Published online Jul 6, 2022. doi: 10.12998/wjcc.v10.i19.6437
Peer-review started: November 11, 2021
First decision: March 3, 2022
Revised: March 23, 2022
Accepted: May 16, 2022
Article in press: May 16, 2022
Published online: July 6, 2022
Processing time: 225 Days and 2.7 Hours
Undifferentiated embryonal sarcoma of the liver (UESL) is a rare and aggressive mesenchymal tumor in children. Herein, we describe our experience in neoadjuvant therapy (NAT) and subsequent surgery for the treatment of UESL in children.
To evaluate the efficacy of NAT and explore a new choice for successful operation of UESL in children.
We retrospectively analyzed six patients newly diagnosed with unresectable UESL who received NAT and then surgery at our center between January 2004 and December 2019. The tumor was considered unresectable if it involved a large part of both lobes of the liver or had invaded the main hepatic vessels or inferior vena cava. The NAT included preoperative transcatheter arterial chemoembolization (TACE) and systemic chemotherapy. The patients were 4 boys and 2 girls with a mean age of 7 years. The longest tumor at presentation ranged from 8.6 to 14.8 cm (mean, 12 cm). Extrahepatic metastases were present in 2 cases. Preoperative systemic chemotherapy was administered 3 wk after TACE. Tumor resection was performed 3 wk after one or two cycles of NAT. The patients received systemic chemotherapy after surgery.
All patients successfully underwent NAT and complete resection. The tumor volumes decreased by 18.2%–68.7%, with a mean decrease of 36% after 1 cycle of NAT (t = 3.524, P = 0.017). According to the Response Evaluation Criteria In Solid Tumors criteria, 4 patients had a partial response and underwent surgery, while 2 had stable disease and received another cycle of NAT before surgery. Massive tumor necrosis was seen on pathological examination of the surgical specimen: > 90% necrosis in two, > 50% necrosis in three, and 25% necrosis in 1, with an average of 71.8%. Post-NAT complications included fever, nausea and vomiting, and mild bone marrow suppression. Elevated alanine transaminase levels occurred in all patients, which returned to normal within 7–10 d after treatment. No cardiac or renal toxicity, severe hepatic dysfunction, bleeding and non-target embolization were observed in the patients. The median follow-up period was 8 years with an overall survival of 100%.
NAT effectively reduced tumor volume, cleared the tumor margin, and caused massive tumor necrosis. This may be a promising choice for successful surgery of UESL in children.
Core Tip: Undifferentiated embryonal sarcoma of the liver (UESL) is a rare and aggressive mesenchymal tumor in children. The prognosis of UESL has previously been considered poor. Modern multimodal treatment and supportive therapy have improved survival. Complete tumor excision plays the central role in the treatment of UESL. In this study, we analyzed our single-center data of children with UESL who received neoadjuvant therapy (NAT) between January 2004 and December 2019, and found that NAT effectively reduced tumor volume, cleared tumor margin, and caused massive tumor necrosis. It may provide a promising choice for successful operation of UESL in children.
- Citation: He M, Cai JB, Lai C, Mao JQ, Xiong JN, Guan ZH, Li LJ, Shu Q, Ying MD, Wang JH. Neoadjuvant transcatheter arterial chemoembolization and systemic chemotherapy for the treatment of undifferentiated embryonal sarcoma of the liver in children. World J Clin Cases 2022; 10(19): 6437-6445
- URL: https://www.wjgnet.com/2307-8960/full/v10/i19/6437.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i19.6437
Undifferentiated embryonal sarcoma of the liver (UESL) is a rare and aggressive mesenchymal tumor that occurs mainly between the ages of 6 and 10 years, without gender predominance[1]. The prognosis of UESL has previously been considered poor[2]. Modern multimodal treatment and supportive therapy have improved survival[3]. Complete tumor excision plays the central role in the treatment of UESL[4]. Transcatheter arterial chemoembolization (TACE) is an effective therapeutic method and has been widely used in the treatment of patients with hepatic malignancy[5,6]. In an attempt to increase resection rate and improve the outcome of UESL, we performed preoperative TACE combined with systematic chemotherapy for the treatment of unresectable UESL in children and evaluated its feasibility and effectiveness.
All the procedures in this study conformed to the ethical requirements of the Ethics Committee of the Children’s Hospital, Zhejiang University School of Medicine and have been approved. The children’s families provided signed informed consent. Six patients with UESL received neoadjuvant therapy (NAT) at our hospital between January 2004 and December 2019. The patients were 4 boys and 2 girls with a mean age of 7 years (range, 3–11 years). The symptoms at admission included abdominal pain, abdominal mass and jaundice. The longest tumor ranged from 8.6 to 14.8 cm (mean, 12 cm). One patient presented with pulmonary metastasis and another with retroperitoneal lymph node metastases. The details of the six patients are summarized in Table 1.
| No. | Age | Sex | Tumor size on admission (mm) | Symptoms | Metastasis | Treatment | Tumor shrunk after NAT | Response after one cycle of NAT | Outcome (follow-up years) |
| 1 | 10 years old | F | 120 | Abdominal mass | None | Neoadjuvant therapy/surgery/chemotherapy | 33% | SD | Alive and event-free at 15 |
| 2 | 3 years and 7 months old | M | 86 | Abdominal pain | Lung metastases | Neoadjuvant therapy/surgery/chemotherapy | 68.70% | PR | Alive and event-free at 11 |
| 3 | 7 years old | F | 96 | Abdominal pain | None | Neoadjuvant therapy/surgery/chemotherapy | 31% | PR | Alive and event-free at 10 |
| 4 | 11 years and 8 months old | M | 137 | Abdominal pain | None | Two cycles of neoadjuvant therapy/surgery/chemotherapy | 18.2% after one cycle; 43.2% after two cycles | SD | Alive and event-free at 7 |
| 5 | 6 years and 8 months old | M | 136 | Abdominal mass | Retroperitoneal lymph node metastasis | Two cycles of neoadjuvant therapy/surgery/chemotherapy | 17.6% after one cycle; 47.3% after two cycles | SD | Alive and event-free at 3 |
| 6 | 5 years and 3 months old | M | 148 | Jaundice | None | Neoadjuvant therapy/surgery/chemotherapy | 48.80% | PR | Alive and event-free at 2 |
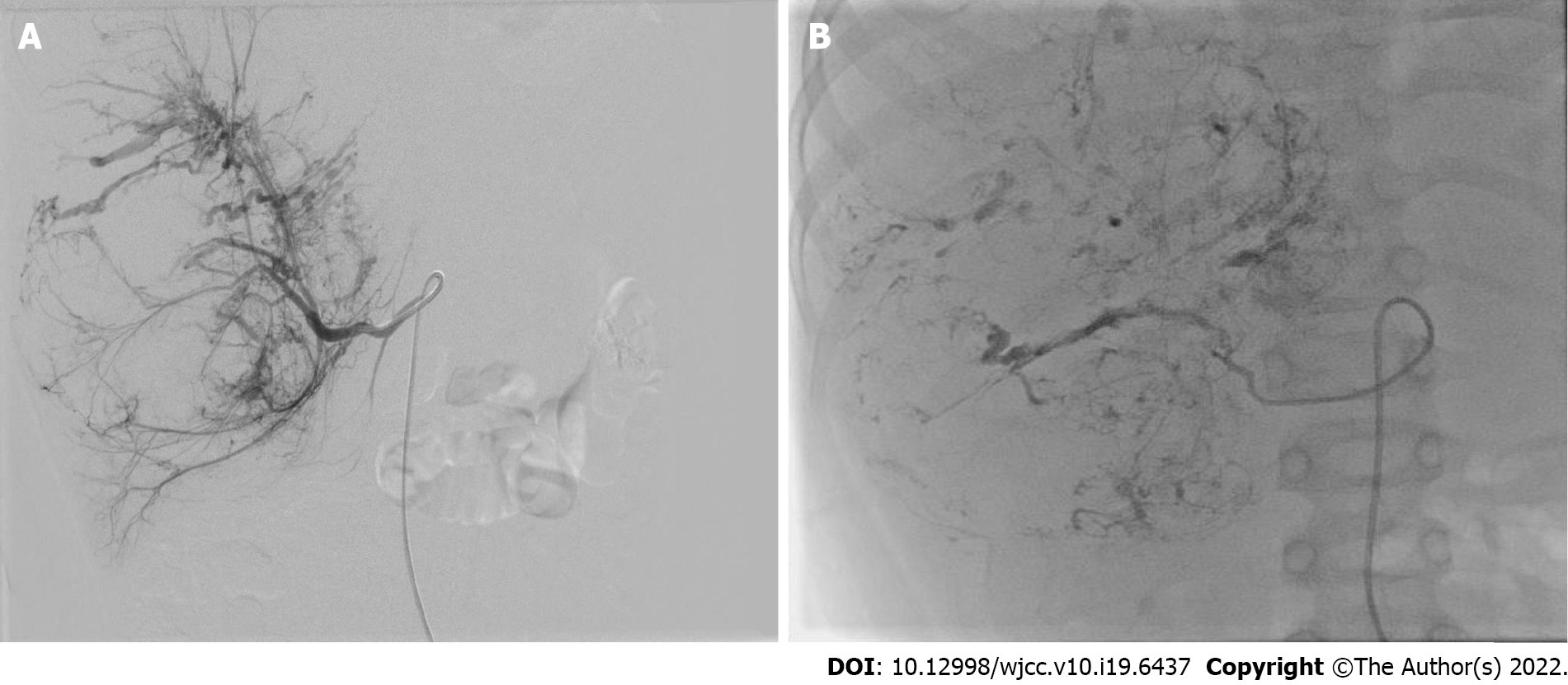
Pathological diagnosis was obtained by percutaneous needle biopsy before treatment in all patients. NAT included preoperative TACE and systemic chemotherapy. TACE was performed under general and sacral block anesthesia. With continuous monitoring, including electrocardiography, blood pressure and oxygen saturation measurements, the femoral artery was catheterized using the Seldinger technique. A 5-F Pigtail catheter was introduced into the abdominal aorta to perform aortography and define the main feeding artery of the tumor, and then a 4-F or 5-F Cobra catheter was advanced toward the tumor-feeding arteries for chemoembolization. The chemoembolic emulsion consisted of pirarubicin 40 mg/m2, vindesine 3 mg/m2, cisplatin 90 mg/m2 and iodized oil 0.5 mL per maximum tumor diameter (cm), which has been proved to have good efficacy and safety in the treatment of UESL and other tumors in our center[5,7]. The drugs were mixed and diluted in 120-180 mL of normal saline, and infused into the feeding artery at a rate of 120 mL/h. After perfusion, the outline of the entire tumor was revealed (Figure 1).
Intravenous chemotherapy was administered 3 wk after TACE if the blood parameters were at normal levels. The chemotherapy regimen consisted of vindesine 3 mg/m2 on day 1, carboplatin 150 mg/m2 and pirarubicin 20 mg/m2 on days 2 and 3. Vindesine was injected every 7 d.
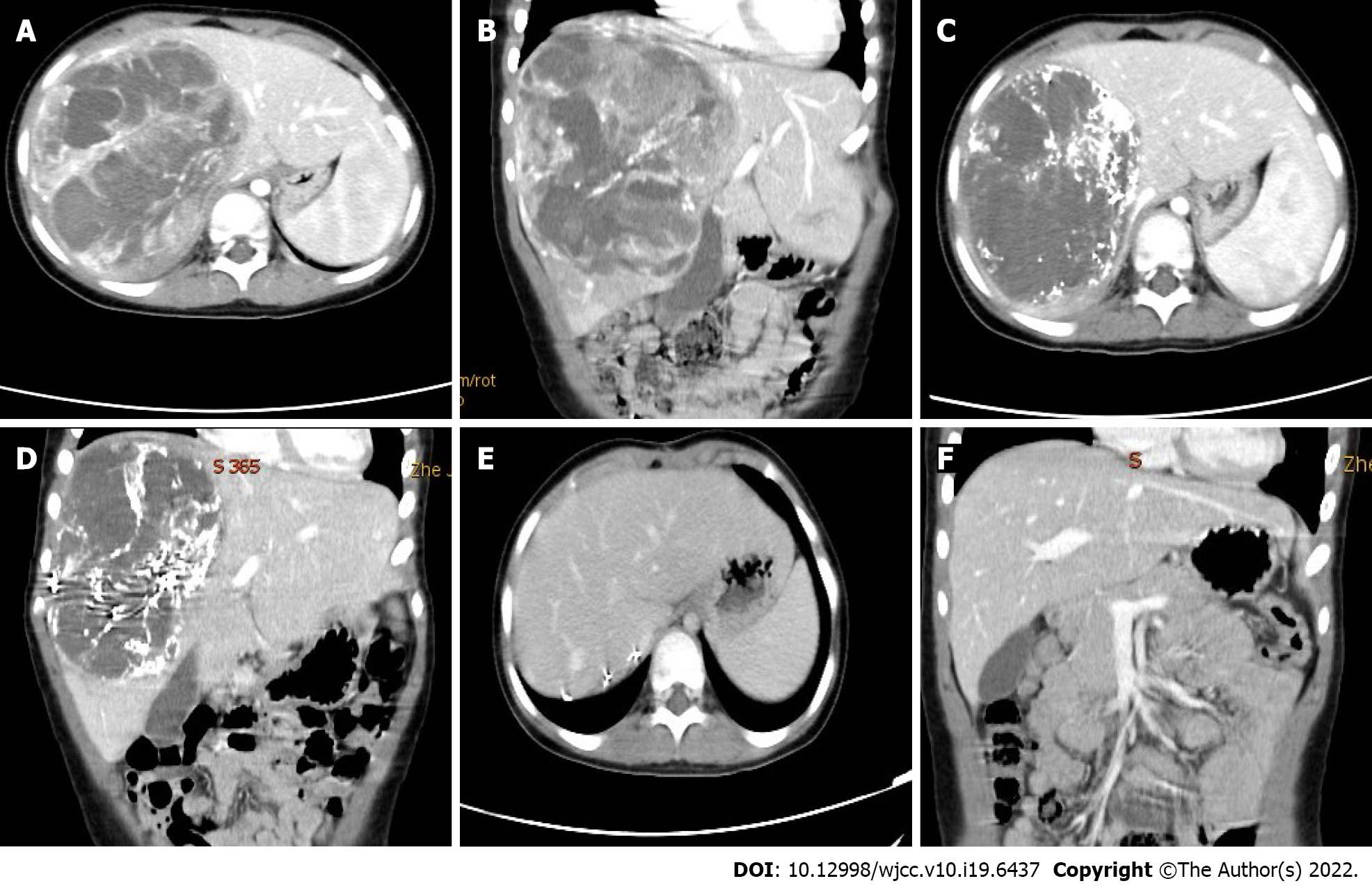
Tumor response was evaluated by enhanced computed tomography (CT) scan or magnetic resonance imaging after every cycle of NAT according to the revised Response Evaluation Criteria In Solid Tumors (RECIST) version 1.1[8]. Surgical resection was carried out after one or two cycles depending on the patient’s response. Postoperative intravenous chemotherapy was given every 3 wk, and the drugs administered were those listed above.
Toxicity was evaluated after each session of NAT. Cardiac function was monitored by echocardiography and ECG; renal function by serum electrolytes, urea, creatinine, and creatinine clearance; and liver function by aspartate aminotransferase and alanine aminotransferase (ALT). Complete blood cell count was repeated weekly to assess bone marrow suppression.
All patients were followed every 2 mo during the first year after comprehensive treatment, every 3 mo during the second and third years, every 6 mo during the fourth and fifth years and annually after 5 years. Follow-up examinations included physical check-up, imaging (abdominal ultrasound and CT, chest X-ray or CT), and laboratory testing (blood and urine analysis, and liver and renal function tests).
TACE was successfully performed in all patients. The tumor volumes decreased by 18.2%–68.7%, with a mean decrease of 36% after 1 cycle of NAT (t = 3.524, P = 0.017). According to the RECIST criteria, 4 patients had a partial response (PR) and underwent surgery, while 2 had stable disease (SD) and received another cycle of NAT before surgery. Retroperitoneal lymph node metastasis and pulmonary metastasis observed in one patient, respectively, were slightly reduced after TACE, and no regrow or other lesions appeared before surgery. Total tumor resection was performed in all patients without positive surgical margins and major postoperative complications. Retroperitoneal lymph node dissection was performed in the patient with lymph node metastasis. The patient with lung metastasis was not treated surgically as the lesions were no longer obvious after chemotherapy. Massive tumor necrosis was seen on pathological examination of the surgical specimen: > 90% necrosis in 2, > 50% necrosis in 3, and 25% necrosis in 1, with an average of 71.8%. Six to 10 courses of regular venous chemotherapy were administered after surgery in patients without metastasis, and 12 courses in the 2 patients with metastases.
The toxic side effects included fever (37.8–39.9 °C) in 5 cases, nausea and vomiting in 3, and mild bone marrow suppression in 3, all these symptoms recovered within 1-2 wk following symptomatic treatment. All patients showed varying degrees of ALT elevation, which returned to normal within 7–10 d after treatment with hepatoprotective drugs. No cardiac or renal toxicity, bleeding or nontarget embolization occurred.
The follow-up duration ranged from 2 to 15 years (median 8 years), and the follow-up rate was 100%. At the last follow-up on February 1, 2021, all patients were alive and disease-free.
Early detection of UESL is difficult, and it is usually found when the tumor becomes larger or metastasizes. It metastasizes in up to 15% of children, usually to the lungs, pleura and peritoneum[9]. Preoperative chemotherapy is currently recommended if a huge unresectable tumor or metastases are present. Although intravenous chemotherapy, as the mainstay of adjuvant treatment for UESL, has improved survival rates, the toxicity induced by systemic chemotherapy may result in early death or potential long-term and late effects[10,11]. In addition, the therapeutic effects in UESL with incomplete resection or distant metastases are still unsatisfactory[12].
TACE was first introduced in the treatment of adult liver tumor in the late 1970s and yielded good results[13]. Since then, reports of TACE in pediatric cases of hepatoblastoma have gradually increased[6,14,15]. In previous studies at our center, preoperative TACE was effective in the treatment of UESL in two cases[5]. Also, neoadjuvant TACE and systemic chemotherapy have been administered in patients with Wilms tumor and clear cell sarcoma of the kidney and achieved satisfactory results[7,16].
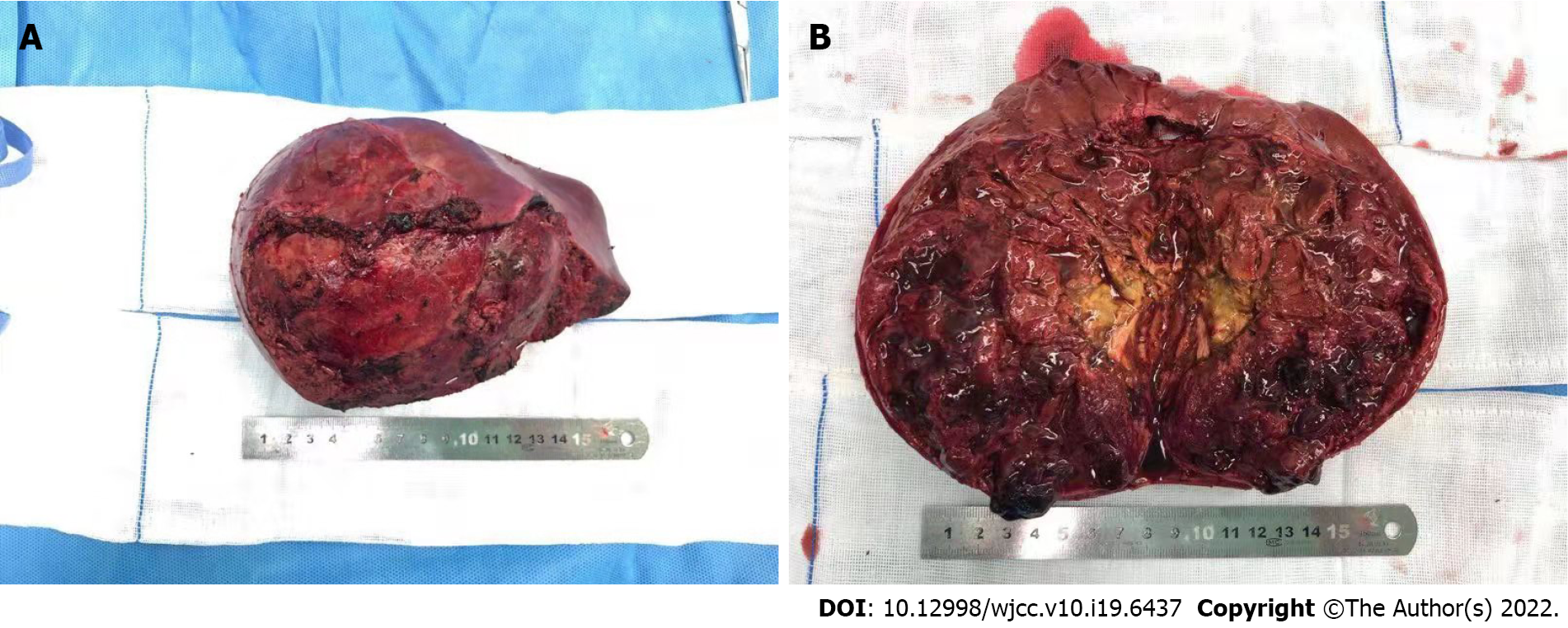
Complete tumor excision plays a central role in the treatment of UESL[4]. TACE can increase the total resection rate in several ways. TACE can deliver a higher concentration of anticancer drugs into the tumor via direct perfusion through the tumor-feeding artery, which reduces the dose of drugs and systemic toxicity[5,6,16]. Lipiodol embolization can reduce the blood supply of tumor and accelerate necrosis. High concentrations of chemotherapeutic drugs and internal ischemia cause the tumor to gradually shrink. After TACE, a pseudo-capsule can be seen around the tumor and a boundary exists between the tumor and normal liver tissue during the operation (Figure 2). These are conducive to complete resection, reducing the rate of positive surgical margins, and diminishing intraoperative bleeding (Figure 3). In this study, all the patients achieved total resection without positive surgical margins and severe bleeding.
TACE plays an important role in the primary lesion but has little effect on distal metastasis[15,17]. In this series, two patients had pulmonary and retroperitoneal lymph node metastasis on admission, respectively. Fortunately, after TACE and before systemic chemotherapy, we found that the metastasis was slightly reduced and no other lesions appeared. Following infusion into the hepatic artery, chemotherapy drugs will still flow through the hepatic vein to the heart and the entire body; thus, the extrahepatic lesions can also be suppressed. However, intravenous chemotherapy is still essential, as the dose of chemotherapy drugs flowing into the body following TACE is limited, and do not achieve an effective concentration. Preoperative intravenous chemotherapy can effectively inhibit primary hepatic and extrahepatic lesions, while postoperative intravenous chemotherapy can reduce the risk of recurrence and improve the survival rate[18,19]. Therefore, systemic chemotherapy can complement TACE. In our series, prolonged postoperative chemotherapy was performed in the two patients who are presently recurrence-free. However, the prognosis is poor, even if the patient receives intravenous chemotherapy, when complete excision of the tumor cannot be achieved[4]. The patient with lung metastases who is currently event-free still requires long-term follow-up.
In recent years drug-eluting beads (DEBs) have been introduced as novel drug-delivery agents for TACE, which can deliver higher concentrations of drugs to the tumor and lower systemic concentrations[20]. Studies have shown a lower incidence of systemic toxicity and improved tolerance with DEB-TACE, but overall survival did not differ from conventional lipiodol-TACE[21,22]. DEB-TACE has been widely used for the treatment of hepatocellular carcinoma in adults, but has not been reported in pediatric liver malignancies, and its clinical safety needs to be confirmed by further clinical trials in children. In addition, DEB-TACE reduces systemic complications by lowering extrahepatic drug concentrations, but it also decreases the inhibition of extrahepatic lesions. Therefore, DEB-TACE was not used in this study. In the near future, DEB-TACE is expected to be applied in the treatment of unresectable intrahepatic malignancy in pediatric patients.
No technical complications including bleeding, vascular injury, or nontarget embolization were noted in this study. Nontarget embolization must be viewed as a critical situation during the TACE procedure, as its appearance may lead to serious consequences[23]. The incidence of nontargeted organ embo
Postembolization syndrome and drug toxicity mainly include fever, abdominal pain, gastrointestinal reaction (nausea and vomiting), myelosuppression and organ damage (cardiotoxicity, renal insufficiency, and hepatic dysfunction)[25]. In our cases, fever was the most common symptom after TACE, possibly due to massive necrosis of the large tumor, which lasted a long time and did not respond to antibiotics. Under such circumstances, short-term small doses of hormones can be used to suppress inflammation and lower prolonged fever. Elevated ALT levels occurred in all of our patients, which returned to normal within 7–10 d after treatment without affecting follow-up treatment. Renal failure, cardiac damage, and severe myelosuppression did not occur. Hence, NAT may be considered a safe preoperative treatment for UESL.
It is noteworthy that two of our patients underwent 2 cycles of NAT before surgery. They achieved SD (tumor shrinkage was approximately 20%) after one cycle of NAT, and still had large tumors with PRETEXT stage III on radiography[26]. After another cycle of NAT, PRs (tumor shrinkage of approximately 35%) were achieved. Therefore, it is suggested that patients who are insensitive to the initial response should be considered for a second round of NAT, which can yield better results. However, unlimited chemotherapy is not desirable. Generally, surgery should be performed after two cycles of NAT, and liver transplantation should be considered if a limited response occurs in a patient with an unresectable tumor.
There were some limitations in this study. Firstly, the data were from a single institution and based on a retrospective analysis. In addition, there was no control group due to the small number of cases, and the assessment of disease response cannot definitely be attributed to TACE or systemic chemotherapy. Further prospective and controlled studies with a larger cohort are needed to compare the efficacy of TACE, systemic chemotherapy and NAT for UESL, respectively.
The present study indicated that the use of NAT effectively reduced tumor volume, cleared tumor margins, and caused massive tumor necrosis. This chemotherapy regimen may be a promising choice for successful surgery of UESL in children.
Undifferentiated embryonal sarcoma of the liver (UESL) is a rare and aggressive mesenchymal tumor in children.Modern multimodal treatment and supportive therapy have improved survival. Complete tumor excision plays the central role in the treatment of UESL.
The prognosis is poor when complete excision of tumor with UESL cannot be achieved. It is imperative to explore new choices for successful operation of UESL in children.
This report aimed to evaluate the efficacy of neoadjuvant therapy (NAT) for UESL and explore a new choice for successful operation of UESL in children.
Six patients who were newly diagnosed and unresectable with UESL received NAT and surgery at our center between January 2004 and December 2019 were retrospectively analyzed. The NAT included preoperative transcatheter arterial chemoembolization (TACE) and systemic chemotherapy. Surgical and survival data would be recorded and analyzed.
The tumor volumes decreased by 18.2%–68.7%, with a mean value of 36% after 1 cycle of NAT (t = 3.524, P = 0.017). Massive tumor necrosis was seen in the pathological examination of the specimen: > 90% necrosis in two, > 50% necrosis in three, and 25% necrosis in 1, with an average percentage of 71.8%. Complete tumor resection was successfully performed in all patients. Preoperative NAT may help complete removal of unresectable UESLs. However, there was no control group due to the small number of cases.
The present study indicated that the use of NAT effectively reduced tumor volume, cleared tumor margin, and caused massive tumor necrosis. It may provide a promising choice for successful operation of UESL in children.
Further investigations with a larger cohort and prospective study are needed to compare the efficacy of TACE, systemic chemotherapy and NAT for UESL.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Marra P, Italy; Zimmitti G, Italy A-Editor: Liu X S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Plant AS, Busuttil RW, Rana A, Nelson SD, Auerbach M, Federman NC. A single-institution retrospective cases series of childhood undifferentiated embryonal liver sarcoma (UELS): success of combined therapy and the use of orthotopic liver transplant. J Pediatr Hematol Oncol. 2013;35:451-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Stocker JT, Ishak KG. Undifferentiated (embryonal) sarcoma of the liver: report of 31 cases. Cancer. 1978;42:336-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 3. | Bisogno G, Pilz T, Perilongo G, Ferrari A, Harms D, Ninfo V, Treuner J, Carli M. Undifferentiated sarcoma of the liver in childhood: a curable disease. Cancer. 2002;94:252-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Murawski M, Scheer M, Leuschner I, Stefanowicz J, Bonar J, Dembowska-Bagińska B, Kaliciński P, Koscielniak E, Czauderna P, Fuchs J. Undifferentiated sarcoma of the liver: Multicenter international experience of the Cooperative Soft-Tissue Sarcoma Group and Polish Paediatric Solid Tumor Group. Pediatr Blood Cancer. 2020;67:e28598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Zhao X, Xiong Q, Wang J, Li MJ, Qin Q, Huang S, Gu W, Shu Q, Tou J. Preoperative Interventional Therapy for Childhood Undifferentiated Embryonal Liver Sarcoma: Two Retrospective Cases from a Single Center. European J Pediatr Surg Rep. 2015;3:90-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Zhang J, Xu F, Chen K, Zhou S, Li H, Niu C, Tan X. An effective approach for treating unresectable hepatoblastoma in infants and children: Pre-operative transcatheter arterial chemoembolization. Oncol Lett. 2013;6:850-854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Li MJ, Zhou YB, Huang Y, Tang DX, Xu S, Wu DH, Zhang YY, Tang HF. A retrospective study of the preoperative treatment of advanced Wilms tumor in children with chemotherapy vs transcatheter arterial chemoembolization alone or combined with short-term systemic chemotherapy. J Vasc Interv Radiol. 2011;22:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 21609] [Article Influence: 1350.6] [Reference Citation Analysis (1)] |
| 9. | Shi Y, Rojas Y, Zhang W, Beierle EA, Doski JJ, Goldfarb M, Goldin AB, Gow KW, Langer M, Meyers RL, Nuchtern JG, Vasudevan SA. Characteristics and outcomes in children with undifferentiated embryonal sarcoma of the liver: A report from the National Cancer Database. Pediatr Blood Cancer. 2017;64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 10. | Tao JJ, Visvanathan K, Wolff AC. Long term side effects of adjuvant chemotherapy in patients with early breast cancer. Breast. 2015;24 Suppl 2:S149-S153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 149] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 11. | Israels T, Chagaluka G, Pidini D, Caron H, de Kraker J, Kamiza S, Borgstein E, Molyneux L. The efficacy and toxicity of SIOP preoperative chemotherapy in Malawian children with a Wilms tumour. Pediatr Blood Cancer. 2012;59:636-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Zhang C, Jia CJ, Xu C, Sheng QJ, Dou XG, Ding Y. Undifferentiated embryonal sarcoma of the liver: Clinical characteristics and outcomes. World J Clin Cases. 2020;8:4763-4772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Wheeler PG, Melia W, Dubbins P, Jones B, Nunnerley H, Johnson P, Williams R. Non-operative arterial embolisation in primary liver tumours. Br Med J. 1979;2:242-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Oue T, Fukuzawa M, Kusafuka T, Kohmoto Y, Okada A, Imura K. Transcatheter arterial chemoembolization in the treatment of hepatoblastoma. J Pediatr Surg. 1998;33:1771-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Tan X, Zhang J, Wen Z, Zou Y, Shen G, Zhou S, Li H, Jiang H. Preoperative transcatheter arterial chemoembolization of hepatoblastoma in infants. J Vasc Interv Radiol. 2014;25:1029-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Wang JH, Li MJ, Tang DX, Xu S, Mao JQ, Cai JB, He M, Shu Q, Lai C. Neoadjuvant transcatheter arterial chemoembolization and systemic chemotherapy for treatment of clear cell sarcoma of the kidney in children. J Pediatr Surg. 2019;54:550-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Li JP, Chu JP, Yang JY, Chen W, Wang Y, Huang YH. Preoperative transcatheter selective arterial chemoembolization in treatment of unresectable hepatoblastoma in infants and children. Cardiovasc Intervent Radiol. 2008;31:1117-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Wu Z, Wei Y, Cai Z, Zhou Y. Long-term survival outcomes of undifferentiated embryonal sarcoma of the liver: a pooled analysis of 308 patients. ANZ J Surg. 2020;90:1615-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 19. | Putra J, Ornvold K. Undifferentiated embryonal sarcoma of the liver: a concise review. Arch Pathol Lab Med. 2015;139:269-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 20. | Song JE, Kim DY. Conventional vs drug-eluting beads transarterial chemoembolization for hepatocellular carcinoma. World J Hepatol. 2017;9:808-814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 21. | Bzeizi KI, Arabi M, Jamshidi N, Albenmousa A, Sanai FM, Al-Hamoudi W, Alghamdi S, Broering D, Alqahtani SA. Conventional Transarterial Chemoembolization Versus Drug-Eluting Beads in Patients with Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 22. | Karalli A, Teiler J, Haji M, Seth E, Brismar TB, Wahlin S, Axelsson R, Stål P. Comparison of lipiodol infusion and drug-eluting beads transarterial chemoembolization of hepatocellular carcinoma in a real-life setting. Scand J Gastroenterol. 2019;54:905-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Nakada S, Allard MA, Lewin M, Awad S, Dahbi N, Nitta H, Cunha AS, Castaing D, Vibert E, Cherqui D, Miyazaki M, Ohtsuka M, Adam R. Ischemic Cholangiopathy Following Transcatheter Arterial Chemoembolization for Recurrent Hepatocellular Carcinoma After Hepatectomy: an Underestimated and Devastating Complication. J Gastrointest Surg. 2020;24:2517-2525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Tarazov PG, Polysalov VN, Prozorovskij KV, Grishchenkova IV, Rozengauz EV. Ischemic complications of transcatheter arterial chemoembolization in liver malignancies. Acta Radiol. 2000;41:156-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Wagnetz U, Jaskolka J, Yang P, Jhaveri KS. Acute ischemic cholecystitis after transarterial chemoembolization of hepatocellular carcinoma: incidence and clinical outcome. J Comput Assist Tomogr. 2010;34:348-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Towbin AJ, Meyers RL, Woodley H, Miyazaki O, Weldon CB, Morland B, Hiyama E, Czauderna P, Roebuck DJ, Tiao GM. 2017 PRETEXT: radiologic staging system for primary hepatic malignancies of childhood revised for the Paediatric Hepatic International Tumour Trial (PHITT). Pediatr Radiol. 2018;48:536-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 169] [Article Influence: 24.1] [Reference Citation Analysis (0)] |