recently, CD19-targeting chimeric antigen receptor T cells (CAR-T) have shown promise in clinical trials. However, approximately 50% of patients treated with CAR-T cells ultimately progress and few salvage therapies are effective.
Here, we report on 7 patients with R/R DLBCL whose disease progressed after CAR-T infusion. They received a PD-1 inhibitor (sintilimab) and a histone deacetylase inhibitor (chidamide). Five of the 7 patients tolerated the treatment without any serious adverse events. Two patients discontinued the treatment due to lung infection and rash. At the 20-mo follow-up, the median overall survival of these 7 patients was 6 mo. Of note, there were 2 complete response rates (CRs) and 2 partial response rates (PRs) during this novel therapy, with an overall response rate (ORR) of 57.1%, and one patient had a durable CR that lasted at least 20 mo.
In conclusion, chidamide combined with sintilimab may be a choice for DLBCL patients progressing after CD19-targeting CAR-T therapy.
Core Tip: We used histone deacetylase inhibitor chidamide combined with PD-1 blockade sintilimab for 7 patients with diffuse large B-cell lymphoma progressing after CART therapy. There are 2 complete response rates (CRs) and 2 PRs during this novel therapy, with an overall response rates of 57.1%, and one patient had a durable CR that lasted at least 20 mo.
- Citation: Hao YY, Chen PP, Yuan XG, Zhao AQ, Liang Y, Liu H, Qian WB. Chidamide and sintilimab combination in diffuse large B-cell lymphoma progressing after chimeric antigen receptor T therapy. World J Clin Cases 2022; 10(19): 6555-6562
- URL: https://www.wjgnet.com/2307-8960/full/v10/i19/6555.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i19.6555
Diffuse large B-cell lymphoma (DLBCL), the most common subtype of non-Hodgkins lymphoma (NHL), is the seventh most common cancer worldwide[1]. Most patients are cured with the combination of rituximab and traditional chemotherapy (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone [R-CHOP]). However, up to 50% of patients become refractory to treatment or relapse after treatment and these R/R DLBCL patients have a poor prognosis[2]. Recently, CD19-targeted chimeric antigen receptor T cells (CAR-T) have shown significant efficacy in patients with R/R DLBCL or another aggressive B-cell lymphoma[3,4]. In the ZUMA-1 study, patients who received CAR-T (Axi-cel) cell therapy showed overall response rates (ORRs) and complete response rates (CRs) of 83% and 58%, respectively, with a median follow-up of 27.1 mo[5]. However, durable complete responses (CRs) are approximately 30% to 40%, indicating that the majority of patients treated with CAR-T cells ultimately progress[6,7]. Unfortunately, there are few appropriate treatment options for patients who develop progressive disease (PD) after CAR-T cell treatment and their median overall survival is only approximately 5 mo[6].
Immune checkpoint inhibitors, especially PD-1 inhibitors, have shown encouraging clinical efficacy in R/R B-cell lymphomas[8]. Recently, Chong et al[9] reported that a patient with R/R DLBCL who developed PD after treatment with CD19-specific CAR-T cells had a significant response following PD-1 blockade therapy. In addition, some recent studies have suggested that patients with large B-cell lymphoma who develop progressive disease after treatment with CD19-specific CAR-T cells may benefit from checkpoint-based therapy[6,7]. Of note, PD-1 inhibitor monotherapy is associated with a low overall response rate among patients with R/R DLBCL which may limit its widespread use in clinical experience[10].
Chidamide is a novel oral selective histone deacetylase inhibitor (HDACi) approved in China for the treatment of relapsed or refractory peripheral T-cell lymphoma (PTCL). Previous studies have shown that HDAC increases tumor antigen presentation, reduces immunosuppressive cell types and augments checkpoint inhibitor therapy[10]. Additionally, chidamide induces growth arrest and apoptosis of lymphogenic tumor cells and enhances antitumor immunity by activating NK cells and CD8+ cytotoxic T cells[12,13]. Furthermore, a recent study showed that chidamide may augment the efficacy of PD-1 inhibitors in soft tissue sarcoma[14]. Therefore, the combination of chidamide and PD-1 inhibitors might be an important option for PD patients post CAR-T cell therapy.
