Published online Jul 6, 2022. doi: 10.12998/wjcc.v10.i19.6520
Peer-review started: September 2, 2021
First decision: December 9, 2021
Revised: January 19, 2022
Accepted: May 16, 2022
Article in press: May 16, 2022
Published online: July 6, 2022
Processing time: 294 Days and 20.4 Hours
Systemic lupus erythematosus (SLE), characterized by the production of autoantibodies and widespread deposition of immune complexes, predominantly affects women of childbearing age. More than one-third of SLE patients present ocular manifestations. Choroidal disease is currently not completely understood, and its precise differentiation from central serous chorioretinopathy is rarely achieved. To date, no more than 60 patients with choroidal involvement have been reported.
A 37-year-old Chinese woman experienced decreased visual acuity bilaterally, accompanied by increasing periorbital swelling and severe conjunctival chemosis. Decreased breath sounds in both bases were detected via auscultation, as well as pitting edema in both ankles. SLE and lupus nephritis were diagnosed based on serositis, renal disorder, leukopenia and positive anti-Smith and anti-nuclear antibodies. Lupus choroidopathy was diagnosed based on ocular presentation and imaging. The patient was treated with systemic corticosteroids, spironolactone, hydroxychloroquine (HCQ), mycophenolate mofetil (MMF), and intravenous immunoglobulin. After 4 wk of hospitalization, the patient was discharged. Indocyanine green angiography showed no leakage from choroidal vessels, and ocular coherence tomography detected low amounts of subretinal fluid right before discharge. The patient was prescribed oral methylprednisolone, HCQ, and MMF. Two months after the first visit, ophthalmological examination revealed a visual acuity of 20/20 bilaterally, and SLE disease activity was well controlled; her symptoms disappeared completely.
Here we presented a case of lupus choroidopathy, successfully treated with systemic corticosteroids, and discussed previously reported cases, focusing on differential diagnosis with a central serous chorioretinopathy.
Core Tip: A 37-year-old Chinese woman with decreased visual acuity bilaterally, was diagnosed with systemic lupus erythematosus (SLE), lupus nephritis and lupus choroidopathy. Patient was successfully treated with systemic corticosteroids, spironolactone, hydroxychloroquine (HCQ), mycophenolate mofetil (MMF) and intravenous immunoglobulin during hospitalization and oral methylprednisolone, HCQ and MMF after discharge. Two months after the first visit, ophthalmological examination revealed a visual acuity of 20/20 bilaterally, and controlled SLE disease activity. Based on the case report and following literature review, lupus choroidopathy was discussed in the context of recurrence of underlying vasculitis; serous chorioretinopathy (CSC) was excluded and condition treated with immunosuppressive agents; spironolactone is discussed to be safe and helpful in both lupus choroidopathy and CSC.
- Citation: Yao Y, Wang HX, Liu LW, Ding YL, Sheng JE, Deng XH, Liu B. Acute choroidal involvement in lupus nephritis: A case report and review of literature. World J Clin Cases 2022; 10(19): 6520-6528
- URL: https://www.wjgnet.com/2307-8960/full/v10/i19/6520.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i19.6520
Systemic lupus erythematosus (SLE) is an immune vasculitis characterized by the production of autoantibodies and widespread deposition of immune complexes[1], and predominantly affects women of childbearing age[2,3]. Its diagnosis requires four of the eleven diagnostic criteria proposed by the American College of Rheumatology[4].
More than one-third of SLE patients present ocular manifestations[5], which correlate to systemic disease activity. Active choroidal vasculitis can coexist with inflammation in other organs, especially in lupus nephropathy or neuropathy[6]. Choroidal disease is currently incompletely understood, and its precise differentiation from central serous chorioretinopathy (CSC) is hardly achieved.
To the best of our knowledge, no more than 60 patients with choroidal involvement have been reported so far worldwide. Herein, we describe a female patient with acute choroidal involvement during a lupus nephritis attack, and retrospectively analyzed the medical records of lupus choroidopathy cases previously reported in the English and Chinese languages from 1977 to July 2019.
The patient was referred to our hospital, because of nausea and oliguria.
About 20 d prior to visiting our hospital on May 7, 2018, a 37-year-old Chinese woman experienced decreased visual acuity bilaterally, accompanied by increasing periorbital swelling; she also gained 4.5 kg over the past 2 wk and complained of worsening anasarca.
She showed leukopenia during routine health checkup five years ago. She underwent no further examination or therapy.
There was no significant family history or ocular disease.
The patient was in poor general condition, including fatigue and malnutrition, heart rate 91 beats/min and breath 17 beats/min. Blood pressure in the right upper arm was 145/87 mmHg. The major systemic findings were periorbital swelling and conjunctival chemosis, decreased breath sounds in both bases detected via auscultation, and pitting edema in both ankles. Visual acuity was “count fingers” at 20 cm OD and “hand motion” OS. On ophthalmologic examination, visual acuity was 2/100 and 1/100 for the right and left eyes, respectively.
Complete blood count showed leucocytes at 1.69 × 109/L (normal range, 3.5 to 9.5 × 109/L), and normal platelet and hemoglobin levels. Laboratory data (May 8, 2018) revealed erythrocyte sedimentation rate at 60 mm/h (normal range, 0 to 20 mm/h), and low total protein and serum albumin levels at 61.7 and 30 g/dL, respectively. Blood urea nitrogen and creatinine levels, and serum potassium amounts were normal. Proteinuria was 0.81 g/24 h and D-dimer was 4.86 µg/mL (normal range, 0-0.5 µg/mL). Moreover, blood tests for anti-nuclear, anti-Smith, anti-SSA, anti-nRNP and anti-Ro52 antibodies were positive. The levels of complements were low (C3 at 19.5 mg/dL and C4 at 2.5 mg/dL). Small focal cerebral ischemia was detected in the frontal lobe bilaterally by magnetic resonance imaging (MRI). The patient declined lumbar puncture. Thoracentesis was performed, and the collected fluid was analyzed by the Rivalta reaction, which showed the presence of exudate.
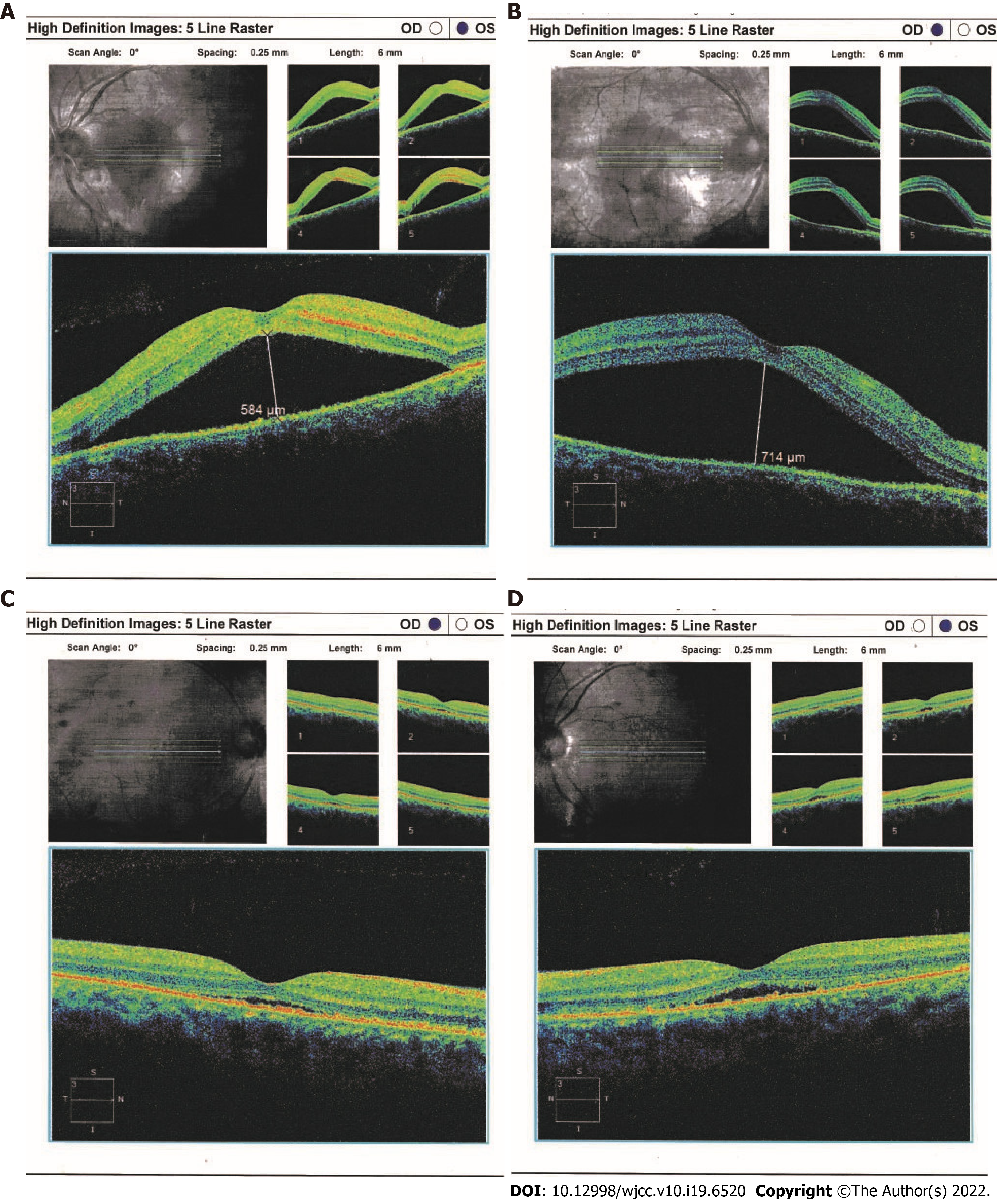
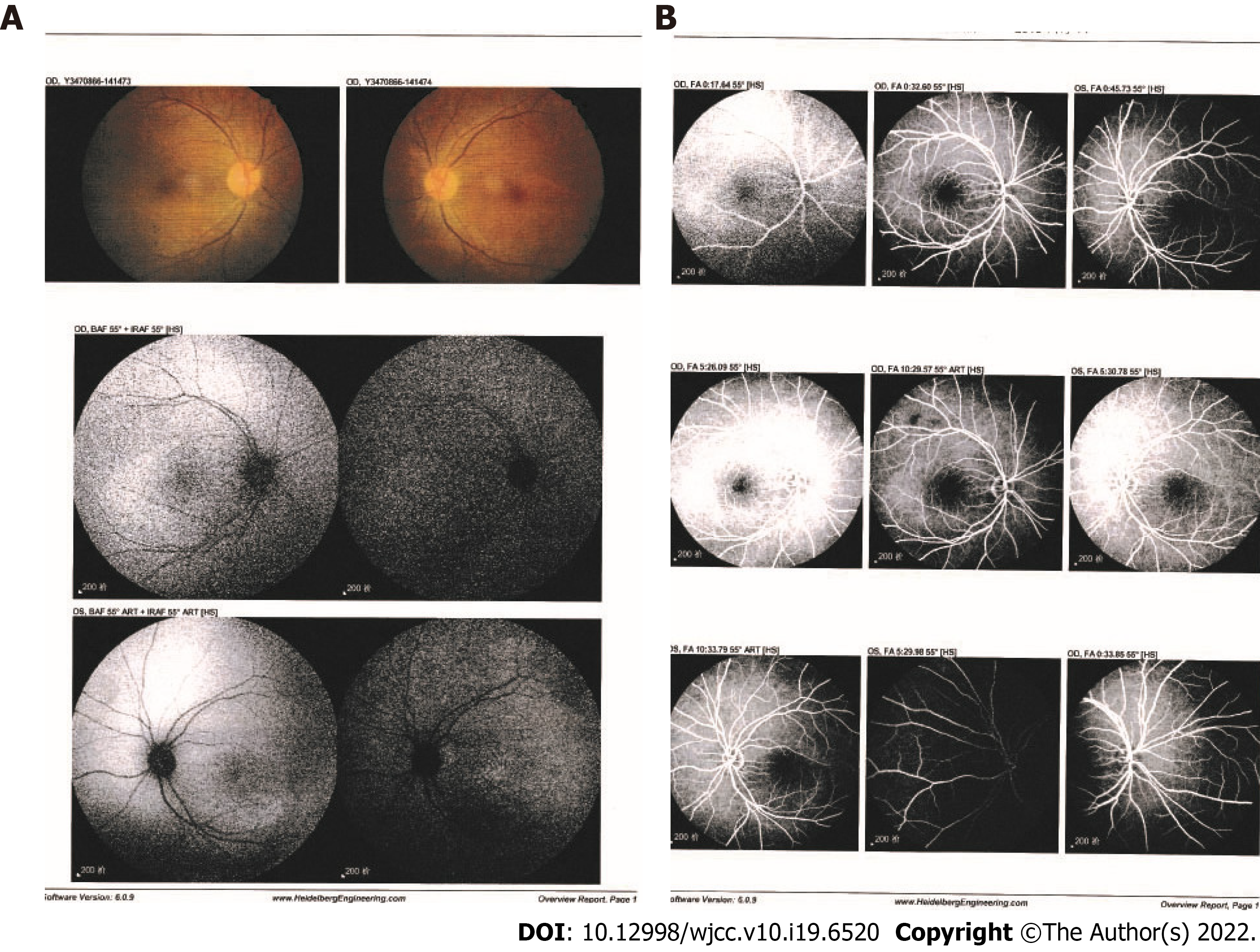
Slit lamp examination showed normal anterior segment, with mildly swollen optic disc. Ocular coherence tomography (OCT) and ophthalmoscopy revealed bilateral serous retinal detachment (Figure 1). Fundus fluorescein angiography (FFA) and indocyanine green angiography (ICGA) were postponed for another two weeks, since the nephrotoxicity of the contrast media used in both tests may aggravate the patient’s renal disease in this acute clinical phase.
We diagnosed the patient with SLE and lupus nephritis based on serositis, renal disorder, leukopenia and positive anti-Smith and anti-nuclear antibodies. Lupus choroidopathy was diagnosed based on ocular presentation and imaging. Cerebral vasculitis was excluded because of near-normal MRI data and the lack of neurological signs.
The patient was treated with methylprednisolone at 80 mg IV qd, hydroxychloroquine (HCQ) at 0.2 g po bid, and mycophenolate mofetil (MMF) at 1500 mg/d. Intravenous immunoglobulin at 400 mg/kg was administered for five days. Seven days after treatment, her symptoms improved gradually. Albumin and diuretics (spironolactone, 2-3 tablets per day) were administered to alleviate edema and nausea.
On June 4, 2018, after 4 wk of hospitalization, the patient was discharged. ICGA showed no leakage from choroidal vessels (Figure 2), and OCT detected little subretinal fluid right before discharge (Figure 1). The following treatment was prescribed: oral methylprednisolone at 40 mg/d followed by a progressive reduction, HCQ at 400 mg/d, and MMF at 1500 mg/d. Two months after the first visit, ophthalmological examination revealed a visual acuity of 20/20 bilaterally, and SLE disease activity was well controlled with related symptoms completely disappearing. Edema subsided and nausea improved. Ophthalmoscopy revealed complete flattening of the previous areas of serous elevations of the retina.
A comprehensive search of reports in English and Chinese from 1977 to July 2019 was conducted in the PubMed database, with lupus, choroid, choroidopathy, retina, and central serous chorioretinopathy as search terms. Cases with doubtful or non-unanimous diagnosis were excluded. We identified 37 relevant manuscripts, and found 56 radiographically-proven cases, including the new case described above. There were 5 men and 51 women. Such female preponderance is well-known[7]. The median age at onset was 35.7 years (range, 15–68), and the median duration of SLE before clinical choroidal vascular disease was 6.4 years (range 0–25 years). Four of the 56 (7%) patients presented with ophthalmological ailment as the initial manifestation before SLE diagnosis. Choroidopathy developed below the age of 20 years in 13% of these patients, between 20 and 45 years in 71%, and after 45 in 16%. Among the 56 patients, twelve were Asian (7 Chinese, and a Cambodian, a Filipino, a Japanese, a Korean, and a Malaysian), seven were African, two were European (Irish and Italian), and one each was Mexican American, unspecified Caucasian, and Mediterranean; 32 patients did not report their ethnic origins.
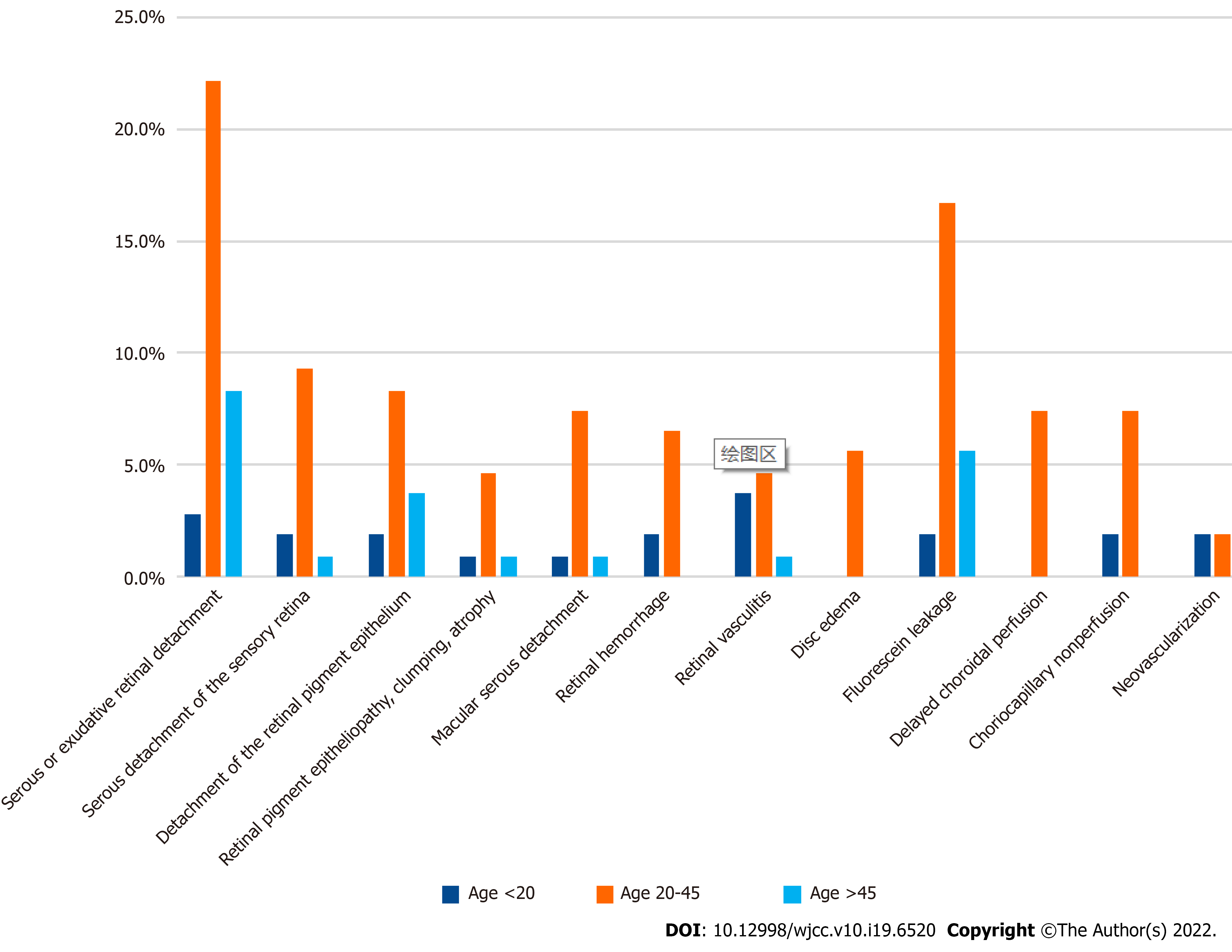
Bilateral involvement was observed in 39 of 56 the cases (69.6%). A total of 108 eyes were involved, and most (83/108, 76.9%) presented decreased visual acuity; 11/108 eyes presented metamorphopsia, 5/108 eyes had visual field loss, and only 2 eyes showed color desaturation. Ophthalmic examination revealed serous or exudative retinal detachment in 36 (64.3%) eyes, serous detachment of the sensory retina in 13 (23.2%), detachment of the retinal pigment epithelium in 15 (26.8%), retinal pigment epitheliopathy and macular serous detachment in 10 (17.9%), retinal vasculitis in 10 (17.8%), retinal hemorrhage in 9 (16.1%), clumping and atrophy in 7 (12.5%), and disc edema in 6 (10.7%). A total of 42 patients underwent FFA and/or ICGA, which showed 26 eyes with fluorescein leakage, 8 with delayed choroidal perfusion, 10 with a choriocapillaris area of nonperfusion, and 4 eyes with neovascularization. The frequencies of different ocular imaging presentations of choroidopathy were analyzed in 56 patients (Figure 3). Only two patients displayed no ophthalmological symptoms although their radiologic findings were consistent with choroid involvement, and the peak period for each symptom is between 20 and 45 years of age.
Lupus nephritis represented the most prominent comorbidity of lupus choroidopathy, occurring in approximately 78.6% of all patients during the course of the disease. Dermatitis, serositis, and arthritis were described in 46.4%, 37.5% and 35.7% of the overall population, respectively. Central nervous system (CNS) lupus affected 33.9% of patients with choroidopathy. Hypertension manifestations were described in 21 of the 56 cases; 18 (32.1%) cases had blood involvement and 5 (8.9%) displayed the Reynolds phenomenon. Systemic Lupus Erythematosus Disease Activity Index scores were 9.8±5.6 in cases. Antiphospholipid antibodies were found in 11 cases, absent in 5, and not assessed in 40.
More than 83% of treated patients with choroidopathy received oral or intravenous corticosteroids, and 35/56 patients were administered one or more immunosuppressive agents. Four patients received targeted agents, including rituximab, infliximab and bevacizumab. Anticoagulation therapy was applied to two patients. Four patients underwent ophthalmic laser treatment. Thirty-five of the 56 patients (62.5%) presented improvement or resolution of lupus choroiditis after systemic disease control. Two patients had no improvement. Three patients showed deterioration of the choroidopathy, likely because of macula or optic nerve involvement[5,8]. Choroidopathy is a less frequent complication of lupus ocular compared with retinopathy; therefore, reports describing SLE patients with choroidal vascular disease are scarce.
Choroidopathy is characterized by unilateral or bilateral blurred vision. Visual prognosis of choroidal involvement depends on the pattern of choroidopathy. For example, vaso-occlusion usually causes poor vision. Bilateral involvement was observed in 69.6% of the assessed cases, corroborating Nguyen et al[7]. Lupus choroidopathy is known to be associated with nephropathy[9], which affected 79% of these patients. Involvement of both the choroid and kidney may be due to their similar structures and pathogeneses[9]. Previous studies have shown that lupus choroidopathy is associated with CNS vasculitis; however, the present data demonstrated that CNS involvement is not more frequent in individuals with choroidopathy compared with other lupus patients. Choroidopathy, as a sign of subclinical, reversible nephropathy or neuropathy, is usually a marker of disease activity and can present as an initial symptom of SLE[10]. Eighty-two percent of patients have complete remission of choroidopathy when the systemic diseases of lupus are controlled[11].
The precise mechanisms of lupus choroidopathy remain debatable, but it is thought to involve the some of the following factors. Firstly, histopathological studies demonstrated the immune complex deposition in the choroid and the presence of autoantibodies against retinal pigment epithelium (RPE)[12]. The inflammatory cells along with the deposition of immunoglobulins and complement in the choroidal vessels might lead to choroidal hyperpermeability, breaking down the blood retinal barrier[8]. Matsuo and colleagues[13] hypothesized that anti-RPE antibodies were involved in the cause of RPE dysfunction which ultimately led to the development of serous retinal detachment. Stefater and colleagues 14 used the Light's criteria to assess the suprachoroidal fluid and proposed that choroidal effusions were exudative in SLE. Low serum protein can lead to a decrease in plasma oncotic pressure, thus, fluid is forced into compartments adjacent to the retina. Polito et al[15] reported that plas
The diagnosis and follow-up of lupus choroidopathy relies mostly on ophthalmic imaging modalities, including OCT, ICGA and FFA. ICGA is extremely valuable for choroidal vascular evaluation and tissue inflammation; however, use of ICGA and FFA to assess choroidopathy, especially in SLE patients with nephropathy, is limited due to nephrotoxicity[19]. OCT provides a non-invasive method to track structural changes in choroidopathy, the qualitative and quantitative assessment of OCT also contributes to the diagnosis and monitoring of lupus choroidopathy[1,19].
Differential diagnosis is vital because a mistake may worsen the ocular symptoms. CSC is also characterized by subretinal fluid accumulation and neurosensory retinal detachment. However, laboratory data are totally normal in CSC, unlike lupus choroidopathy. The treatments of these diseases are completely different. Glucocorticoids can efficiently reduce macular edema, but also aggravate subretinal fluid accumulation in CSC patients. The main hypothesis is that glucocorticoids may also regulate ion and water channels in the eye by mineralocorticoid receptor (MR) activation, leading to an abnormal edema effect in CSC[20]. In SLE patients previously administered steroid therapy, CSC is hardly distinguishable clinically from lupus choroidopathy. Ultimately, CSC can be completely excluded and lupus choroidopathy confirmed only after a good response to steroids[21]. Our patient received no steroid treatment before, and recovered after steroid administration, so CSC was excluded.
Most patients with choroidopathy show improvement or complete remission of serous detachment and choroidopathy after systemic disease control[7]. Therefore, controlling systemic disease with sufficient immunosuppression is the first step in the treatment of lupus choroidal disease. Because choroidopathy is primarily consistent with the active phase of SLE, its treatment is based on the typical regimen used in active SLE cases. This treatment includes systemic corticosteroids, immunosuppressive drugs and biological agents. Topical eye therapy is a good choice. Systemic steroid therapy is thought to be effective for SLE choroiditis, but causes serous retinal detachment. Therefore, SLE patients treated with corticosteroids have a higher risk of developing CSC[22-28]. Hopefully, use of systemic glucocorticoids would be reduced in the future and gradually replaced by other immunosuppressive or bio
MR antagonists are efficient in reducing subretinal fluid associated with CSC[30]. Therefore, we propose spironolactone application in SLE patients at disease onset to prevent serous retinal detachment although current evidence is insufficient. Aldosterone receptor blockade is safe and well tolerated in progressive murine lupus nephritis, and results in alleviated clinical proteinuria, reduced serum levels of autoantibodies, and decreased kidney damage[31].
The incidence of central serous chorioretinopathy is low and most clinicians are not well aware of it. Moreover, in some of lupus comorbidities steroid use can lead to iatrogenic impairment. Here we presented a case of lupus choroidopathy, successfully treated with systemic corticosteroids and spironolactone, with detailed discussion of previously reported cases and a focus on differential diagnosis with a central serous chorioretinopathy. Those findings contribute to the development of multidisciplinary approach for lupus choroidopathy patients and might be useful not only for rheumatologists but also for ophthalmologists who require understanding of the eye performance in SLE.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Immunology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cimen SG, Turkey; Dauyey K, Kazakhstan; Kupeli S, Turkey S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
| 1. | Ozturk B, Bozkurt B, Karademir Z, Kerimoglu H. Follow-up of lupus choroidopathy with optical coherence tomography. Lupus. 2011;20:1076-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Pons-Estel GJ, Alarcón GS, Scofield L, Reinlib L, Cooper GS. Understanding the epidemiology and progression of systemic lupus erythematosus. Semin Arthritis Rheum. 2010;39:257-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 672] [Cited by in RCA: 598] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 3. | Danchenko N, Satia JA, Anthony MS. Epidemiology of systemic lupus erythematosus: a comparison of worldwide disease burden. Lupus. 2006;15:308-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 534] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 4. | Guidelines for referral and management of systemic lupus erythematosus in adults. American College of Rheumatology Ad Hoc Committee on Systemic Lupus Erythematosus Guidelines. Arthritis Rheum. 1999;42:1785-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Silpa-archa S, Lee JJ, Foster CS. Ocular manifestations in systemic lupus erythematosus. Br J Ophthalmol. 2016;100:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 125] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 6. | Jeyachandran D, Natarajan G, Balasubramaniyan T, Thanigachalam D. Rare Ocular Manifestations of Systemic Lupus Erythematosus--Two Case Reports. J Assoc Physicians India. 2014;62:52-54. [PubMed] |
| 7. | Nguyen QD, Uy HS, Akpek EK, Harper SL, Zacks DN, Foster CS. Choroidopathy of systemic lupus erythematosus. Lupus. 2000;9:288-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 87] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Dammacco R. Systemic lupus erythematosus and ocular involvement: an overview. Clin Exp Med. 2018;18:135-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 114] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 9. | Han YS, min Yang C, Lee SH, Shin JH, Moon SW, Kang JH. Secondary angle closure glaucoma by lupus choroidopathy as an initial presentation of systemic lupus erythematosus: a case report. BMC Ophthalmol. 2015;15:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Lee KR, Peng LY, Iqbal TB, Subrayan V. Role of Angiography in Systemic Lupus Erythematosus-Induced Choroiditis. Ocul Immunol Inflamm. 2018;26:1146-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Edouard S, Douat J, Sailler L, Arlet P, Astudillo L. Bilateral choroidopathy in systemic lupus erythematosus. Lupus. 2011;20:1209-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Shoughy SS, Tabbara KF. Ocular findings in systemic lupus erythematosus. Saudi J Ophthalmol. 2016;30:117-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Matsuo T, Nakayama T, Koyama T, Matsuo N. Multifocal pigment epithelial damages with serous retinal detachment in systemic lupus erythematosus. Ophthalmologica. 1987;195:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Stefater JA, Eliott D, Kim LA. Drainage and analysis of suprachoroidal fluid in a patient with acute systemic lupus erythematous. Am J Ophthalmol Case Rep. 2016;5:29-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Polito MS, Machetta F, Fea AM, Eandi CM. Hypertensive choroidopathy in atypical hemolytic-uremic syndrome. Eur J Ophthalmol. 2021;31:NP63-NP66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Rezkallah A, Kodjikian L, Abukhashabah A, Denis P, Mathis T. Hypertensive choroidopathy: Multimodal imaging and the contribution of wide-field swept-source oct-angiography. Am J Ophthalmol Case Rep. 2019;13:131-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Silva RA, Moshfeghi DM. Antiphospholipid antibody-associated choroidopathy. Eye (Lond). 2014;28:773-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Hirabayashi Y, Saito S, Takeshita MW, Kodera T, Munakata Y, Ishii T, Fujii H, Shimura M, Sasaki T. Mononeuritis multiplex, protein-losing gastroenteropathy, and choroidopathy seen together in a case of systemic lupus erythematosus. Mod Rheumatol. 2003;13:265-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Kouprianoff S, Chiquet C, Bouillet L, Romanet JP. OCT follow-up of systemic lupus erythematosus choroidopathy. Ocul Immunol Inflamm. 2010;18:113-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Daruich A, Matet A, Dirani A, Bousquet E, Zhao M, Farman N, Jaisser F, Behar-Cohen F. Central serous chorioretinopathy: Recent findings and new physiopathology hypothesis. Prog Retin Eye Res. 2015;48:82-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 676] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 21. | Hasanreisoglu M, Gulpinar Ikiz GD, Kucuk H, Varan O, Ozdek S. Acute lupus choroidopathy: multimodal imaging and differential diagnosis from central serous chorioretinopathy. Int Ophthalmol. 2018;38:369-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Carvalho-Recchia CA, Yannuzzi LA, Negrão S, Spaide RF, Freund KB, Rodriguez-Coleman H, Lenharo M, Iida T. Corticosteroids and central serous chorioretinopathy. Ophthalmology. 2002;109:1834-1837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 229] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 23. | Haimovici R, Koh S, Gagnon DR, Lehrfeld T, Wellik S, Central Serous Chorioretinopathy Case-Control Study G. Risk factors for central serous chorioretinopathy: a case-control study. Ophthalmology. 2004;111:244-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 335] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 24. | Karadimas P, Bouzas EA. Glucocorticoid use represents a risk factor for central serous chorioretinopathy: a prospective, case-control study. Graefes Arch Clin Exp Ophthalmol. 2004;242:800-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Kitzmann AS, Pulido JS, Diehl NN, Hodge DO, Burke JP. The incidence of central serous chorioretinopathy in Olmsted County, Minnesota, 1980-2002. Ophthalmology. 2008;115:169-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 309] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 26. | Tittl MK, Spaide RF, Wong D, Pilotto E, Yannuzzi LA, Fisher YL, Freund B, Guyer DR, Slakter JS, Sorenson JA. Systemic findings associated with central serous chorioretinopathy. Am J Ophthalmol. 1999;128:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 264] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 27. | Tsai DC, Chen SJ, Huang CC, Chou P, Chung CM, Chan WL, Huang PH, Lin SJ, Chen JW, Chen TJ, Leu HB. Risk of central serous chorioretinopathy in adults prescribed oral corticosteroids: a population-based study in Taiwan. Retina. 2014;34:1867-1874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Wakakura M, Song E, Ishikawa S. Corticosteroid-induced central serous chorioretinopathy. Jpn J Ophthalmol. 1997;41:180-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Donnithorne KJ, Read RW, Lowe R, Weiser P, Cron RQ, Beukelman T. Retinal vasculitis in two pediatric patients with systemic lupus erythematosus: a case report. Pediatr Rheumatol Online J. 2013;11:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Zhao M, Célérier I, Bousquet E, Jeanny JC, Jonet L, Savoldelli M, Offret O, Curan A, Farman N, Jaisser F, Behar-Cohen F. Mineralocorticoid receptor is involved in rat and human ocular chorioretinopathy. J Clin Invest. 2012;122:2672-2679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 297] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 31. | Trune DR, Kempton JB. Blocking the glucocorticoid receptor with RU-486 does not prevent glucocorticoid control of autoimmune mouse hearing loss. Audiol Neurootol. 2009;14:423-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |