Published online Jul 6, 2022. doi: 10.12998/wjcc.v10.i19.6514
Peer-review started: August 12, 2021
First decision: October 3, 2021
Revised: October 16, 2021
Accepted: May 14, 2022
Article in press: May 14, 2022
Published online: July 6, 2022
Processing time: 315 Days and 17.7 Hours
Endoscopic ultrasound (EUS)-guided radiofrequency ablation (RFA) has recently been proposed as a local treatment for functional pancreatic neuroendocrine neoplasms in patients unfit for surgery, in order to obtain clinical syndrome regression. Data on the safety and long-term effectiveness of this approach are scarce, and EUS-RFA procedures are not standardized.
The present case series reports 3 elderly patients with a pancreatic insulinoma and comorbidities, locally treated by EUS-guided RFA with clinical success in terms of hypoglycemic symptoms. RFA procedures were performed during deep sedation, under EUS control with a 19 G needle, an electrode 5-mm in size at a power of 30 W and multiple RFA applications during the same session in order to treat the whole area of the lesions. Immediate relief of symptoms was evident in 2 patients after the first EUS-RFA, while in the third patient a second endoscopic treatment was needed. All 3 patients are symptom-free without need of medications after 24 mo of follow-up with imaging follow-up showing no disease recurrence. A single adverse event of intraprocedural bleeding occurred, which was successfully treated endoscopically.
EUS-RFA represents an effective and safe alternative to surgery for the treatment of insulinomas in elderly patients at high surgical risk. However, larger multicenter studies with longer follow-up are needed in order to better assess its safety and clinical success.
Core Tip: Endoscopic ultrasound (EUS)-guided radiofrequency ablation (RFA) has been proposed as a local treatment for functional pancreatic neuroendocrine neoplasms in patients unfit for surgery. However, data on safety and long-term effectiveness are scarce and procedures are not standardized. The present case series reports 3 elderly patients with comorbidities diagnosed with a pancreatic insulinoma who received local treatment by EUS-guided RFA with a standardized protocol, with clinical success in terms of hypoglycemic symptoms over a relatively long follow-up. Effective EUS-RFA represents an alternative to surgery for the treatment of insulinoma in elderly patients at high surgical risk. However, larger multicenter studies with longer follow-up are needed in order to assess the safety and clinical success of this treatment.
- Citation: Rossi G, Petrone MC, Capurso G, Partelli S, Falconi M, Arcidiacono PG. Endoscopic ultrasound radiofrequency ablation of pancreatic insulinoma in elderly patients: Three case reports. World J Clin Cases 2022; 10(19): 6514-6519
- URL: https://www.wjgnet.com/2307-8960/full/v10/i19/6514.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i19.6514
The incidence of pancreatic neuroendocrine neoplasms (p-NENs) has increased over the last decades due to advances in imaging methods[1]. Non-functional p-NENs that were typically diagnosed at advanced stages when the volume of the lesions determined symptoms, are now often incidentally diagnosed as small (< 2 cm) lesions and whether any treatment should be pursued is debatable[2].
Functional p-NENs (F-pNENs) are usually recognized at early stages, due to the presence of a specific syndrome[3]. Surgery is always indicated in symptomatic cases as the gold standard. However, given the high morbidity and mortality of pancreatic surgery, alternative treatments such as endoscopic ultrasound (EUS)-guided radiofrequency ablation (RFA) can be considered in order to obtain resolution of the syndrome in elderly patients with comorbidities and high surgical risks. The current literature[4] is scarce regarding data on EUS-RFA treatment of F-pNENs. Therefore, safety concerns remain and long-term data on the efficacy of this treatment are needed[5]. Moreover, specific RFA settings (particularly in terms of ablation power) are not standardized.
This is a case series presenting data on the feasibility, safety and clinical efficacy of EUS-guided RFA to induce relief of the clinical syndrome in 3 elderly patients with symptomatic pancreatic insulinomas at high surgical risk.
Three elderly patients with symptomatic pancreatic insulinomas underwent a total of 4 EUS-RFA procedures performed after failure or limited control with medical treatments.
Case 1: An 84-year-old male patient had repeated episodes of syncope for 3 years, associated with blood glucose < 20 mg/dL and neuroglycopenic symptoms with prompt relief of symptoms following the administration of glucose. The diagnosis of insulinoma was supported by a preoperative fasting test.
Case 2: An 82-year-old male patient, with 2 previous episodes of syncope and marked hypoglycemia (glucose = 38 and 32 mg/dL) was referred to our center. A fasting test confirmed the diagnosis of insulinoma with glucose and C-peptide levels (glucose 40 mg/dL, C-peptide 0.7 ng/mL).
Case 3: An 84-year-old female patient was referred to our center after two years of symptomatic hypoglycemic episodes (glucose < 30 mg/dL). A fasting test was suggestive of pancreatic insulinoma, with neuroglycopenic symptoms after fasting associated with levels of glucose 30 mg/dL and C-peptide 0.9 ng/mL (normal values: 1.1-4.4 ng/mL).
Case 1: The patient had chronic renal failure and severe ischemic heart disease.
Case 2 and case 3: These 2 patients were affected by severe chronic obstructive pneumopathy disease.
No family history of NENs was present in these cases.
Case 1 had moderate obesity. The other two patients did not present specific signs at physical examination.
All three patients had consistent and constant neuroglycopenic symptoms and diagnosis was supported by elevated insulin, C-peptide and proinsulin blood levels at the preoperative fasting test. The same plasma markers were monitored after EUS-guided RFA to support the relief of hypoglycemic symptoms and clinical syndrome.
All the patients underwent magnetic resonance imaging (MRI) with administration of contrast medium and the lesions were diagnosed as likely p-NENs ranging in size from 9 to 14 mm.
The patients were referred to our multidisciplinary neuroendocrine tumor board, and due to their age and comorbidities it was decided to treat the lesions with EUS-RFA at the Hospital’s Endosonography Unit.