In the present study, we report on seven patients who developed PD after CD19-targeted CAR-T therapy. They received treatment with chidamide (Chipscreen Bioscience Ltd, Shenzhen, China) and sintilimab (Innovent Biologics, Suzhou, China), which had not been reported previously.
In this study, we performed a retrospective analysis of an R/R B-cell lymphoma cohort of 7 DLBCL patients with disease progression after CD19-targeted CAR-T therapy. This study was approved by the Medical Ethics Committee of the First Affiliated Hospital of Zhejiang University School of Medicine. The median age of the 7 patients was 55 years (range 46-68) and 4 of the 7 patients were female.
All patients were diagnosed with DLBCL (nonGCB), including one patient who had follicular lymphoma (grade 3B) that transformed to DLBCL.
One patient (14.3%) relapsed after autologous hematopoietic cell transplantation before CAR-T therapy. All patients received lymphodepletion chemotherapy consisting of cyclophosphamide (500 mg/m2) and fludarabine (30 mg/m2) for 3 d before CAR-T infusion. The indications for CAR-T cell therapy were as follows: no response to CAR-T cells (n = 3, 42.9%), relapse or progression after initial PR (n = 2, 28.6%), and relapse or progression after initial CR (n = 2, 28.6%). The median time from CAR-T to treatment with chidamide and sintilimab therapy was 4 mo. Four patients were positive for PD-L1 expression on tumor cells (range, 3-10%), and three were negative before chidamide and sintilimab treatment. Three patients (42.9%) received bridging therapy before chidamide and sintilimab treatment. These bridging chemotherapies included GDP (gemcitabine, dexamethasone, cisplatin) and ICE (ifosfamide, carboplatin, and etoposide).
No specific personal and family history.
The Eastern Cooperative Oncology Group (ECOG) score of the 7 patients is showed in Table 1.
| Characteristic | Patient | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Age (yr) | 68 | 69 | 60 | 55 | 55 | 46 | 48 |
| Sex | Male | Male | Male | Female | Female | Female | Female |
| ECOG PS | 1 | 0 | 0 | 1 | 2 | 1 | 0 |
| Diagnosis/subtype | DLBCL (nonGCB) | DLBCL (nonGCB) | DLBCL (nonGCB) | DLBCL (nonGCB) | FL transformed to DLBCL (nonGCB) | DLBCL (nonGCB) | DLBCL (nonGCB) |
| Disease stage | IVA | IIA | IVB | IVB | IVB | IIIA | IIA |
| IPI | 4 | 4 | 4 | 4 | 2 | 4 | 2 |
| PD-L1 (%) | 10 | 3 | 0 | 0 | 5 | 0 | 10 |
| Pre-CAR-T therapies | (1) R2-CHOP; and (2) RCHOP | (1) RCHOP; and (2) RECOP | (1) RCHOP; (2) RGDP; and (3) Ibrutinib | (1) RCHOP | (1) RCHOP; (2) R-DA-EPOCH; (3) R-GeMox; (4) R-ABVD; (5) R2-MTX-CTX; and (6) Ibrutinib | (1) RCHOP; (2) R-DA-EPOCH; and (3) R-Hyper-CVAD | (1) RCHOP; (2) DA-EPOCH; (3) Hyper-CVAD; and (4) AutoHSCT |
| Response to CART | Relapse after CR post CART | Relapse after PR post CART | No response after CART | Relapse after PR post CART | No response after CART | No response after CART | Relapse after CR post CART |
| Time from CAR-T to chidamide and sintilimab therapy (mo) | 9 | 4 | 3 | 6 | 3 | 4 | 15 |
| Bridging treatment | NO | GDP | ICE | ICE | NO | NO | NO |
| Response to chidamide and sintilimab therapy | PR | PD | PD | PR | CR | PD | CR |
No additional laboratory examinations were performed on the 7 patients.
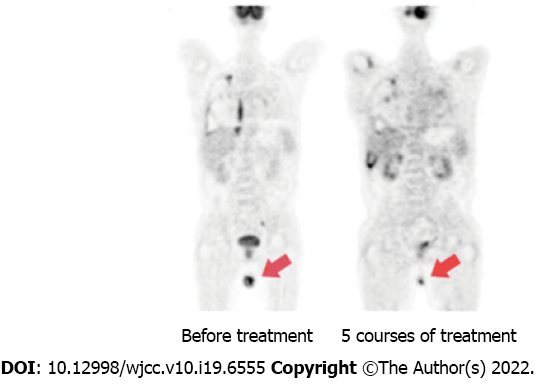
After 5 courses of treatment, PET-CT showed continued partial response (PR) with only a little hypermetabolism in the testes.
7 DLBCL patients with disease progression after CD19-targeted CAR-T therapy.
This novel therapy comprises chidamide (30 mg orally twice per week) and sintilimab (200 mg every 3 wk). Adverse events (AEs) of chidamide and sintilimab mainly included thrombocytopenia, leucopenia, neutropenia and fatigue[13]. All patients experienced treatment-related AEs of any grade during the treatment and five of the patients tolerated the AE’s. Two of the patients developed severe treatment-related AEs including lung infection and rash. Therefore, they discontinued treatment despite having a better therapeutic effect. Nevertheless, one of these two patients remained in CR 1 year after stopping treatment, and the other patient developed PD 8 mo after stopping treatment. No patient died from the treatment-related AEs. Five of the seven patients died due to disease progression. Based on the safety profile of these seven patients, the regimen was deemed a safe study protocol.
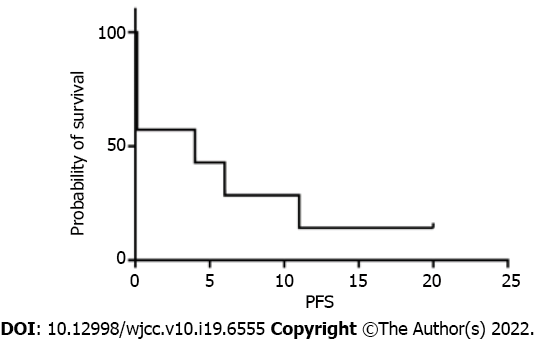
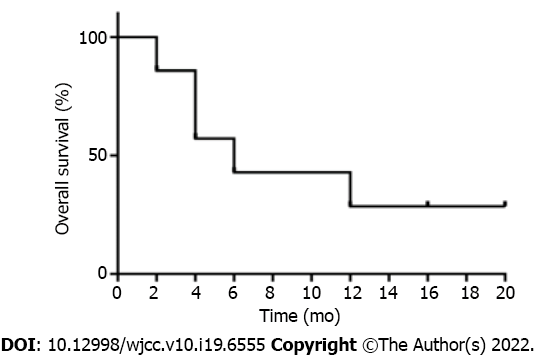
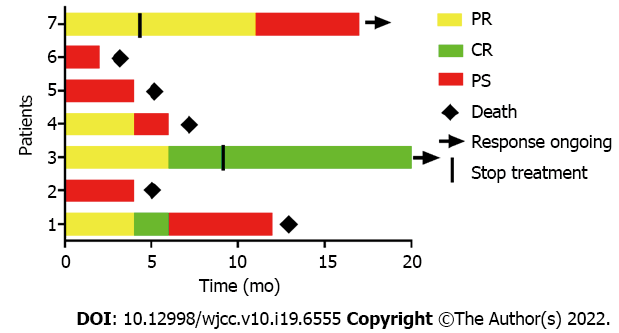
Four of the seven patients (57.1%) achieved objective response within 2 courses of chidamide and sintilimab therapy, and two of them achieved CR within 4 and 5 courses, respectively. The median progression-free survival (PFS) and OS were 4 and 6 mo, respectively (Figures 1 and 2). Among all patients (Figure 3), 2 patients had persistent treatment-related AEs during the treatment period at the last follow-up. One patient was a 60-year-old male with R/R DLBCL (nonGCB, IV B, IPI 4). He underwent fludarabine-cyclophosphamide lymphodepletion followed by infusion of CD19-targeted CAR-T cells after relapsing on 3 prior treatments. He tolerated the treatment without CRS and ICANS. However, on day +90, PET-CT showed his PD. He received ICE treatment but could not tolerate it. Then, we treated him with chidamide and sintilimab. After 2 courses of treatment, he had PR. After 5 courses of treatment, PET-CT showed continued PR with only a little hypermetabolism in the testes (Figure 4). After 6 mo, he probably reached CR due to the absence of enlarged lymph nodes on ultrasound. However, due to a serious lung infection, he stopped treatment after 8 mo, and he received no additional treatment for his disease. To our surprise, 1 year after he stopped the treatment, he was still alive and felt well with no PD. The other patient was a 48-year-old female with R/R DLBCL (nonGCB, IIA, IPI 2) for 2.5 years. She had received 4 prior chemotherapies, including autoHSCT, before CD19 CAR-T. Biopsy of the breast mass confirmed DLBCL with CD19 and PD-L1 (10%) expression. She underwent standard lymphodepletion and CD19 CAR-T. She did not develop any CRS or ICANS. PET-CT revealed PR on day +30 and CR on day +90. However, after 15 mo, CT revealed PD. Then, she received chidamide and sintilimab therapy. After 4 courses, she had a PR on ultrasound. However, due to serious mouth ulcers and rash, she stopped the treatment after 4 mo. Since then, she did not undergo further treatment. Unfortunately, 8 mo later, she had PD on PET-CT.
To date, four CD19-CAR-T products have been approved by the US Food and Drug Administration (FDA) as treatments for R/R B-cell lymphomas, including 4-1BB-based axicabtagene ciloleucel, tisagenlecleucel, lisocabtagene maraleucel (liso-cel), and CD28-based KTE-X19[3,5,15,16]. Nastoupil et al[17] reported at a median follow-up of 12.9 mo that axi-cel in 275 R/R LBCL patients treated with standard therapy exhibited ORRs and CRs of 82% and 64%, respectively. Recent data show that the 5-year ORR of patients with R/R B-cell lymphoma treated with CD19-targeted CAR-T cells (tisagenlecleucel) was 58%, and 46% had CR[18]. Despite the efficacy of CAR-T therapy in patients with R/R DLBCL, approximately 50% of patients still experience PD after CAR-T therapy[7]. For these patients, the median OS was only 5.3 mo and treatment options remain limited[6].
Three reasons for nonresponse or relapse in patients receiving CD19-targeted CAR-T infusions are (1) loss or downregulation of CD19 expression; (2) poor T-cell function; and (3) immune-suppressed tumor microenvironment. In the ZUMA-1 study, 4 of 16 patients (25%) relapsed post-CAR-T (axi-cel) due to loss of CD19 expression[19]. In a large cohort of 136 patients who had PD after CAR-T infusion, CD19 loss accounted for approximately 30% of progression cases[7]. Poor T-cell function is another reason explaining the failure of CAR-T therapies. A study found that many patients had insufficient CAR-T expansion capacity, suggesting that intrinsic causes of T cells contribute to treatment failure[20]. Recently, many studies have found that the tumor microenvironment (TME) plays an important role in cancer progression and therapy resistance. In the TME, tumor-derived extracellular vesicles (TEXs) act as communication vehicles to transfer information between cancer cells and other cells and their unique functions have gained increasing attention such as, promoting tumorigenesis and metastasis, modulating antitumor immunity and neutralizing drugs to compromise therapeutic effects[21,22]. Immunosuppressive molecules carried by TEXs can inhibit T cells and CAR-T cells.
Salvage therapies post axi-cel include checkpoint inhibitors, lenalidomide, chemotherapy, radiation, venetoclax, ibrutinib or a second CAR-T. Among these salvage therapies, checkpoint inhibitor-based therapy appears to be the most effective treatment, with an ORR of 46% and CR of 18%[7]. Of note, an alternative CD19-targeted CAR-T therapy for disease progression after the first CD19-CAR-T therapy may have a limited response. Chow et al[6] reported 3 patients with R/R DLBCL whose disease progressed after CD19-CART therapy who were treated with an alternative CD19-CAR-T product for salvage treatment. The dates of PD in these three patients were +160, +21 and +6 d after the second CD19 CAR-T infusion, respectively. This result shows that no meaningful responses were observed with salvage therapy using alternative CD19-CAR-T products.
In our study, there were 2 CRs and 2 PRs during this novel therapy, with an ORR of 57.1% and CR of 28.6%. One patient had a durable CR that lasted at least 20 mo, which may be slightly better than other therapies. Anti-PD-1/PD-L1 immunotherapy has emerged as a new treatment option for R/R lymphoma, especially in combination with other drugs. Of note, in our study, PD-L1 expression was 10% and 0% in the 2 patients who achieved CR, indicating that higher tumor PD-L1 expression might not be necessary for effective chidamide plus sintilimab combination therapy. Chidamide, a subtype-selective HDACi, not only directly exerts anticancer activity but also exerts multiple immunomodulatory effects. On the one hand, it enhances the antitumor ability of immune cells by enhancing the intratumoral infiltration of CD8+ T cells and macrophages, upregulating costimulatory molecules, promoting tumor-specific T cell-mediated cancer cell killing and sensitizing tumor cells to NK cell lysis. On the other hand, it inhibits intratumoral infiltration of myeloid-derived suppressor cells (MDSCs), primary M2 macrophages and T-regulatory cells[23-25]. HDACis may also enhance checkpoint inhibitor therapy. Yan et al[26] reported that a patient with NK/T-cell lymphoma resistant to pegaspargase and immunotherapy had a durable response to sintilimab and chidamide. Currently, a clinical trial is evaluating the feasibility of combination therapy with HDACis and PD1 inhibitors[27].
One limitation of our study is the small number of patients in our single-center experience. Another limitation is that the choice of therapy may be influenced by multiple factors such as physician preference, cost, the proximity of treatment centers and even coronavirus disease 2019[28], which may influence therapeutic effects and disease assessment. Future studies should incorporate multicenter cohorts and randomized clinical studies.
In summary, the outcome after PD of CD19-targeted CAR-T therapy is poor. Despite the limited number of meaningful responses observed, chidamide and sintilimab may be salvage treatment options. To our knowledge, this is the first report on the outcome of chidamide and sintilimab treatment in patients with PD following CD19-specific CAR-T therapy. These data may inform novel interventions for the treatment of this group of patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Hematology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chudek JT, Poland; Moschovi MA, Greece; Shomura M, Japan S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Wu YXJ
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13300] [Cited by in RCA: 15474] [Article Influence: 2579.0] [Reference Citation Analysis (2)] |
| 2. | Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800-1808. Blood. 2018;131:587-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, Jäger U, Jaglowski S, Andreadis C, Westin JR, Fleury I, Bachanova V, Foley SR, Ho PJ, Mielke S, Magenau JM, Holte H, Pantano S, Pacaud LB, Awasthi R, Chu J, Anak Ö, Salles G, Maziarz RT; JULIET Investigators. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med. 2019;380:45-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1919] [Cited by in RCA: 2855] [Article Influence: 475.8] [Reference Citation Analysis (0)] |
| 4. | Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak Ö, Brogdon JL, Pruteanu-Malinici I, Bhoj V, Landsburg D, Wasik M, Levine BL, Lacey SF, Melenhorst JJ, Porter DL, June CH. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N Engl J Med. 2017;377:2545-2554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1462] [Article Influence: 182.8] [Reference Citation Analysis (0)] |
| 5. | Locke, F. L., et al, Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20:31-42. [DOI] [Full Text] |
| 6. | Chow VA. Outcomes of patients with large B-cell lymphomas and progressive disease following CD19-specific CAR T-cell therapy. Am J Hematol. 2019;94:E209-E213. [RCA] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 7. | Spiegel JY. Outcomes of patients with large B-cell lymphoma progressing after axicabtagene ciloleucel therapy. Blood. 2021;137:1832-1835. [DOI] [Full Text] |
| 8. | Lesokhin AM. Nivolumab in Patients With Relapsed or Refractory Hematologic Malignancy: Preliminary Results of a Phase Ib Study. J Clin Oncol. 2016;34:2698-2704. [DOI] [Full Text] |
| 9. | Chong EA, Melenhorst JJ, Lacey SF, Ambrose DE, Gonzalez V, Levine BL, June CH, Schuster SJ. PD-1 blockade modulates chimeric antigen receptor (CAR)-modified T cells: refueling the CAR. Blood. 2017;129:1039-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 396] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 10. | Ansell SM, Minnema MC, Johnson P, Timmerman JM, Armand P, Shipp MA, Rodig SJ, Ligon AH, Roemer MGM, Reddy N, Cohen JB, Assouline S, Poon M, Sharma M, Kato K, Samakoglu S, Sumbul A, Grigg A. Nivolumab for Relapsed/Refractory Diffuse Large B-Cell Lymphoma in Patients Ineligible for or Having Failed Autologous Transplantation: A Single-Arm, Phase II Study. J Clin Oncol. 2019;37:481-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 275] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 11. | Briere D, Sudhakar N, Woods DM, Hallin J, Engstrom LD, Aranda R, Chiang H, Sodré AL, Olson P, Weber JS, Christensen JG. The class I/IV HDAC inhibitor mocetinostat increases tumor antigen presentation, decreases immune suppressive cell types and augments checkpoint inhibitor therapy. Cancer Immunol Immunother. 2018;67:381-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 12. | Ning ZQ, Li ZB, Newman MJ, Shan S, Wang XH, Pan DS, Zhang J, Dong M, Du X, Lu XP. Chidamide (CS055/HBI-8000): a new histone deacetylase inhibitor of the benzamide class with antitumor activity and the ability to enhance immune cell-mediated tumor cell cytotoxicity. Cancer Chemother Pharmacol. 2012;69:901-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 217] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 13. | Shi Y. Results from a multicenter, open-label, pivotal phase II study of chidamide in relapsed or refractory peripheral T-cell lymphoma. Ann Oncol. 2015;26:1766-1771. [DOI] [Full Text] |
| 14. | Que Y, Zhang XL, Liu ZX, Zhao JJ, Pan QZ, Wen XZ, Xiao W, Xu BS, Hong DC, Guo TH, Shen LJ, Fan WJ, Chen HY, Weng DS, Xu HR, Zhou PH, Zhang YZ, Niu XH, Zhang X. Frequent amplification of HDAC genes and efficacy of HDAC inhibitor chidamide and PD-1 blockade combination in soft tissue sarcoma. J Immunother Cancer. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 15. | Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, Timmerman JM, Holmes H, Jaglowski S, Flinn IW, McSweeney PA, Miklos DB, Pagel JM, Kersten MJ, Milpied N, Fung H, Topp MS, Houot R, Beitinjaneh A, Peng W, Zheng L, Rossi JM, Jain RK, Rao AV, Reagan PM. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N Engl J Med. 2020;382:1331-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 1277] [Article Influence: 255.4] [Reference Citation Analysis (0)] |
| 16. | Abramson JS. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396:839-852. [RCA] [DOI] [Full Text] [Cited by in Crossref: 772] [Cited by in RCA: 1574] [Article Influence: 314.8] [Reference Citation Analysis (0)] |
| 17. | Nastoupil LJ, Jain MD, Feng L, Spiegel JY, Ghobadi A, Lin Y, Dahiya S, Lunning M, Lekakis L, Reagan P, Oluwole O, McGuirk J, Deol A, Sehgal AR, Goy A, Hill BT, Vu K, Andreadis C, Munoz J, Westin J, Chavez JC, Cashen A, Bennani NN, Rapoport AP, Vose JM, Miklos DB, Neelapu SS, Locke FL. Standard-of-Care Axicabtagene Ciloleucel for Relapsed or Refractory Large B-Cell Lymphoma: Results From the US Lymphoma CAR T Consortium. J Clin Oncol. 2020;38:3119-3128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 578] [Article Influence: 115.6] [Reference Citation Analysis (0)] |
| 18. | Chong EA, Ruella M, Schuster SJ; Lymphoma Program Investigators at the University of Pennsylvania. Five-Year Outcomes for Refractory B-Cell Lymphomas with CAR T-Cell Therapy. N Engl J Med. 2021;384:673-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 226] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 19. | Neelapu SS, Rossi JM, Jacobson CA, Locke FL, Miklos DB, Reagan PM, Rodig SJ, Lekakis LJ, Flinn LW, Zheng LQ, Milletti F, Chang E, Xue A, Plaks V, Kim JJ, Bot A. CD19-Loss with Preservation of Other B Cell Lineage Features in Patients with Large B Cell Lymphoma Who Relapsed Post-Axi-Cel. Blood. 2019;134:203-203. [RCA] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 20. | Oak J. Target Antigen Downregulation and Other Mechanisms of Failure after Axicabtagene Ciloleucel (CAR19) Therapy. Blood. 2018;132:4656-4656. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Ali S, Toews K, Schwiebert S, Klaus A, Winkler A, Grunewald L, Oevermann L, Deubzer HE, Tüns A, Jensen MC, Henssen AG, Eggert A, Schulte JH, Schwich E, Rebmann V, Schramm A, Künkele A. Tumor-Derived Extracellular Vesicles Impair CD171-Specific CD4+ CAR T Cell Efficacy. Front Immunol. 2020;11:531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Cox MJ, Lucien F, Sakemura R, Boysen JC, Kim Y, Horvei P, Manriquez Roman C, Hansen MJ, Tapper EE, Siegler EL, Forsman C, Crotts SB, Schick KJ, Hefazi M, Ruff MW, Can I, Adada M, Bezerra E, Kankeu Fonkoua LA, Nevala WK, Braggio E, Ding W, Parikh SA, Kay NE, Kenderian SS. Leukemic extracellular vesicles induce chimeric antigen receptor T cell dysfunction in chronic lymphocytic leukemia. Mol Ther. 2021;29:1529-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 23. | Knox T, Sahakian E, Banik D, Hadley M, Palmer E, Noonepalle S, Kim J, Powers J, Gracia-Hernandez M, Oliveira V, Cheng F, Chen J, Barinka C, Pinilla-Ibarz J, Lee NH, Kozikowski A, Villagra A. Author Correction: Selective HDAC6 inhibitors improve anti-PD-1 immune checkpoint blockade therapy by decreasing the anti-inflammatory phenotype of macrophages and down-regulation of immunosuppressive proteins in tumor cells. Sci Rep. 2019;9:14824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Wang X, Waschke BC, Woolaver RA, Chen SMY, Chen Z, Wang JH. HDAC inhibitors overcome immunotherapy resistance in B-cell lymphoma. Protein Cell. 2020;11:472-482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 25. | Kim YD, Park SM, Ha HC, Lee AR, Won H, Cha H, Cho S, Cho JM. HDAC Inhibitor, CG-745, Enhances the Anti-Cancer Effect of Anti-PD-1 Immune Checkpoint Inhibitor by Modulation of the Immune Microenvironment. J Cancer. 2020;11:4059-4072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 26. | Yan Z, Yao S, Liu Y, Zhang J, Li P, Wang H, Chu J, Zhao S, Yao Z. Durable Response to Sintilimab and Chidamide in a Patient With Pegaspargase- and Immunotherapy-Resistant NK/T-Cell Lymphoma: Case Report and Literature Review. Front Oncol. 2020;10:608304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Jespersen H, Olofsson Bagge R, Ullenhag G, Carneiro A, Helgadottir H, Ljuslinder I, Levin M, All-Eriksson C, Andersson B, Stierner U, Nilsson LM, Nilsson JA, Ny L. Concomitant use of pembrolizumab and entinostat in adult patients with metastatic uveal melanoma (PEMDAC study): protocol for a multicenter phase II open label study. BMC Cancer. 2019;19:415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 28. | Bao C, Tao X, Cui W, Hao Y, Zheng S, Yi B, Pan T, Young KH, Qian W. Natural killer cells associated with SARS-CoV-2 viral RNA shedding, antibody response and mortality in COVID-19 patients. Exp Hematol Oncol. 2021;10:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |