Published online Jul 6, 2022. doi: 10.12998/wjcc.v10.i19.6385
Peer-review started: February 1, 2022
First decision: March 15, 2022
Revised: March 21, 2022
Accepted: April 9, 2022
Article in press: April 9, 2022
Published online: July 6, 2022
Processing time: 142 Days and 19.1 Hours
The intestinal mucosal barrier is the first line of defense against numerous harmful substances, and it contributes to the maintenance of intestinal homeostasis. Recent studies reported that structural and functional changes in the intestinal mucosal barrier were involved in the pathogenesis of several intestinal diseases. However, no study thoroughly evaluated this barrier in patients with functional constipation (FC).
To investigate the intestinal mucosal barrier in FC, including the mucus barrier, intercellular junctions, mucosal immunity and gut permeability.
Forty FC patients who fulfilled the Rome IV criteria and 24 healthy controls were recruited in the Department of Gastroenterology of China-Japan Friendship Hospital. The colonic mucus barrier, intercellular junctions in the colonic epithelium, mucosal immune state and gut permeability in FC patients were comprehensively examined. Goblet cells were stained with Alcian Blue/Periodic acid Schiff (AB/PAS) and counted. The ultrastructure of intercellular junctional complexes was observed under an electron microscope. Occludin and zonula occludens-1 (ZO-1) in the colonic mucosa were located and quantified using immunohistochemistry and quantitative real-time polymerase chain reaction. Colonic CD3+ intraepithelial lymphocytes (IELs) and CD3+ lymphocytes in the lamina propria were identified and counted using immunofluorescence. The serum levels of D-lactic acid and zonulin were detected using enzyme-linked immunosorbent assay.
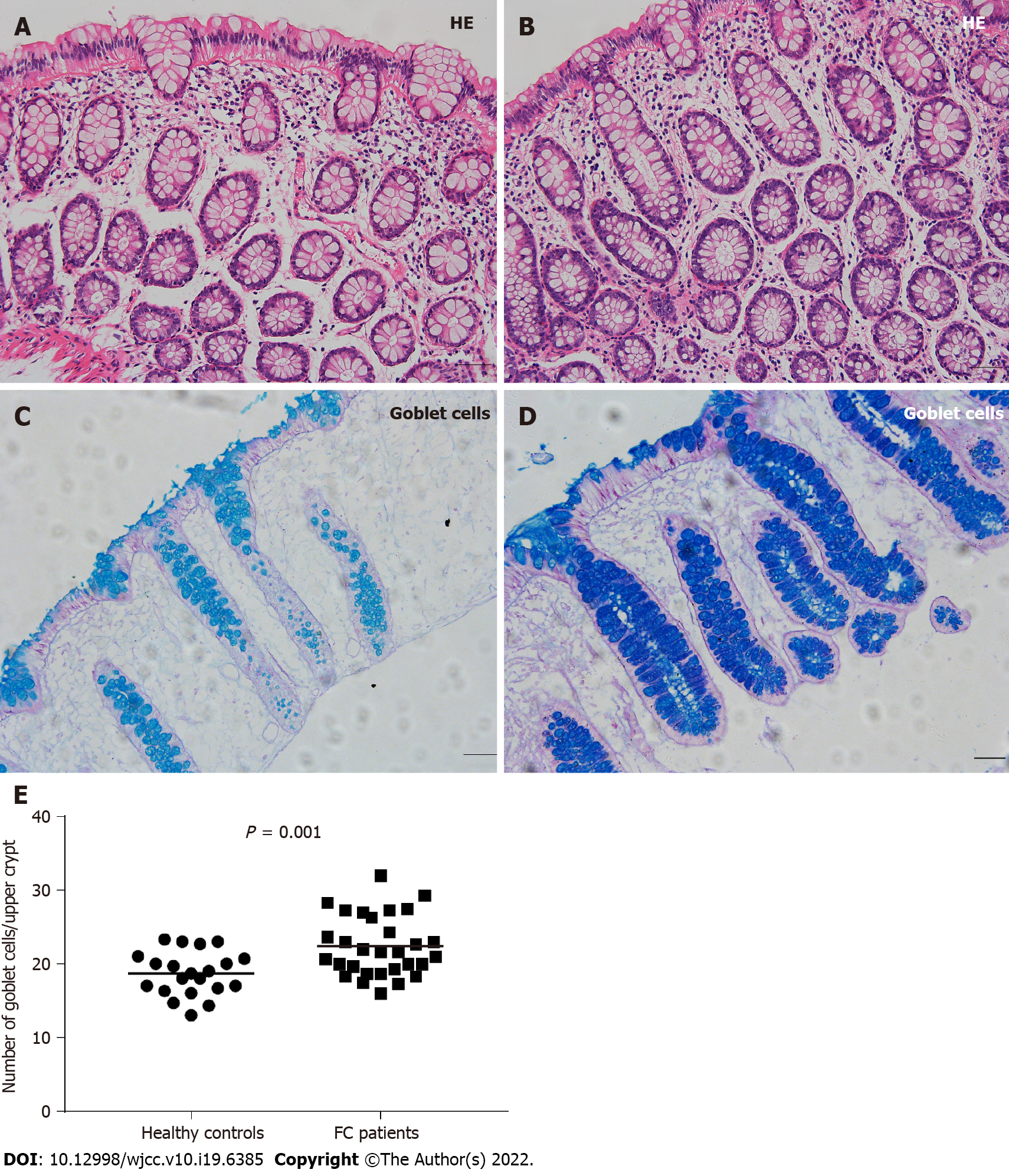
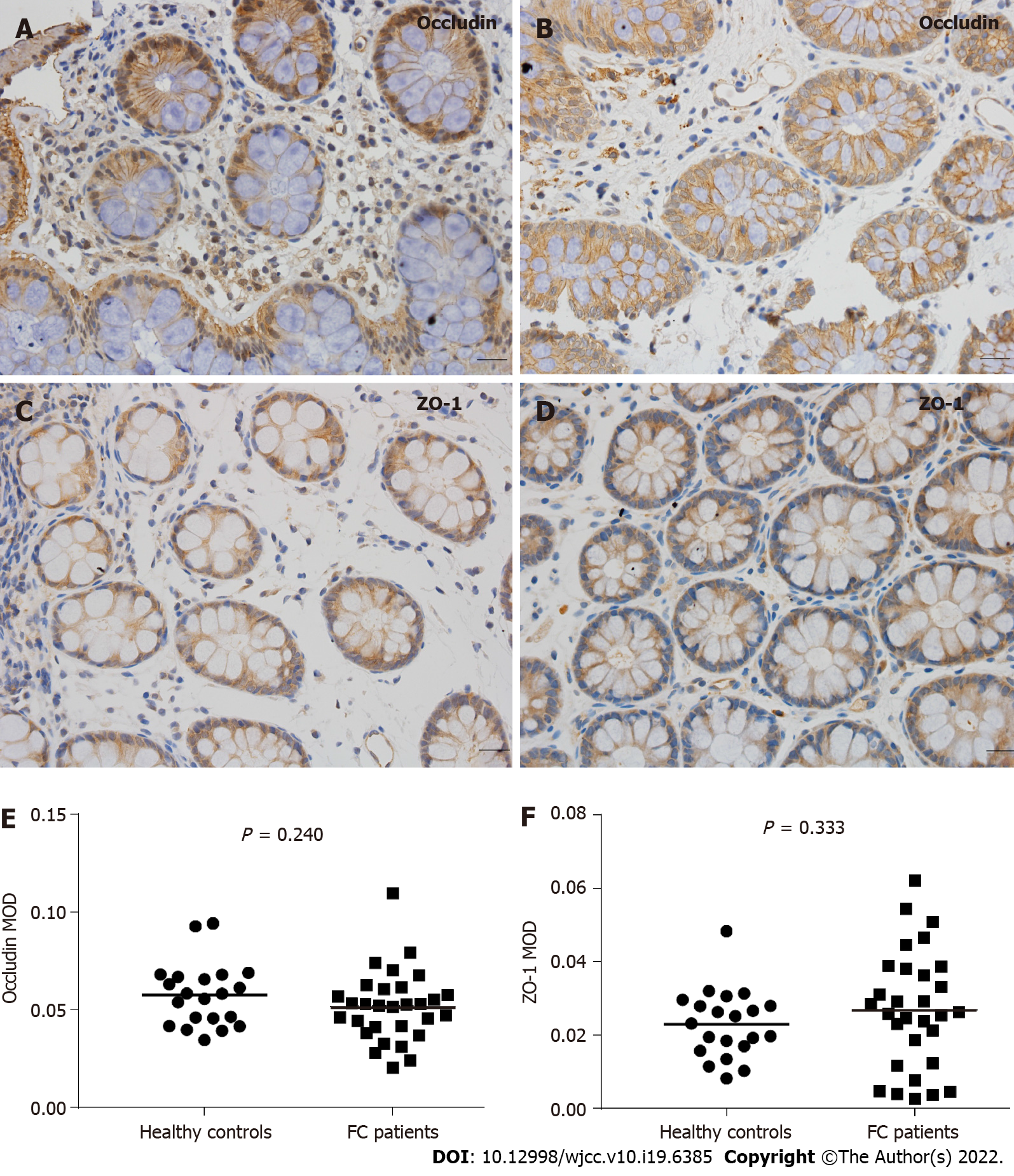
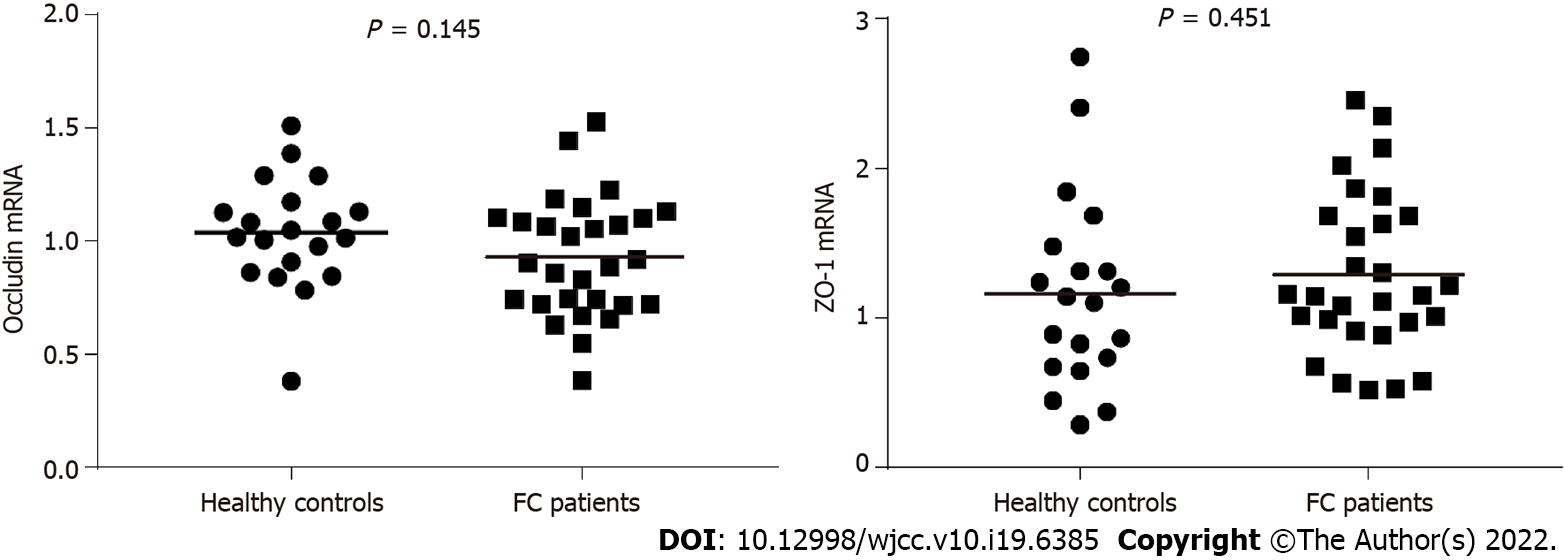
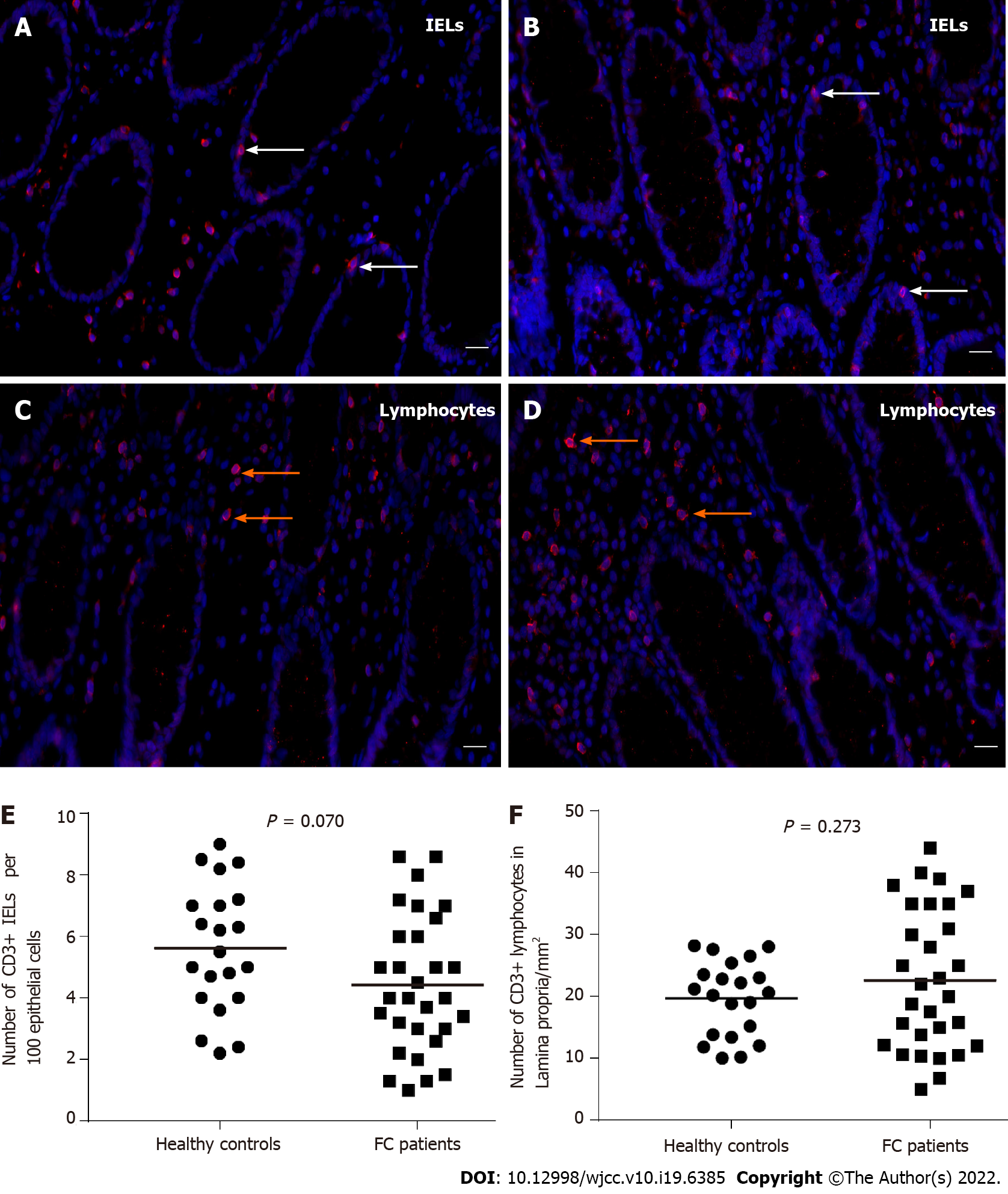
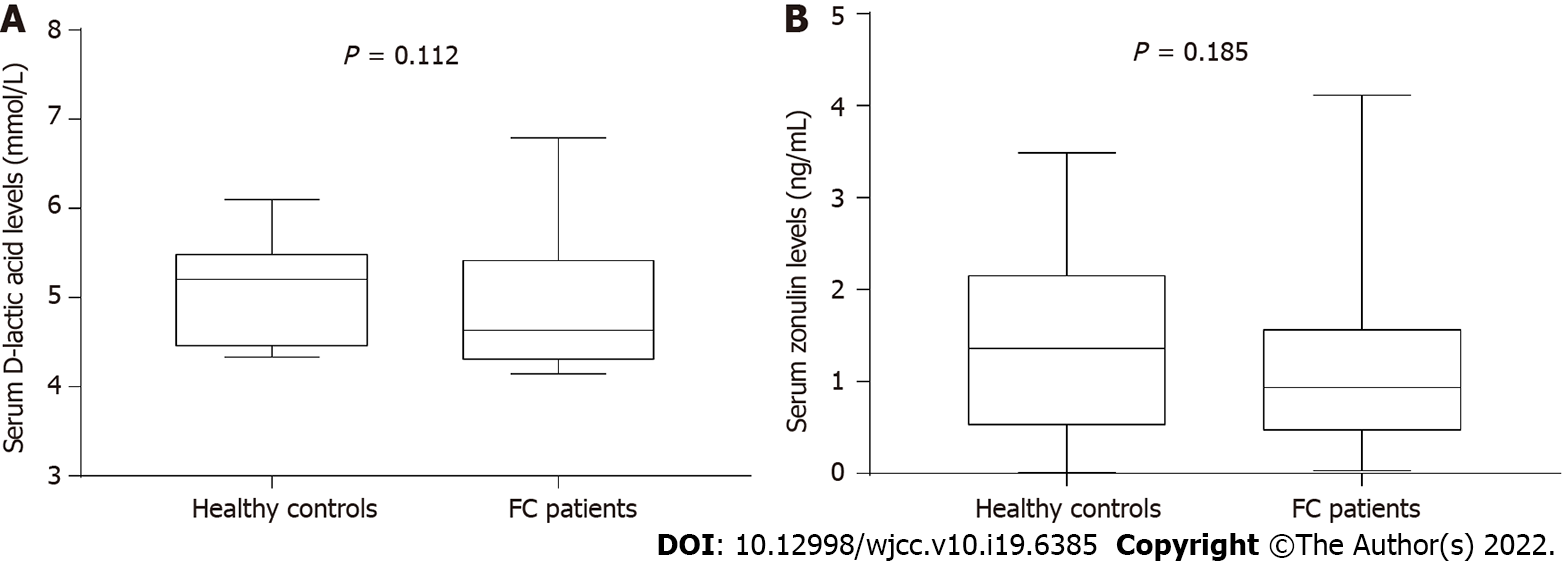
Compared to healthy controls, the staining of mucus secreted by goblet cells was darker in FC patients, and the number of goblet cells per upper crypt in the colonic mucosa was significantly increased in FC patients (control, 18.67 ± 2.99; FC, 22.42 ± 4.09; P = 0.001). The intercellular junctional complexes in the colonic epithelium were integral in FC patients. The distribution of mucosal occludin and ZO-1 was not altered in FC patients. No significant differences were found in occludin (control, 5.76E-2 ± 1.62E-2; FC, 5.17E-2 ± 1.80E-2; P = 0.240) and ZO-1 (control, 2.29E-2 ± 0.93E-2; FC, 2.68E-2 ± 1.60E-2; P = 0.333) protein expression between the two groups. The mRNA levels in occludin and ZO-1 were not modified in FC patients compared to healthy controls (P = 0.145, P = 0.451, respectively). No significant differences were observed in the number of CD3+ IELs per 100 epithelial cells (control, 5.62 ± 2.06; FC, 4.50 ± 2.16; P = 0.070) and CD3+ lamina propria lymphocytes (control, 19.69 ± 6.04/mm2; FC, 22.70 ± 11.38/mm2; P = 0.273). There were no significant differences in serum D-lactic acid [control, 5.21 (4.46, 5.49) mmol/L; FC, 4.63 (4.31, 5.42) mmol/L; P = 0.112] or zonulin [control, 1.36 (0.53, 2.15) ng/mL; FC, 0.94 (0.47, 1.56) ng/mL; P = 0.185] levels between FC patients and healthy controls.
The intestinal mucosal barrier in FC patients exhibits a compensatory increase in goblet cells and integral intercellular junctions without activation of mucosal immunity or increased gut per
Core Tip: The present study investigated the intestinal mucosal barrier in functional constipation (FC) patients for the first time, including the mucus barrier, the intestinal epithelial barrier, the mucosal immune state and gut permeability. FC patients exhibited a significant increase in goblet cells and integral intercellular junctional complexes. There were no significant alterations in the localization and expression of occludin and zonula occludens-1 in FC patients. No significant increase in CD3+ intraepithelial lymphocytes, CD3+ lamina propria lymphocytes, serum D-lactic acid or zonulin levels was found in FC patients, which indicated no mucosal immune activation or increased gut permeability.
- Citation: Wang JK, Wei W, Zhao DY, Wang HF, Zhang YL, Lei JP, Yao SK. Intestinal mucosal barrier in functional constipation: Dose it change? World J Clin Cases 2022; 10(19): 6385-6398
- URL: https://www.wjgnet.com/2307-8960/full/v10/i19/6385.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i19.6385
The intestinal mucosal barrier has selective absorption and secretory functions, and it is the first line of defense against potentially harmful substances, including antigens, proinflammatory factors and pathogenic agents[1,2]. This barrier also contributes to the maintenance of normal intestinal permeability and inner homeostasis. The efficiency of the barrier depends on the integrity and coordinated interaction of important constituents, including luminal microorganisms, the mucus barrier, the intestinal epithelial barrier and mucosal immune cells[3]. The commensal flora inhibits the colonization of pathogens and influences nutrient acquisition, energy regulation and epithelial repair of the host[4,5]. The mucus barrier is a thick hydrated gel overlying the intestinal epithelium, and it primarily consists of mucins secreted by goblet cells and numerous immune mediators, which provide a habitat for commensal microorganisms, lubricate the gut and prevent pathogenic microorganisms from adhering to the intestinal epithelium and the subsequent transepithelial invasion[6]. The intestinal epithelial barrier, which is below the mucus layer, consists of an epithelial cell monolayer and intercellular junctions, and it is essential to the intestinal mucosal barrier. For example, tight junctions (TJs) are composed of multiprotein complexes (e.g., occludin, claudins, junctional adhesion molecules and tricellulin) and are the most apical intercellular junctional complexes (TJs, adherent junctions, desmosomes and gap junctions)[7,8]. TJs play a key role in maintaining the polarity of the epithelial barrier and regulating paracellular permeability. As a cytosolic adaptor protein, zonula occludens-1 (ZO-1) interacts with TJ-associated transmembrane proteins, and participates in TJ formation. Previous data have suggested that the downregulation of occludin and ZO-1 is associated with an increased permeability[9,10]. The claudin family contains 24 members in humans with intricate functional interplay, and the results regarding their regulation of intestinal permeability are controversial[11-13]. Therefore, we focused on occludin and ZO-1 in the present study. A series of immune cells, such as intraepithelial lymphocytes (IELs) and lamina propria lymphocytes, monitor and respond to the invasion of foreign substances but acquire tolerance to harmless antigens[14,15]. The elements of this barrier intrinsically interact with each other.
Disruption of the intestinal mucosal barrier results in the disturbance of gut permeability and the invasion of pathogenic antigens into mucosal tissues, which activates local immune activities and induces severe inflammatory responses[2]. This cascade has attracted the interest of researchers to investigate the intestinal mucosal barrier in different conditions. For example, patients with inflammatory bowel disease (IBD) exhibit an altered composition of the mucus layer, goblet cell depletion or hyperplasia and changes in the expression and distribution of TJ proteins[16,17]. Occludin and ZO-1 are markedly decreased in diarrhea-predominant irritable bowel syndrome (IBS-D) patients[13,18]. Recent studies observed significantly elevated immune cells in the colonic mucosa in patients with IBS-D, celiac disease and IBD compared to healthy controls, which indicates immune activation and a severe inflammatory response[19-21]. Therefore, the intestinal mucosal barrier may be involved in the occurrence and development of some intestinal disorders, and changes in the intestinal mucosal barrier in functional constipation (FC) should be examined.
FC is a functional bowel disorder that exhibits common pathophysiological mechanisms, including colonic dysmotility, rectal hyposensitivity and dyssynergic defecation, which ultimately lead to the prolonged retention of intestinal contents in the lumen, including gut microorganisms[22,23]. These changes inevitably influence the metabolism, proliferation and maintenance of the intestinal mucosal barrier[24,25]. Although limited studies have focused on the alterations of gut microbiota and metabolites in FC, no study thoroughly examined other components of the intestinal mucosal barrier in FC patients[26,27].
Therefore, the present study comprehensively investigated the intestinal mucosal barrier in FC, including the mucus barrier, intercellular junctions, mucosal immune state and gut permeability. In the present study, the following experiments were performed: (1) Counting the goblet cells, IELs and lamina propria lymphocytes in the colonic mucosa; (2) Observing the ultrastructure of intercellular junctional complexes; (3) Evaluating the distribution and expression of occludin and ZO-1 in the colonic epithelium; and (4) Analyzing serum D-lactic acid and zonulin levels in FC patients and healthy controls.
Forty patients (age 25-65 years; 8 males and 32 females) who met the Rome IV criteria[28] for FC were consecutively recruited in this prospective case-control study from the Department of Gastroenterology of China-Japan Friendship Hospital between September 2020 and June 2021. FC patients with the following criteria were excluded: severe organic diseases (including gastrointestinal and other major organ disorders); personal history of major abdominal or pelvic surgeries, except for cholecystectomy and appendectomy; pregnant or lactating females; severe psychiatric disorders or abuse of alcohol. Patients with metabolic diseases (e.g., diabetes, hypothyroidism, or hypokalemia) and neuromuscular diseases were also excluded. Twenty-four healthy controls (age 25-60 years; 7 males and 17 females) were enrolled via public advertisements. The controls denied having digestive symptoms, organic or functional gastrointestinal diseases, or metabolic, endocrine, or immunological diseases.
Venous blood samples from all subjects were obtained in the fasting state. All subjects underwent colonoscopy after standard bowel preparation with polyethylene glycol electrolyte powder. Thirty patients and 21 healthy controls underwent colonic biopsy. Two to three mucosal biopsy specimens were taken from the rectosigmoid junction for hematoxylin and eosin (HE) staining, ultrastructural observation under an electron microscope, Alcian Blue/Periodic acid Schiff (AB/PAS) staining, immunohistochemistry, immunofluorescence, and quantitative real-time polymerase chain reaction (qRT-PCR).
The Ethics Committee of the China-Japan Friendship Hospital approved the study (No. 2019-64-K44), which was performed in accordance with the guidelines of the Declaration of Helsinki. Written informed consent was obtained from all subjects.
Biopsy specimens were fixed in 4% paraformaldehyde, embedded in paraffin, and cut into 4-µm thick sections. The sections were stained with HE for routine histology, and goblet cells were stained with AB/PAS. Acidic mucus in the cytoplasm was stained blue. The number of goblet cells was counted for a 150-µm distance from the surface epithelium of longitudinally cut crypts, and the mean results of 3 crypts were analyzed for each subject[29]. Two independent observers evaluated all sections in a blinded manner.
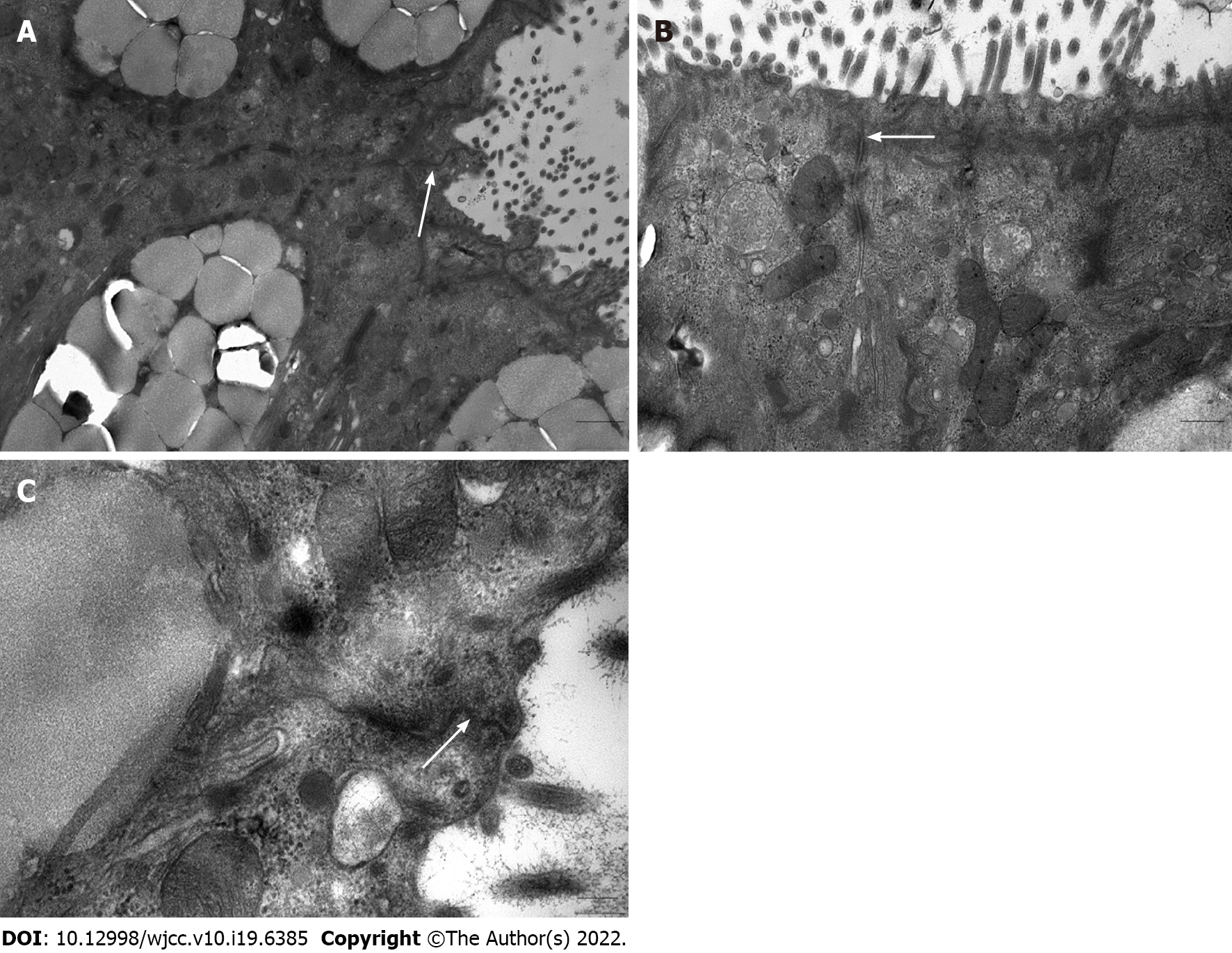
Mucosal tissues were cut into 1-mm3 pieces, immediately immersed in 2.5% glutaraldehyde at 4 ˚C for 2 h, washed three times with 0.1 M Phosphate Buffered Saline (PBS) for 30 min and postfixed with 1% osmium acid. After washing twice with distilled water for 5 min, the specimens were dehydrated in a graded series of acetone: Twice in 50% acetone for 10 min, twice in 70% acetone for 10 min, three times in 90% acetone for 10 min and three times in pure acetone for 10 min. Following resin penetration and embedding, the embedding models were moved to a 60 ˚C oven for polymerization for longer than 48 h. Sections (0.5 μm) were cut and positioned under a light microscope after staining with 1% toluidine blue. Ultrathin sections of 70 nm were cut, and the tissues were placed on 150-mesh cuprum grids with formvar film. After uranyl acetate and lead citrate staining, the sections were observed using a JEM-1400 Plus (JEOL, Tokyo, Japan) electron microscope, and images were captured.
Following deparaffinization, antigen retrieval, endogenous peroxidase inhibition and serum blocking, the sections were incubated with primary antibodies (anti-occludin, 1:700, Servicebio, Wuhan, China; anti-ZO-1, 1:200, HuaBio, Hangzhou, China) overnight at 4 °C. After washing with PBS, the sections were incubated with a horseradish peroxidase-labeled goat anti-rabbit antibody (1:200; Servicebio, Wuhan, China) at room temperature for 50 min. Diaminobenzidine (DAB) chromogenic reaction, nuclear counterstaining, dehydration and mounting were performed, and the slides were observed using an Olympus BX53 microscope (Olympus, Tokyo, Japan). For each slide, the mean optical density (MOD) of the positive staining area from five nonoverlapping, randomly selected fields was considered the expression level of occludin and ZO-1. Two independent observers analyzed the images using Image-Pro Plus 6.0 software (Media Cybernetics, Bethesda, MD, United States).
Mucosal total RNA was extracted from colonic tissues using RNA extraction (Servicebio, Wuhan, China). After reverse transcription using Servicebio® RT First Strand cDNA Synthesis Kit (Servicebio, Wuhan, China) according to the manufacturer’s instructions, quantitative PCR was performed using 2×SYBR Green qPCR Master Mix (Servicebio, Wuhan, China) in the Real-Time PCR System (Bio–Rad Laboratories, California, United States). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the endogenous reference. The following specific primers for target genes were used: Occludin (forward 5′-TTCCTATAAATCCACGCCGG-3′, and reverse 5′- TGTCTCAAAGTTACCACCGCTG-3′), ZO-1 (forward 5′-TTCCAGCCAGCCTGCTAAAC-3′, and reverse 5′-CAATAGCGTAGCCCGTTCATCT-3′), GAPDH (forward 5′- GGAAGCTTGTCATCAATGGAAATC-3′, and reverse 5′-TGATGACCCTTTTGGCTCCC-3′).
After deparaffinization, rehydration, antigen retrieval, and serum blocking, the sections were incubated with the primary antibody overnight at 4 °C. The primary antibody was a rabbit monoclonal anti-CD3G antibody (1:100; Abclonal, Wuhan, China). The sections were incubated with a Cy3-conjugated goat anti-rabbit IgG (H+L) (1:300; Servicebio, Wuhan, China) at room temperature for 50 minutes in the dark. Nuclei were counterstained with 4’,6-diamidino-2-phenylindole, and spontaneous fluorescence quenching was performed. The slides were observed under a Nikon Eclipse C1 fluorescence microscope (Nikon, Tokyo, Japan), and images were collected using a Nikon DS-U3 system (Nikon, Tokyo, Japan).
Two independent observers in a blinded manner counted the cells according to previous studies[20,30]. The number of IELs per 100 epithelial cells was counted for at least 500 epithelial cells, and the average was calculated. Lymphocytes in the lamina propria were counted in five nonoverlapping high-power fields (400× magnification; field area, 0.111 mm2), and the mean of these 5 values was calculated. The results were expressed as counts per square millimeter (/mm2).
Subsequent to centrifugation, the blood supernatants were collected and stored at −80℃ until assay. Serum D-lactic acid and zonulin levels were quantified with commercially available enzyme-linked immunosorbent assay (ELISA) Kits (D-lactic acid, Camilo Biological, Nanjing, China; zonulin, Cusabio, Wuhan, China).
Statistical analysis was performed using SPSS software, version 22.0 (SPSS Inc., Chicago, IL, United States) and statistical charts were generated using GraphPad Prism software, version 7.0 (GraphPad Software Inc., La Jolla, CA, United States). Continuous data are presented as the mean ± standard deviation (SD) if normally distributed or the median (Q1, Q3) if not. Statistical comparisons between groups were performed using independent Student’s t-test or nonparametric Mann-Whitney U-test according to the data distribution and homogeneity of variance. The Chi-square test was used to analyze dichotomous data. P < 0.05 was considered statistically significant.
Forty FC patients (age 25-65 years; mean age, 42.18 years; 8 males and 32 females) and 24 healthy controls (age 25-60 years; mean age, 40.04 years; 7 males and 17 females) participated in the study. No significant differences were found between the two groups in age, sex or body mass index (P = 0.504, P = 0.402, and P = 0.295, respectively). The median duration of disease was 19.53 years. The characteristics of the patients are shown in Table 1.
| Healthy controls | FC patients | P value | |
| n | 24 | 40 | NA |
| Age (yr) | 40.04 ± 11.57 | 42.18 ± 12.70 | 0.504 |
| Sex (male: female) | 7:17 | 1:4 | 0.402 |
| Body mass index (kg/m2) | 22.62 ± 3.28 | 21.83 ± 2.68 | 0.295 |
| Duration of disease (yr) | NA | 19.53 (10, 29) | NA |
Thirty FC patients and 21 healthy controls received colonic mucosal biopsy. All biopsy specimens were observed to be normal (Figure 1A and B). Figure 1C and D show that the cytoplasm of goblet cells was filled with blue, thick mucus. Mucus staining in the FC group was darker than the control group. The number of goblet cells per upper crypt in the colonic mucosa was also significantly increased in FC patients (22.42 ± 4.09) compared to controls (18.67 ± 2.99) (P = 0.001) (Figure 1E).
We randomly selected 5 colonic mucosal specimens from FC patients for further observation under an electron microscope. As shown in Figure 2, the intercellular junctional complexes in the colonic mucosa were continuous and integral and exhibited a regular arrangement. No interruption or widened gaps were found, which was consistent with the normal transmission electron microscopy images[8].
Figure 3A-D indicates that colonic mucosal occludin and ZO-1 were primarily present in the cell membrane and cytoplasmic membrane, and no significant changes or differences were found in the cellular distribution between the two groups.
The protein levels of occludin and ZO-1 were quantified based on the MOD values evaluated by image analysis software, as shown in Figure 3E and F. Compared to the healthy controls, there were no significant differences in colonic mucosal occludin (control, 5.76E-2 ± 1.62E-2; FC, 5.17E-2 ± 1.80E-2; P = 0.240) or ZO-1 (control, 2.29E-2 ± 0.93E-2; FC, 2.68E-2 ± 1.60E-2; P = 0.333) expression in FC patients.
Consistent with the results of immunohistochemical analysis, the mRNA levels in occludin and ZO-1 were not changed in FC patients compared to control values (P = 0.145, P = 0.451, respectively) (Figure 4).
As shown in Figure 5A-D, we observed that CD3+ IELs resided at the basolateral side of intestinal epithelial cells and CD3+ lymphocytes scattered in the lamina propria. Moreover, the mean number of IELs per 100 epithelial cells for healthy controls was 5.62 ± 2.06 and for FC patients was 4.50 ± 2.16, with no significant difference (P = 0.070) (Figure 5E). Likewise, CD3+ lamina propria lymphocyte count was not significantly different between the two groups (control, 19.69 ± 6.04/mm2; FC, 22.70 ± 11.38/mm2; P = 0.273) (Figure 5F).
There were no significant differences in the median serum level of D-lactic acid [control, 5.21 (4.46, 5.49) mmol/L; FC, 4.63 (4.31, 5.42) mmol/L; P = 0.112] or zonulin [control, 1.36 (0.53, 2.15) ng/mL; FC, 0.94 (0.47, 1.56) ng/mL; P = 0.185] between FC patients and healthy controls (Figure 6).
Limited evidence reported structural changes in the gut microbiome in constipation patients, but no studies thoroughly investigated the intestinal mucosal barrier in FC patients. The present study evaluated the intestinal mucosal barrier in FC patients from different perspectives using comprehensive methods, including immunohistochemical and immunofluorescence analyses, qRT-PCR, ultrastructural observation under an electron microscope and ELISA, and compared these parameters with healthy controls. First, the number of goblet cells per upper crypt in the colonic epithelium was significantly increased in FC patients, along with the darker mucus staining. Ultrastructural observations confirmed that the intercellular junctional complexes in the colonic epithelium were not interrupted or widened in FC patients. Compared with the healthy controls, there were no statistically significant differences in the mRNA or protein expression levels of occludin or ZO-1 in FC patients. There were no significant increases in the number of CD3+ IELs or CD3+ lymphocytes in the lamina propria in FC patients. No significant differences were found in the serum D-lactic acid or zonulin levels between the two groups. To the best of our knowledge, this study provides the first comprehensive evidence that the intestinal mucosal barrier in FC patients may show a compensatory increase in mucus production and secretion and integral intercellular junctional complexes in the colonic epithelium without activation of mucosal immunity or increased gut permeability.
Goblet cells are especially abundant in the upper crypts of the colon and are the major producers of the mucus overlying the intestinal epithelium, which provide the first line of defense against potentially harmful substances. Mucins are the main component of mucus, which also consists of secretory immunoglobulin A (sIgA) and antimicrobial products that give the barrier its gut-lubricating properties and provides a habitat for gut commensal bacteria (the outer mucus layer) while keeping pathogenic substances away from the epithelium (the inner mucus layer)[31]. The significant increase in goblet cell counts in FC patients suggests an increased secretion of colonic mucus to a certain extent, which is consistent with the darker mucus staining. Under physiological circumstances, mucus secretion and degradation are in equilibrium as the intestinal contents flow. However, reduced colonic motility in FC patients with a longer disease duration (median duration, 19.53 years) leads to long retention of the intestinal contents, which disrupts the balance of the mucus barrier. The body demands more mucus secretion by goblet cells to lubricate the gut, promote colonic emptying and separate the intestinal mucosa from pathogenic substances. Therefore, perhaps the increase in goblet cell counts is a compensatory mechanism to compensate for the relative insufficiency of mucus volume in FC patients. Another possible explanation is the complex interactions of colonic microorganisms with the intestinal mucosal barrier. For example, B. thetaiotaomicron and Faecalibacterium prausnitzii influence mucus production by augmenting goblet cell differentiation and inducing expression of genes involved in mucin glycosylation[32]. Bacterial metabolites, such as SCFAs, are linked to the mucus biosynthesis and cell growth[33,34]. Significant butyrate-producing genera, Roseburia and Faecalibacterium, also tended to increase in constipated patients[35]. These findings suggest that the alterations in colonic microbiota and metabolites in FC patients might be involved in the increase in goblet cell counts and mucus. But of note, there is no consensus on the specific gut microbiota characteristics of patients with FC. Further studies are needed to provide definitive evidence for associations between gut microbiota in FC and increased goblet cells.
The intestinal epithelial barrier is the critical component of the mucosal barrier that separates the underlying tissues from luminal antigens[3]. TJs are multiprotein complexes that are embedded into the plasma membrane of adjacent cells to maintain the integrity of the intestinal epithelial barrier and modulate paracellular permeability, and ZO-1 primarily mediates TJ-actin cytoskeleton interactions[36]. The present study observed that intercellular junctional complexes in the colonic epithelium in FC patients were intact and regularly arranged with no widened gaps, which is consistent with a previous study that showed normal intercellular structures of the ascending colon mucosa in constipation-predominant IBS (IBS-C) patients[37]. Claudins are important structural components of TJs, presenting a tissue-specific expression pattern[38]. However, as noted in previous studies, heterogeneous claudin species exhibit different functional properties with mutual influence and most cells express more than two claudins in various combinations (i.e., claudin clusters)[11,39], which makes it difficult to analyze the effect of a single claudin under pathological conditions. Therefore, in the present study, we focused on occludin and ZO-1. Compared with the healthy controls, there were no significant alterations in the localization, protein and mRNA expression of occludin and ZO-1 in the colonic mucosa in FC patients. These data are consistent with previous studies that showed unaltered protein and mRNA expression in occludin and ZO-1 in IBS-C patients[18,40]. Peters et al[40] also observed that females with IBS-C had a normal colonic barrier using complementary in vivo and ex vivo techniques. Therefore, the cellular distribution and expression of TJs may not be changed in patients with FC or IBS-C.
Mucosal barrier function is further supported by mucosal immune cells, and IELs and lamina propria lymphocytes play important roles due to their proximity to the barrier[41]. Once pathogens invade mucosal tissues, these immune cells respond quickly to activate local immune responses and induce severe inflammatory responses. FC patients in our study showed no significant increase in CD3+ IEL or CD3+ lamina propria lymphocyte counts compared with healthy controls, which indicates a lack of mucosal immune activation. However, these results are different from a previous study that found an association of chronic constipation with immune activation[42]. Possible explanations include the fact that we evaluated mucosal immune cells and they focused on the systemic immune response by analyzing concentrations of serum T lymphocytes. Given that the systemic immunity is affected by many factors and it cannot reflect the true immune state of the colonic mucosa, we believe that a direct assessment of the mucosal immune status is more reliable.
Gut permeability is defined as the ability of the intestinal mucosal surface to be penetrated by a solute. An increased permeability indicates the disruption of the intestinal mucosal barrier. Currently, several serological biomarkers have been identified as reliable indicators to assess gut permeability[3]. In the present study, we selected commonly used serum markers, namely, D-lactic acid and zonulin, to reflect gut permeability in FC patients. D-lactic acid is produced by some gut bacteria, and it enters the blood circulation when the intestinal epithelial barrier is impaired[43]. D-lactic acid levels are elevated in patients with acute perforated appendicitis, acute mesenteric ischemia, and necrotizing enterocolitis, who suffer severe intestinal injury[44-46]. Zonulin is a human counterpart of Vibrio Cholerae zonula occludens toxin and is involved in the modulation of intestinal TJs[47]. Gluten and bacterial colonization in the small intestine are powerful luminal stimuli that trigger zonulin release[48,49]. Higher serum zonulin levels are associated with increased permeability in several disorders, including celiac disease, IBD and type 1 diabetes[50-52]. In the present study, we found that FC patients had neither higher D-lactic acid nor zonulin levels than healthy controls, which indirectly indicated that the intestinal epithelial barrier was not impaired.
Overall, the increase in goblet cell counts and mucus secretion in FC patients thickens the mucus layer covering the intestinal epithelium, which blocks the invasion of pathogenic substances by creating a physical barrier and neutralizing the pathogenic bacteria via the secretion of antibacterial products or a direct immunological effect. These alterations may explain why FC patients in the present study had integral intercellular junctional complexes and normal gut permeability without the activation of mucosal immunity. In turn, the maintenance of intestinal barrier functions in FC patients protects the body from bacterial translocation and enterogenic infection.
The present study had several limitations. First, future studies should fully investigate the changes in intestinal intercellular junctional proteins in FC patients, such as claudins. Second, specific molecular mechanisms or signaling pathways underlying the increase in goblet cells and the maintenance of an intact epithelial barrier in FC patients should be further explored. Third, it will be more meaningful to combine multiple approaches to assess intestinal barrier function (i.e., gut permeability) in FC patients, such as intestinal fatty acid binding protein and orally ingested probes assessed in urine. Finally, due to the limited tissue availability, relatively small sample size may cause the lack of statistical difference in the results. Further studies in a larger sample should be performed to validate our conclusions. To counterbalance the limitations, we performed a comprehensive analysis of the intestinal mucosal barrier in FC patients using multiple methods, which resulted in convincing conclusions.
In summary, for the first time, we comprehensively investigated the intestinal mucosal barrier in FC patients, including the mucus barrier, the intestinal epithelial barrier, the mucosal immune state and gut permeability. Specifically, we demonstrated a compensatory increase in goblet cell counts but no alterations in intercellular junctions (including the expression of occludin and ZO-1), activation of mucosal immunity or increased gut permeability in FC patients. These results are important considering the alterations of gut microbiota and metabolites in FC patients, but no severe enterogenic infection was induced by bacterial translocation. Further studies are needed to examine the molecular mechanisms underlying these changes, such as the interaction between gut microbiota in FC patients and the mucosal barrier, and further evaluate the intestinal barrier in FC patients from a functional level.
The intestinal mucosal barrier prevents potentially harmful substances in the intestinal lumen from passing through the epithelium to the underlying tissue while allowing the selective absorption and secretion of nutrients and fluids. Disruption of this barrier alters intestinal permeability and activates the immune system in some chronic intestinal disorders. However, no studies have thoroughly explored the intestinal mucosal barrier in patients with functional constipation (FC).
The integrity of the intestinal mucosal barrier contributes to the maintenance of normal intestinal permeability and inner homeostasis. Few studies have investigated this barrier in FC patients. The main experimental procedures of the present study were as follows: counting the goblet cells, CD3+ intraepithelial lymphocytes (IELs) and CD3+ lamina propria lymphocytes in the colonic mucosa in FC patients and healthy controls, observing the ultrastructure of intercellular junctional complexes in FC patients, evaluating the expression of occludin and zonula occludens-1 (ZO-1), and analyzing serum D-lactic acid and zonulin levels in FC patients and healthy controls. These findings may provide the first comprehensive insights into the alterations of the intestinal mucosal barrier in FC patients.
The present study aimed to comprehensively investigate the intestinal mucosal barrier in FC patients, including the mucus barrier, intercellular junctions, mucosal immunity and gut permeability.
Subjects underwent colonoscopy and colonic mucosal biopsy. Goblet cells were stained with Alcian Blue/Periodic acid Schiff (AB/PAS) and counted. The ultrastructure of intercellular junctional complexes was observed under an electron microscope. Occludin and ZO-1 in the colonic mucosa were located and quantified using immunohistochemistry and quantitative real-time polymerase chain reaction (qRT-PCR). Colonic CD3+ IELs and CD3+ lymphocytes in the lamina propria were identified and counted using immunofluorescence. The serum levels of D-lactic acid and zonulin were assayed using enzyme-linked immunosorbent assay.
Compared to healthy controls, the staining of mucus secreted by goblet cells was darker and the number of goblet cells in the colonic mucosa was significantly increased in FC patients. The intercellular junctional complexes in the colonic epithelium were integral in FC patients. There were no significant alterations in the localization, protein and mRNA expression of occludin and ZO-1 in the colonic mucosa in FC patients compared to healthy controls. No significant differences were observed in the number of CD3+ IELs and CD3+ lamina propria lymphocytes between the two groups. There were no significant differences in serum D-lactic acid or zonulin levels between FC patients and healthy controls.
This study provides the first comprehensive evidence that the intestinal mucosal barrier in FC patients shows a compensatory increase in mucus production and secretion as well as integral intercellular junctional complexes in the colonic epithelium without activation of mucosal immunity or increased gut permeability.
The present study thoroughly investigated the key components of the intestinal mucosal barrier in FC patients. In the future, the molecular mechanisms underlying the alterations of this barrier, such as the interaction between gut microbiota in FC patients and the mucosal barrier, need to be explored. Further studies should also evaluate the intestinal barrier in FC patients from a functional level.
We thank Dr. Du SY, Dr. Li YM, Dr. Qin G and Dr. Bai RX for enrollment of participants.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gassler N, Germany; Kreisel W, Germany S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 2709] [Article Influence: 169.3] [Reference Citation Analysis (0)] |
| 2. | D'Antongiovanni V, Pellegrini C, Fornai M, Colucci R, Blandizzi C, Antonioli L, Bernardini N. Intestinal epithelial barrier and neuromuscular compartment in health and disease. World J Gastroenterol. 2020;26:1564-1579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (51)] |
| 3. | Schoultz I, Keita ÅV. The Intestinal Barrier and Current Techniques for the Assessment of Gut Permeability. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 329] [Cited by in RCA: 295] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 4. | Collier-Hyams LS, Neish AS. Innate immune relationship between commensal flora and the mammalian intestinal epithelium. Cell Mol Life Sci. 2005;62:1339-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Tappenden KA, Deutsch AS. The physiological relevance of the intestinal microbiota--contributions to human health. J Am Coll Nutr. 2007;26:679S-683S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Johansson ME, Hansson GC. Immunological aspects of intestinal mucus and mucins. Nat Rev Immunol. 2016;16:639-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 644] [Article Influence: 71.6] [Reference Citation Analysis (0)] |
| 7. | Schulzke JD, Fromm M. Tight junctions: molecular structure meets function. Ann N Y Acad Sci. 2009;1165:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Salvo Romero E, Alonso Cotoner C, Pardo Camacho C, Casado Bedmar M, Vicario M. The intestinal barrier function and its involvement in digestive disease. Rev Esp Enferm Dig. 2015;107:686-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (2)] |
| 9. | Hou Q, Huang Y, Zhu S, Li P, Chen X, Hou Z, Liu F. MiR-144 Increases Intestinal Permeability in IBS-D Rats by Targeting OCLN and ZO1. Cell Physiol Biochem. 2017;44:2256-2268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 10. | Yamamoto-Furusho JK, Mendivil EJ, Fonseca-Camarillo G. Differential expression of occludin in patients with ulcerative colitis and healthy controls. Inflamm Bowel Dis. 2012;18:E1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Markov AG, Aschenbach JR, Amasheh S. Claudin clusters as determinants of epithelial barrier function. IUBMB Life. 2015;67:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Martínez C, Lobo B, Pigrau M, Ramos L, González-Castro AM, Alonso C, Guilarte M, Guilá M, de Torres I, Azpiroz F, Santos J, Vicario M. Diarrhoea-predominant irritable bowel syndrome: an organic disorder with structural abnormalities in the jejunal epithelial barrier. Gut. 2013;62:1160-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 209] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 13. | Zhu H, Xiao X, Shi Y, Wu Y, Huang Y, Li D, Xiong F, He G, Chai Y, Tang H. Inhibition of miRNA-29a regulates intestinal barrier function in diarrhea-predominant irritable bowel syndrome by upregulating ZO-1 and CLDN1. Exp Ther Med. 2020;20:155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Olivares-Villagómez D, Van Kaer L. Intestinal Intraepithelial Lymphocytes: Sentinels of the Mucosal Barrier. Trends Immunol. 2018;39:264-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 186] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 15. | Van Kaer L, Olivares-Villagómez D. Development, Homeostasis, and Functions of Intestinal Intraepithelial Lymphocytes. J Immunol. 2018;200:2235-2244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (1)] |
| 16. | Sünderhauf A, Hicken M, Schlichting H, Skibbe K, Ragab M, Raschdorf A, Hirose M, Schäffler H, Bokemeyer A, Bettenworth D, Savitt AG, Perner S, Ibrahim S, Peerschke EI, Ghebrehiwet B, Derer S, Sina C. Loss of Mucosal p32/gC1qR/HABP1 Triggers Energy Deficiency and Impairs Goblet Cell Differentiation in Ulcerative Colitis. Cell Mol Gastroenterol Hepatol. 2021;12:229-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Dorofeyev AE, Vasilenko IV, Rassokhina OA, Kondratiuk RB. Mucosal barrier in ulcerative colitis and Crohn's disease. Gastroenterol Res Pract. 2013;2013:431231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 18. | Bertiaux-Vandaële N, Youmba SB, Belmonte L, Lecleire S, Antonietti M, Gourcerol G, Leroi AM, Déchelotte P, Ménard JF, Ducrotté P, Coëffier M. The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease subtype. Am J Gastroenterol. 2011;106:2165-2173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 218] [Article Influence: 15.6] [Reference Citation Analysis (1)] |
| 19. | Ohman L, Isaksson S, Lindmark AC, Posserud I, Stotzer PO, Strid H, Sjövall H, Simrén M. T-cell activation in patients with irritable bowel syndrome. Am J Gastroenterol. 2009;104:1205-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 20. | Hossein-Nataj H, Masjedi M, Emami MH, Mokhtari M, Alsahebfosoul F. Cell Density Counts of the Intestinal Intraepithelial Lymphocytes in the Celiac Patients. Iran J Immunol. 2019;16:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Ahn JY, Lee KH, Choi CH, Kim JW, Lee HW, Kim MK, Kwon GY, Han S, Kim SE, Kim SM, Chang SK. Colonic mucosal immune activity in irritable bowel syndrome: comparison with healthy controls and patients with ulcerative colitis. Dig Dis Sci. 2014;59:1001-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Pannemans J, Masuy I, Tack J. Functional Constipation: Individualising Assessment and Treatment. Drugs. 2020;80:947-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Vriesman MH, Koppen IJN, Camilleri M, Di Lorenzo C, Benninga MA. Management of functional constipation in children and adults. Nat Rev Gastroenterol Hepatol. 2020;17:21-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 249] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 24. | Martens EC, Neumann M, Desai MS. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat Rev Microbiol. 2018;16:457-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 481] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 25. | Adak A, Khan MR. An insight into gut microbiota and its functionalities. Cell Mol Life Sci. 2019;76:473-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 841] [Article Influence: 140.2] [Reference Citation Analysis (0)] |
| 26. | Choi CH, Chang SK. Alteration of gut microbiota and efficacy of probiotics in functional constipation. J Neurogastroenterol Motil. 2015;21:4-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Quigley EM. The enteric microbiota in the pathogenesis and management of constipation. Best Pract Res Clin Gastroenterol. 2011;25:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | Mearin F, Lacy BE, Chang L, Chey WD, Lembo AJ, Simren M, Spiller R. Bowel Disorders. Gastroenterology. 2016;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1781] [Cited by in RCA: 1897] [Article Influence: 210.8] [Reference Citation Analysis (3)] |
| 29. | Johansson ME, Gustafsson JK, Holmén-Larsson J, Jabbar KS, Xia L, Xu H, Ghishan FK, Carvalho FA, Gewirtz AT, Sjövall H, Hansson GC. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. 2014;63:281-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 582] [Cited by in RCA: 736] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 30. | Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 805] [Cited by in RCA: 817] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 31. | Zhang M, Wu C. The relationship between intestinal goblet cells and the immune response. Biosci Rep. 2020;40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 32. | Wrzosek L, Miquel S, Noordine ML, Bouet S, Joncquel Chevalier-Curt M, Robert V, Philippe C, Bridonneau C, Cherbuy C, Robbe-Masselot C, Langella P, Thomas M. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013;11:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 418] [Cited by in RCA: 567] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 33. | Johansson ME, Jakobsson HE, Holmén-Larsson J, Schütte A, Ermund A, Rodríguez-Piñeiro AM, Arike L, Wising C, Svensson F, Bäckhed F, Hansson GC. Normalization of Host Intestinal Mucus Layers Requires Long-Term Microbial Colonization. Cell Host Microbe. 2015;18:582-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 367] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 34. | Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ. The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett. 2002;217:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 931] [Cited by in RCA: 957] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 35. | Zhu L, Liu W, Alkhouri R, Baker RD, Bard JE, Quigley EM, Baker SS. Structural changes in the gut microbiome of constipated patients. Physiol Genomics. 2014;46:679-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 266] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 36. | Nusrat A, Turner JR, Madara JL. Molecular physiology and pathophysiology of tight junctions. IV. Regulation of tight junctions by extracellular stimuli: nutrients, cytokines, and immune cells. Am J Physiol Gastrointest Liver Physiol. 2000;279:G851-G857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 351] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 37. | Cheng P, Yao J, Wang C, Zhang L, Kong W. Molecular and cellular mechanisms of tight junction dysfunction in the irritable bowel syndrome. Mol Med Rep. 2015;12:3257-3264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (5)] |
| 38. | Rahner C, Mitic LL, Anderson JM. Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology. 2001;120:411-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 419] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 39. | Furuse M, Sasaki H, Tsukita S. Manner of interaction of heterogeneous claudin species within and between tight junction strands. J Cell Biol. 1999;147:891-903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 561] [Cited by in RCA: 552] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 40. | Peters SA, Edogawa S, Sundt WJ, Dyer RB, Dalenberg DA, Mazzone A, Singh RJ, Moses N, Smyrk TC, Weber C, Linden DR, MacNaughton WK, Turner JR, Camilleri M, Katzka DA, Farrugia G, Grover M. Constipation-Predominant Irritable Bowel Syndrome Females Have Normal Colonic Barrier and Secretory Function. Am J Gastroenterol. 2017;112:913-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 41. | Mowat AM, Agace WW. Regional specialization within the intestinal immune system. Nat Rev Immunol. 2014;14:667-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 828] [Cited by in RCA: 1170] [Article Influence: 106.4] [Reference Citation Analysis (0)] |
| 42. | Khalif IL, Quigley EM, Konovitch EA, Maximova ID. Alterations in the colonic flora and intestinal permeability and evidence of immune activation in chronic constipation. Dig Liver Dis. 2005;37:838-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 272] [Article Influence: 13.6] [Reference Citation Analysis (2)] |
| 43. | Yao YM, Yu Y, Wu Y, Lu LR, Sheng ZY. Plasma D (-)-lactate as a new marker for diagnosis of acute intestinal injury following ischemia-reperfusion. World J Gastroenterol. 1997;3:225-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | Demircan M, Cetin S, Uguralp S, Sezgin N, Karaman A, Gozukara EM. Plasma D-lactic acid level: a useful marker to distinguish perforated from acute simple appendicitis. Asian J Surg. 2004;27:303-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Murray MJ, Gonze MD, Nowak LR, Cobb CF. Serum D(-)-lactate levels as an aid to diagnosing acute intestinal ischemia. Am J Surg. 1994;167:575-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 154] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 46. | Garcia J, Smith FR, Cucinell SA. Urinary D-lactate excretion in infants with necrotizing enterocolitis. J Pediatr. 1984;104:268-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Wang W, Uzzau S, Goldblum SE, Fasano A. Human zonulin, a potential modulator of intestinal tight junctions. J Cell Sci. 2000;113 Pt 24:4435-4440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 246] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 48. | Drago S, El Asmar R, Di Pierro M, Grazia Clemente M, Tripathi A, Sapone A, Thakar M, Iacono G, Carroccio A, D'Agate C, Not T, Zampini L, Catassi C, Fasano A. Gliadin, zonulin and gut permeability: Effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand J Gastroenterol. 2006;41:408-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 341] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 49. | El Asmar R, Panigrahi P, Bamford P, Berti I, Not T, Coppa GV, Catassi C, Fasano A. Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology. 2002;123:1607-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 256] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 50. | Duerksen DR, Wilhelm-Boyles C, Veitch R, Kryszak D, Parry DM. A comparison of antibody testing, permeability testing, and zonulin levels with small-bowel biopsy in celiac disease patients on a gluten-free diet. Dig Dis Sci. 2010;55:1026-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Caviglia GP, Dughera F, Ribaldone DG, Rosso C, Abate ML, Pellicano R, Bresso F, Smedile A, Saracco GM, Astegiano M. Serum zonulin in patients with inflammatory bowel disease: a pilot study. Minerva Med. 2019;110:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 52. | Visser J, Rozing J, Sapone A, Lammers K, Fasano A. Tight junctions, intestinal permeability, and autoimmunity: celiac disease and type 1 diabetes paradigms. Ann N Y Acad Sci. 2009;1165:195-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 175] [Article Influence: 10.9] [Reference Citation Analysis (0)] |