A cytological diagnosis of insulinoma was obtained with EUS-FNA in case 2. In case 1 and case 3 the clinical, biochemical and radiological findings were considered typical for insulinoma and multidisciplinary evaluation considered biopsies unnecessary as cited in international guidelines[2,6].
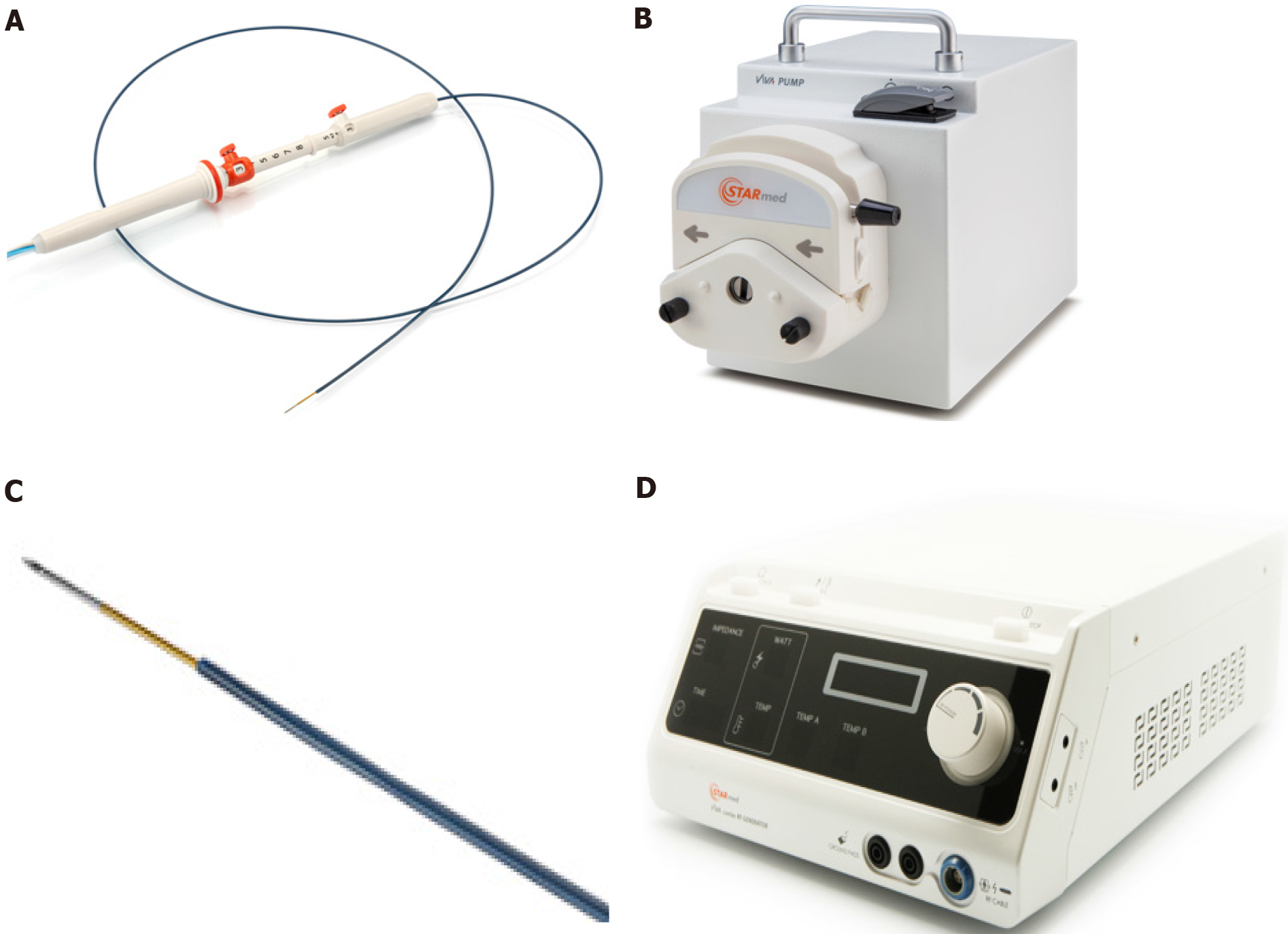
During the endoscopic procedure the patients underwent deep sedation and were placed in the left lateral position. In each case, RFA was delivered by a 19-gauge needle (EUSRA; STARmed Co., Ltd., Goyang, Korea), with a 5 mm-active monopolar electrode on the distal part of the probe (delivering the ablation). The needle was inserted in the operative channel of a therapeutic EUS-scope (Pentax EG-3870UTK or Pentax 38J10UT), connected to an ultrasound platform (Hitachi Arietta 750 or Hitachi Arietta 850). The needle was also connected to a RFA generator (VIVA; STARmed Co., Ltd., Goyang, Korea) delivering the thermal energy to ablate the lesions and was also connected to a peristaltic pump infusing cold saline solution (at 0 °C, to avoid tissue charring around the probe, maximizing the lesion ablation volume). Figure 1 describes the RFA system. The generator was set at 30 W of power in all procedures and treatment was applied for different times depending on tissue impedance (system was stopped at impedance > 500 Ω, resulting in an ineffective treatment), until a complete “cloud effect” was obtained in the lesion area (multiple RFA applications were performed during the same endoscopic session). Each patient underwent a computed tomography (CT) scan 24-72 h after the RFA procedure, in order to assess the size of the necrotic area inside the lesions and exclude complications.
One single endoscopic session was conducted with 4 subsequent EUS-RFA applications at 3 W for 12-16-12-10 s each and stopped when the impedance increased.
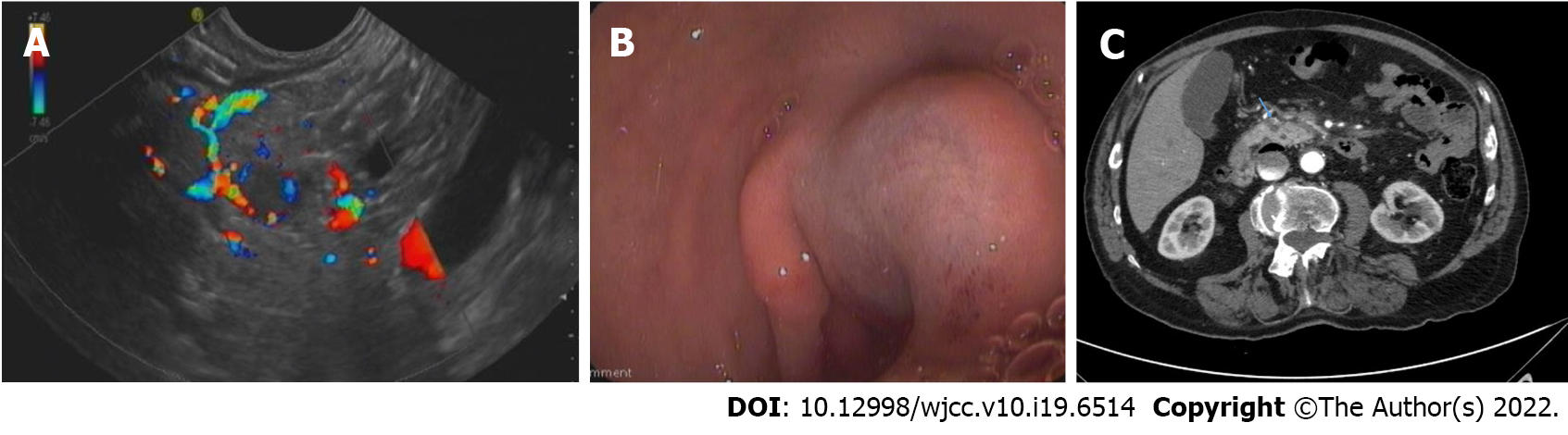
A first RFA procedure was performed with 3 applications lasting 20, 15 and 15 s each at a power of 30 W. Complete relief of symptoms was not obtained, while a 72-h CT scan showed a 7 mm hypodense necrotic area. Blood tests were consistent with ablation failure. A second EUS-RFA session was performed after 1 mo. Four RFA applications were carried out for 10, 8, 6, and 8 s, respectively, until complete covering of the pancreatic insulinoma by a hyperechoic cloud was observed. Possibly due to the proximity between the lesion and gastroduodenal artery (Figure 2A), immediate post-procedural bleeding was endoscopically evidenced with a submucosal hematoma located at the superior duodenal genus, due to a side-branch artery injury. Bleeding was immediately treated by mechanical (metallic clip) and injective (adrenalin dilution: 1:10000) hemostatic therapy with success (Figure 2B).
The procedure was performed by 3 applications lasting 6 s each at the standard power of 30 W.
No immediate or late complications occurred and immediate clinical success with syndrome relief was obtained. A CT scan performed 48-h after the procedure showed a 14 mm hypodense necrotic area in the pancreatic tail, as the outcome of the procedure. A subsequent diagnostic EUS performed after 3 mo, showed a total non-vascularized 12 mm area on contrast enhancement (Sonovue®, Bracco) at the site of the previous RFA (Video). The patient is still asymptomatic (with mild hyperglycemia), after 27-mo of clinical follow-up. No further radiological examinations were performed due to chronic renal failure and related-risks of CT or MRI-contrast medium administration.
The patient showed relief of hypoglycemic symptoms immediately after the second procedure with normalization of glucose blood levels. A CT scan performed 72 h after RFA revealed an 8-mm hypodense necrotic area at the site of the lesion, without evidence of bleeding (Figure 2C). The patient refused further radiological follow-up and complete symptom relief persists at 24 mo with normalization of biochemical tests.
A CT scan with contrast enhancement was performed 72 h after RFA and confirmed the presence of a 13-mm necrotic area inside the lesion, and complete relief from hypoglycemic symptoms was obtained. After 15 mo the patient remains asymptomatic without the need for treatment. A contrast-enhanced MRI performed 14 mo after the procedure confirmed the complete disappearance of the treated lesion in the pancreatic body.
EUS-RFA represents a potentially useful and safe option to treat insulinomas and related symptoms in patients at high surgical risk, especially in cases of pancreatic head/neck lesions, requiring a Whipple resection. EUS-RFA is relatively safe, although specific care needs to be paid to the bleeding risk of such hypervascularized lesions. Usually RFA-related complications can be endoscopically treated by a highly experienced endoscopist. In the present series, EUS-RFA led to symptom relief during a relatively long follow-up, with a single endoscopic session in 2 patients and 2 endoscopic sessions in the remaining patient. Notably, while most of the published case series on this topic did not present specific and standardized ablation settings[7-10], in the present study we standardized the setting of the ablation power in line with previous ex-vivo animal[11] and human studies (unpublished data), with the application of 30 W and stopping energy delivery when tissue impedance increased. All 3 patients are symptom-free after more than 12 mo of clinical and biochemical follow-up and the lesion is no longer visible after 14 mo in one of the patients who underwent radiological examination.
Larger multicenter studies with a longer and standardized follow-up are needed in order to confirm the safety and long-term clinical success of EUS-RFA in patients with p-NENs. The results of a large ongoing multicenter study endorsed by the European Neuroendocrine Tumour Society are eagerly awaited (ClinicalTrials.gov Identifier: NCT03834701).
Provenance and peer review: Unsolicited article; Externally peer reviewed
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kuraoka N, Japan; Rathnaswami A, India A-Editor: Makker J S-Editor: Wang JJ L-Editor: Webster JR P-Editor: Wang JJ
| 1. | Fitzgerald TL, Hickner ZJ, Schmitz M, Kort EJ. Changing incidence of pancreatic neoplasms: a 16-year review of statewide tumor registry. Pancreas. 2008;37:134-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 140] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 2. | Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, Kos-Kudla B, Kwekkeboom D, Rindi G, Klöppel G, Reed N, Kianmanesh R, Jensen RT; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology. 2016;103:153-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1097] [Cited by in RCA: 985] [Article Influence: 109.4] [Reference Citation Analysis (1)] |
| 3. | Lee DW, Kim MK, Kim HG. Diagnosis of Pancreatic Neuroendocrine Tumors. Clin Endosc. 2017;50:537-545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 4. | Imperatore N, de Nucci G, Mandelli ED, de Leone A, Zito FP, Lombardi G, Manes G. Endoscopic ultrasound-guided radiofrequency ablation of pancreatic neuroendocrine tumors: a systematic review of the literature. Endosc Int Open. 2020;8:E1759-E1764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 5. | Larghi A, Rizzatti G, Rimbaş M, Crino SF, Gasbarrini A, Costamagna G. EUS-guided radiofrequency ablation as an alternative to surgery for pancreatic neuroendocrine neoplasms: Who should we treat? Endosc Ultrasound. 2019;8:220-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 6. | Jensen RT, Cadiot G, Brandi ML, de Herder WW, Kaltsas G, Komminoth P, Scoazec JY, Salazar R, Sauvanet A, Kianmanesh R; Barcelona Consensus Conference participants. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: functional pancreatic endocrine tumor syndromes. Neuroendocrinology. 2012;95:98-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 465] [Cited by in RCA: 374] [Article Influence: 28.8] [Reference Citation Analysis (1)] |
| 7. | Lakhtakia S, Ramchandani M, Galasso D, Gupta R, Venugopal S, Kalpala R, Reddy DN. EUS-guided radiofrequency ablation for management of pancreatic insulinoma by using a novel needle electrode (with videos). Gastrointest Endosc. 2016;83:234-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 8. | Choi JH, Seo DW, Song TJ, Park DH, Lee SS, Lee SK, Kim MH. Endoscopic ultrasound-guided radiofrequency ablation for management of benign solid pancreatic tumors. Endoscopy. 2018;50:1099-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 9. | Oleinikov K, Dancour A, Epshtein J, Benson A, Mazeh H, Tal I, Matalon S, Benbassat CA, Livovsky DM, Goldin E, Gross DJ, Jacob H, Grozinsky-Glasberg S. Endoscopic Ultrasound-Guided Radiofrequency Ablation: A New Therapeutic Approach for Pancreatic Neuroendocrine Tumors. J Clin Endocrinol Metab. 2019;104:2637-2647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 10. | Furnica RM, Deprez P, Maiter D, Vandeleene B, Borbath I. Endoscopic ultrasound-guided radiofrequency ablation: An effective and safe alternative for the treatment of benign insulinoma. Ann Endocrinol (Paris). 2020;81:567-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Rossi G, Petrone MC; Capurso G, Albarello L, Testoni SGG, Archibugi L, Lena MS, Doglioni C, Arcidiacono PG. Standardization of a Radiofrequency Ablation Tool in an Ex-Vivo Porcine Liver Model. Gastrointest Disord. 2020;2:300-309. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |