Published online Jun 26, 2022. doi: 10.12998/wjcc.v10.i18.6269
Peer-review started: December 13, 2021
First decision: February 14, 2022
Revised: February 23, 2022
Accepted: April 22, 2022
Article in press: April 22, 2022
Published online: June 26, 2022
Processing time: 185 Days and 11.1 Hours
Immunoglobulin G4 related disease (IgG4-RD) is a fibroinflammatory disease with markedly elevated serum IgG4 levels and fibrous tissue proliferation, accompanied by numerous plasma cells. IgG4 related hypertrophic pachymeningitis (IgG4-RHP) is relatively rare and indistinguishable from other phymatoid diseases before the operation. The risk of long-term immunosuppression needs to be balanced with disease activity.
A 40-year-old man presented with headache and bilateral abducent paralysis. He was also diagnosed with pulmonary tuberculosis 10 years ago and was on regular treatment for the same. Before the operation and steroid therapy, the patient was suspected of having tubercular meningitis at a local hospital. A clivus lesion was found via brain magnetic resonance imaging (MRI) at this presentation. He was preliminarily diagnosed with meningioma and underwent Gamma Knife Surgery. Transnasal endoscopic resection was performed to treat deterioration of nerve function. Postoperative pathologic examination suggested IgG4-RD. Moreover, the serum IgG4 was elevated at 1.90 g/L (reference range: 0.035-1.500 g/L). After steroid therapy for 2 mo, the lesion size diminished on MRI, and the function of bilateral abducent nerves recovered.
IgG4-RHP is relatively rare and indistinguishable before the operation. Elevated serum IgG4 levels and imaging examination help in the diagnosis of IgG4-RHP. Surgery is necessary when lesions progress and patients start to develop cranial nerve function deficit.
Core Tip: Immunoglobulin G4 related disease (IgG4-RD) is a fibroinflammatory disease with markedly elevated serum IgG4 levels and fibrous tissue proliferation, accompanied by numerous plasma cells. It is known to affect multiple organs. IgG4-related hypertrophic pachymeningitis (IgG4-RHP) is relatively rare and indistinguishable from IgG4-RD before the operation. Herein, we present a rare case of IgG4-RHP with intact magnetic resonance imaging and pathologic images. The case highlighted the differential diagnosis with other phymatoid lesions such as meningioma, fungal infection, and tuberculosis and the importance of comprehensive multidisciplinary treatment. Surgery becomes necessary when lesions progress and patients start to develop cranial nerve function deficit.
- Citation: Yu Y, Lv L, Yin SL, Chen C, Jiang S, Zhou PZ. Clivus-involved immunoglobulin G4 related hypertrophic pachymeningitis mimicking meningioma: A case report. World J Clin Cases 2022; 10(18): 6269-6276
- URL: https://www.wjgnet.com/2307-8960/full/v10/i18/6269.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i18.6269
Immunoglobulin G4 related disease (IgG4-RD) was initially noticed in patients with autoimmune pancreatitis in 2001 and formally named in 2010, classified as sarcoidosis with different manifestations in several organs and the same pathological characteristics[1,2]. The main characteristic of IgG4-RD is elevated levels of serum IgG4. Moreover, the lesions are often tumescent with abundant IgG4-positive plasma cells and fibrosis. Such inflammatory lesions can be seen in the pancreas, kidney, lungs, salivary glands, and other organs. Specifically, the conditions of IgG4-RD in the central nervous system are meningitis and hypophysitis[3]. As for the IgG4-related hypertrophic pachymeningitis (IgG4-RHP), the clinical and imaging manifestation is similar to that of meningioma, posing a challenge for preoperative diagnosis[4,5]. Additionally, the time and scope of operation should be considered carefully. Finally, this disease is related to some bacterial infections, such as tuberculosis. And we need to weight the pros and cons between these infections and corticosteroid therapy for IgG4-RD. Herein, we report a rare case with IgG4-RHP at the clivus area mimicking meningioma and discuss the relevant literature.
A 40 year-old man was admitted for headache, bilateral temporal visual field defect, and limited abduction in both eyes.
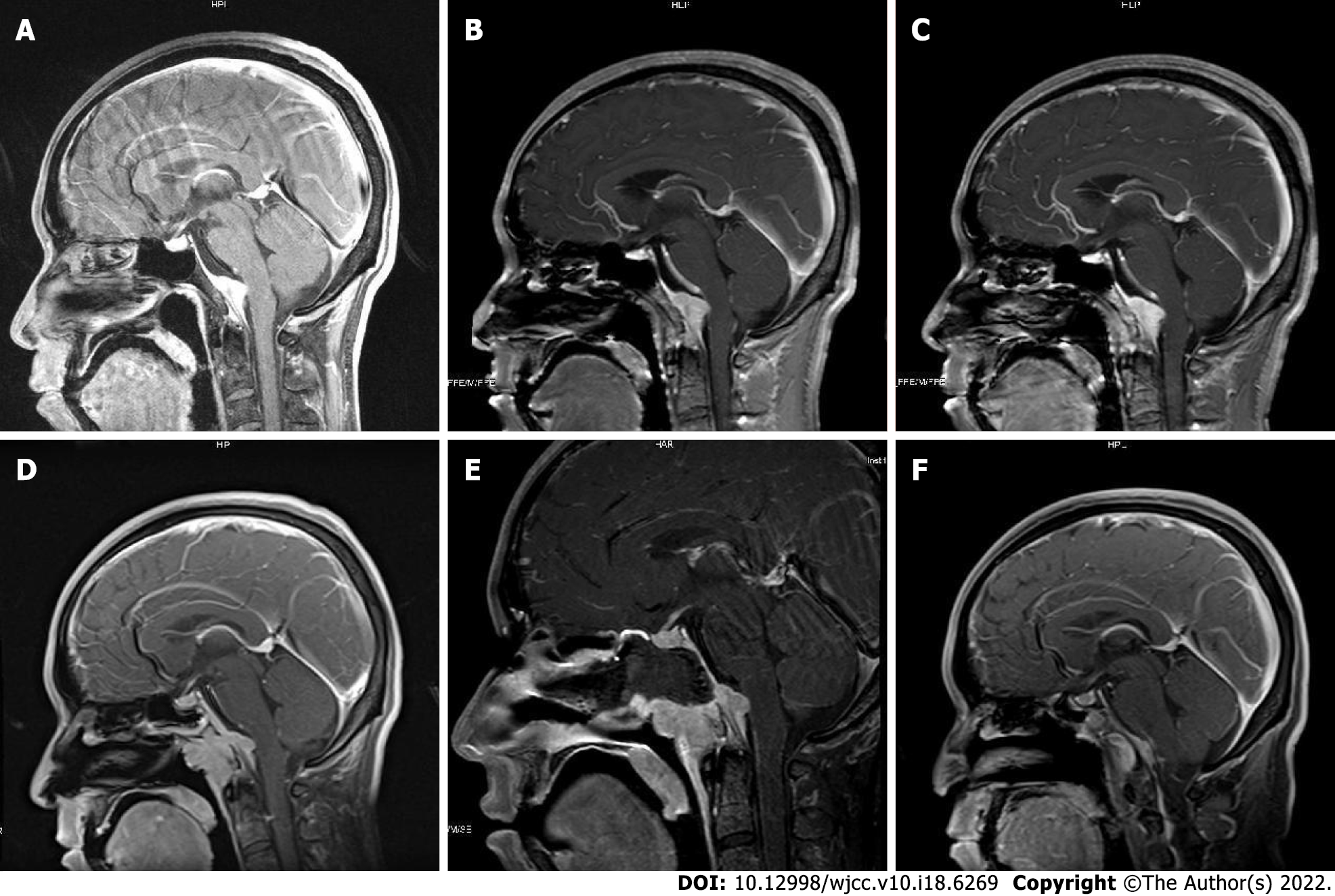
Five years before the present admission, the patient started to experience discontinuous and aggravating headache. Owing to symptomatic deterioration, the patient was admitted to the neurology department of a local hospital. Because the patient also had a history of pulmonary tuberculosis, he was suspected of having tuberculosis meningitis and treated with anti-tuberculosis drugs at the local hospital. However, the symptom did not alleviate. Upon presentation to our hospital, the patient underwent a brain magnetic resonance imaging (MRI) scan that showed the presence of a clival lesion measuring 2.6 × 1 cm2 with isointense signal on T1-weighted (T1WI) and T2-weighted (T2WI) imaging; accordingly, he was diagnosed with meningioma. The lesion was homogenously enhanced on contrast MRI with a dural tail sign (Figure 1). Because there was no cranial nerve function defect, the patients chose to undergo Gamma Knife Surgery at a dose of 11 Gy at the 45% isodose line, and regular follow-up was planned.
The patient had suffered from pulmonary tuberculosis 11 years ago and accepted standard anti-tuberculosis treatment for 1 year.
No other particular personal and family history was reported.
This patient showed right abducens paralysis, hoarse voice, bitemporal hemianopsia, and slight swallowing difficulty. No other positive signs were found.
Lumbar puncture was performed and we found that the number of karyocytes (mainly mononuclear cells) and protein levels in cerebrospinal fluid had risen (Table 1).
| 2014 | 2016 | 2020 | |
| General characters | Normal | Normal | Normal |
| Karyocytes (106/L) | 120 | 90 | 1 |
| Mononuclear cells (%) | 97.0 | 92.0 | - |
| Multinucleate cells (%) | 3.0 | 8.0 | - |
| Pus cells | Unseen | Unseen | Unseen |
| Protein quantification (g/L) | 1.02 | 0.69 | 0.55 |
| Glucose (mmol/L) | 2.55 | 4.07 | 3.95 |
| Chloride (mmol/L) | 127.2 | 124.4 | 127 |
| Acid-fast bacillus | None | None | None |
| Ink staining | None | None | None |
| Gram staining | None | None | None |
| Fungal and bacterial culture | Undone | Negative | Negative |
| TB-DNA | Undone | Negative | Negative |
After pathological results showed IgG4-RD, further systemic evaluation was performed to find other lesions associated with IgG4-RD. The serum IgG level was 17.20 g/L (reference range: 8.00-15.50 g/L), and the serum level of IgG4 was 1.90 g/L (reference range: 0.035-1.500 g/L). Tuberculosis associated gamma interferon release assay showed positive results with TB-IGRA (T-N) at 414.21 pg/mL.
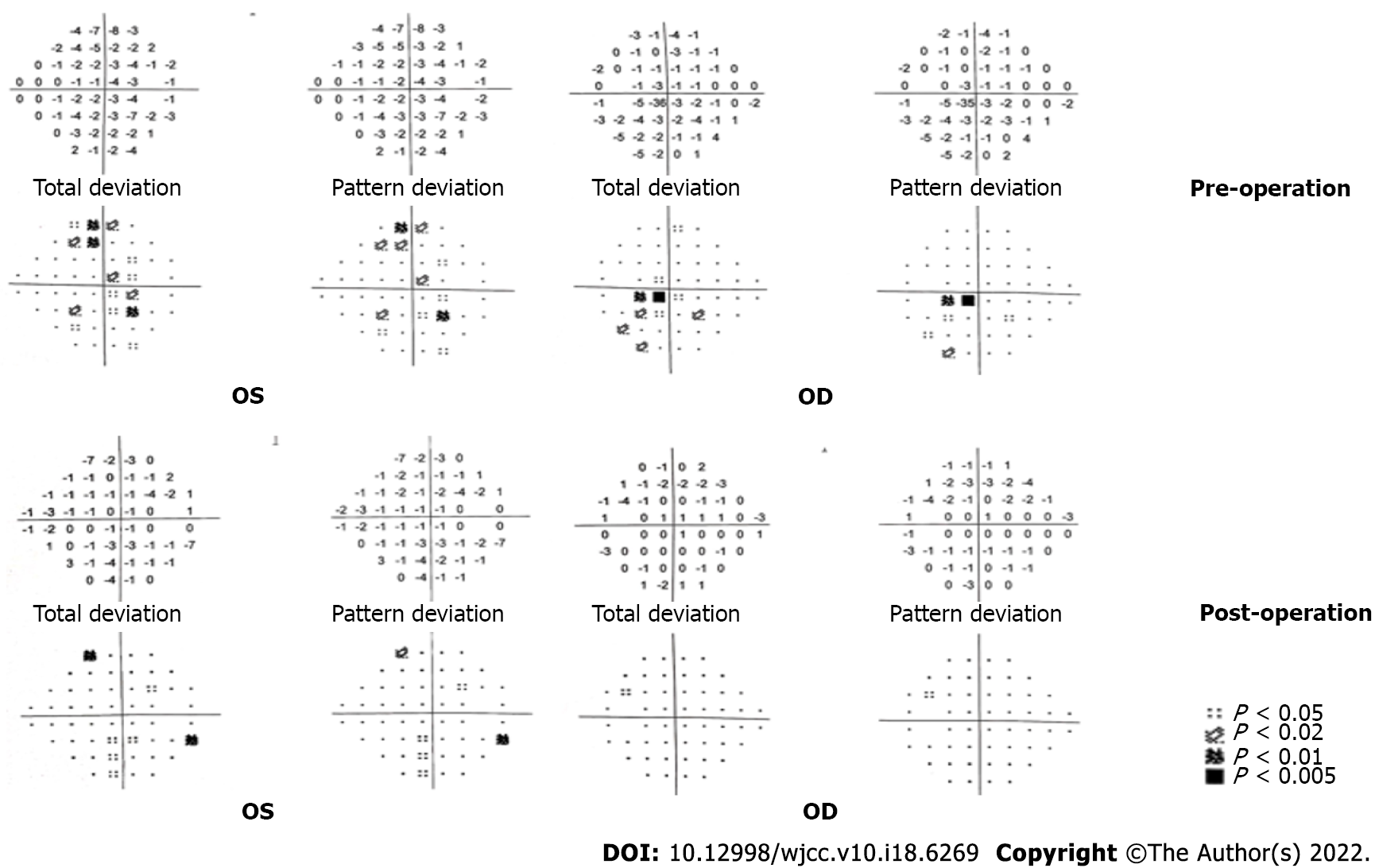
After admission, routine laboratory testing and preoperative preparation were carried out. A repeat brain MRI scan showed that the lesion became larger, measured 3.8 cm × 2.9 cm × 2.9 cm, and compressed the adjacent brain stem (Figure 1). Further, small pneumatoceles in the upper lobe of the right lung were detected by thorax computed tomography (CT). Moreover, the examination of visual field confirmed binocular hemianopia (Figure 2). No other positive results were found.
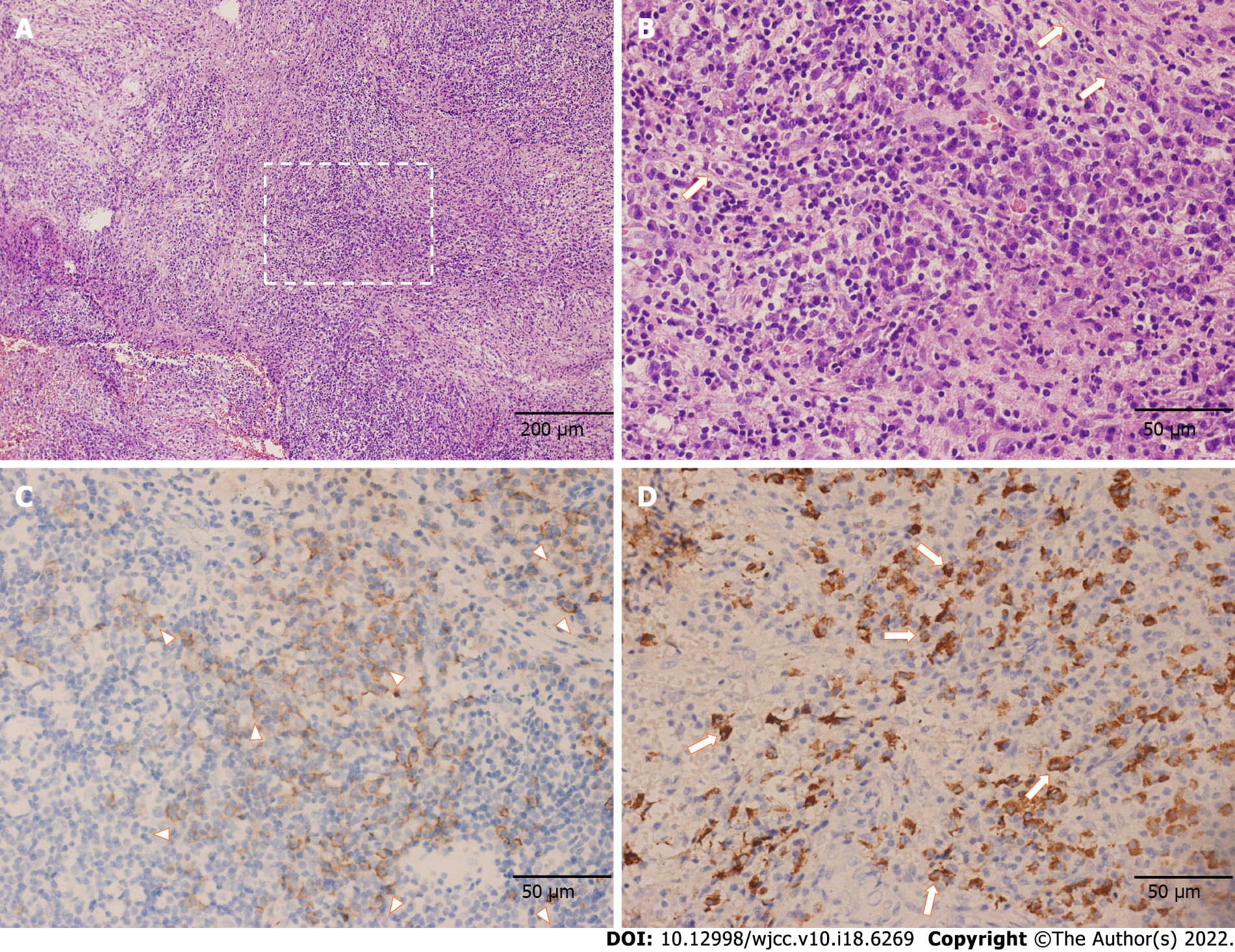
The postoperative pathology confirmed the proliferation of fibrous tissue accompanied by numerous lymphocytes and plasma cells, which is displayed in Figure 3. Immunohistochemical staining showed positive results for CD138 and IgG4. Gene rearrangement test showed negative results for IgH. Thus, IgG4-RD was finally diagnosed.
The patient underwent transnasal endoscopic approach resection which aimed to partially remove the lesion for pathology analysis and alleviate the headache caused by meningeal tension. During the operation, we found that the lesion extended to the sphenoid sinus and nasopharynx without a clear boundary. Notably, the local mucosa was edematous and tight. The clivus bone had been partially damaged, and the clivus epidural was thicker. The intraoperative frozen section examination revealed the proliferation of spindle cells accompanied by many lymphocytes and plasma cells.
For the diagnosis of IgG4-RD, solu medrol was administrated at a dose of 80 mg per day, and methotrexate was administrated at 10 mg every week. Famotidine, calcium carbonate, and vitamin D3 tablets were prescribed against adverse reactions during the treatment. After discharge from the hospital, the solu medrol was tapered over 4 wk to 50 mg per day.
The patient confirmed that his headache and hoarse voice gradually improved after 1 mo. The follow-up was arranged 3 mo after the operation, which showed that the abduction movement could be achieved for binocular vision. Brain MRI showed that the residual lesion obviously shrunk (Figure 1). The change for bilateral visual fields is displayed in Figure 2.
IgG4-RD is a condition that affects multiple organs, and its clinical manifestations often vary across different organs. Reportedly, several kinds of bacterial infection can be causative factors for this disease related to stimulation with Toll-like receptor ligands[6,7]. Several previous studies have also reported the comorbidity of IgG4-RD with tuberculosis, as seen in our patient[8-11].
IgG4-RD of the CNS is mainly related to IgG4-related hypertrophic pachymeningitis and hypophysitis. Among them, IgG4-RHP is relatively rare, with the primary clinical manifestation of headache and other nerve function disabilities. Moreover, it was apparent that the cranial nerve function could partially recover once the disease was in remission. At the first onset of the disease, multi-organ disease is not widespread (57%)[6]. Therefore, regular follow-up and systemic evaluation is crucial.
Through this case, we summarize the differential diagnoses of IgG4-RHP, such as meningioma, tuberculosis meningitis, fungal meningitis, and metastatic tumor. Furthermore, the complete MRI images showed the lesion alteration during treatment. However, there are limited reports of this rare disease in the literature. Higher evidence-based studies are needed to promote the diagnosis and treatment of IgG4-RHP.
Measuring the serum concentration of IgG4, radiological examination, and pathological screening are important for diagnosis. It is difficult to distinguish IgG4-RHP and meningioma before the operation and pathologic examination. The serum level of IgG4 can facilitate diagnosis, but it does not always show an increase. As reported by Wallace et al[12], the sensitivity and specificity of serum IgG4 were 90% and 60%, respectively. Moreover, the negative predictive value and positive predictive value of the serum IgG4 assay were 96% and 34%, respectively, which could be helpful and convenient to exclude the diagnosis of IgG4-RD related to the CNS[12]. It is also helpful to distinguish tuberculosis and IgG4-RD based on the fact that serum IgG4 does not significantly increase in tuberculosis[13]. Further, imaging results could be a crucial clue for preoperative diagnosis. Lumbar puncture provides the necessary information for differentiation from CNS infections and malignant tumors. IgG4 levels in cerebrospinal fluid have been reported to be elevated[14]. However, the concentration of IgG4 in cerebrospinal fluid could not distinguish this disease from other inflammatory pachymeningitis[6].
Radiology examination plays an essential role in diagnosis. The lesion could be observed as linear dural thickening or a bulging mass. The linear dural thickened lesion appears both in the brain and spine. The tumoral lesion is frequently located in the clivus area. The heterogeneity was observed on MRI because of active inflammation. Typically, T1WI MRI would exhibit a hyperintense or isointense signal. Hypertrophic pachymeningitis usually shows thickening meninges and hypointensity on T2WI MRI, while it would become relative hyperintense when the inflammation aggravates[3,4,6,7,15]. The lesion would be homogenously enhanced on enhanced MRI. In this case, the lesion showed an isointensite signal on T1WI and T2WI and was homogenously enhanced on contrast MRI with a dural tail sign. CT showed that the skull was involved apparently and the lesion appeared hyperdense when contrast-enhanced CT was performed. In case of a meningioma, CT frequently displays that the lesion is isodense or has slightly higher density with a round, leafy, or flat shape[3,6]. Calcification becomes visible in some tumors[6]. Meningioma has similar characteristics as an IgG4-RHP lesion. T1WI often shows isointense or mildly hypointense signal, and T2WI usually shows isointense or mildly hyperintense signal. Besides, the meningioma could be markedly characterized by the tail of the meninges.
It is advisable to focus on some characteristics to help distinguish between meningioma and IgG4-RHP. We noticed that the symptoms of IgG4-RHP were severe and diverse, while those of meningioma were not as varied. These symptoms were due to inflammatory irritation and compression of the adjacent nerves and dura mater[16]. Another characteristic of IgG4-RHP was that the tail signal was broader than meningioma on MRI for the diffuse inflammation along with the dura mater. The meningioma lesion seems relatively confined and phymatoid compared with IgG4-RHP. Moreover, the IgG4-RHP lesion frequently involves extracranial parts.
Other diseases, such as metastatic tumors and fungal infections, should also be considered. It was observed that metastatic tumors could spread and proliferate along the meninges, causing various severe symptoms. In this situation, the history of malignant tumor provided clues to the diagnosis. Likewise, a CNS fungal infection can show similar features, which can be identified by examining the cerebrospinal fluid.
CNS tuberculosis is another antidiastole. Patients with tuberculous meningitis often have a fever, headache, and focal neurological symptoms. And tuberculous meningitis is often secondary to pulmonary or intestinal tuberculosis. As for radiology examination, CT often exhibits nodular or punctate calcifications and hydrocephalus, and enhanced scans are often accompanied by meningeal strengthening. MRI frequently shows a hypointense T1WI signal and hyperintense T2WI signal. The enhancement scan could display irregular bar or nodular strengthening lesions of the meninges. Cerebrospinal fluid is essential for the diagnosis of tuberculous meningitis. Moreover, TB-IGRA could facilitate this diagnosis.
The purpose of the operation was not only to perform a biopsy but also to alleviate symptoms. We know that the lesion would stretch meninges and then cause headache. Similarly, the lesion compresses cranial nerves to cause relevant symptoms. The resection can reduce meningeal tension, release compression, and finally alleviate headache and nerve deficits. Further, it is suitable to use the transnasal endoscopic approach for a clival lesion in IgG4-RHP. When the lesion is too broad to remove completely, it is sensible to leave some parts in order to maintain the integrity of the dura mater, which can prevent severe complications such as cerebrospinal fluid leakage and intracranial infection.
Glucocorticoids and immunosuppressants can be used for the non-surgical treatment, such as prednisolone (0.6 mg/kg/d) for 4 wk. The dose of steroid was gradually decreased through 3-6 mo and the dose was finally maintained at 2.5 to 5.0 mg/d for 3 years[17]. Other immunosuppressants should be considered, such as methotrexate, cyclophosphamide, mycophenolate mofetil, and azathioprine[6,7]. Another consensus recommended utilizing calcium carbonate and vitamin D3 tablets to prevent glucocorticoid-induced osteoporosis[18,19]. Additionally, it is essential to exclude some latent infections before using glucocorticoids and immunosuppressants. In this case, the patient had a history of tuberculosis and we performed the chest CT and TB-IGRA to ensure the absence of any current underlying infection. In future, when similar patients with the imaging characteristics described in this report are encountered, measurement of serum IgG4 levels may be helpful for diagnosis.
IgG4-RHP is a relatively rare disease that seems complicated to diagnose preoperatively. The purpose of surgery is to obtain the specimens required for pathological examination and plan the follow-up treatment. It is essential to perform a rigorous follow-up and systematic assessment of the whole body.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Khan S, India; Velázquez-Saornil J, Spain S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR
| 1. | Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, Fukushima M, Nikaido T, Nakayama K, Usuda N, Kiyosawa K. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2026] [Cited by in RCA: 1876] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 2. | Takahashi H, Yamamoto M, Suzuki C, Naishiro Y, Shinomura Y, Imai K. The birthday of a new syndrome: IgG4-related diseases constitute a clinical entity. Autoimmun Rev. 2010;9:591-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Wallace ZS, Carruthers MN, Khosroshahi A, Carruthers R, Shinagare S, Stemmer-Rachamimov A, Deshpande V, Stone JH. IgG4-related disease and hypertrophic pachymeningitis. Medicine (Baltimore). 2013;92:206-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 4. | Tang H, Ding G, Xiong J, Zhu H, Hua L, Xie Q, Gong Y. Clivus Inflammatory Pseudotumor Associated with Immunoglobulin G4-Related Disease. World Neurosurg. 2018;118:71-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Cha YJ, Lee SK, Chang JH, Kim SH. Report of a rare case of atypical lymphoplasmacyte-rich meningioma in the tentorium mimicking idiopathic hypertrophic pachymeningitis. Brain Tumor Pathol. 2016;33:216-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Lu LX, Della-Torre E, Stone JH, Clark SW. IgG4-related hypertrophic pachymeningitis: clinical features, diagnostic criteria, and treatment. JAMA Neurol. 2014;71:785-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 162] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 7. | Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366:539-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1856] [Cited by in RCA: 1866] [Article Influence: 143.5] [Reference Citation Analysis (83)] |
| 8. | Bae K, Jung An H, Jeon KN, Hyun Song D, Kim SH, Kim HC. Coexistence of nontuberculous mycobacterium and IgG4-related disease in a solitary pulmonary nodule: A case report. Medicine (Baltimore). 2019;98:e18179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Bajema KL, Daniel JC, Hussain S, Dworkin RJ. IgG4-related pulmonary and salivary disease associated with pulmonary tuberculosis. Ann Am Thorac Soc. 2014;11:1165-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Kawano M, Yamada K, Kakuchi Y, Ito K, Hamano R, Fujii H, Inoue R, Matsumura M, Takahira M, Zen Y, Yachie A, Nakashima A, Yamagishi M. A case of immunoglobulin G4-related chronic sclerosing sialadenitis and dacryoadenitis associated with tuberculosis. Mod Rheumatol. 2009;19:87-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Imai T, Yumura W, Takemoto F, Kotoda A, Imai R, Inoue M, Hironaka M, Muto S, Kusano E. A case of IgG4-related tubulointerstitial nephritis with left hydronephrosis after a remission of urinary tract tuberculosis. Rheumatol Int. 2013;33:2141-2144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Wallace ZS, Mattoo H, Carruthers M, Mahajan VS, Della Torre E, Lee H, Kulikova M, Deshpande V, Pillai S, Stone JH. Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann Rheum Dis. 2015;74:190-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 319] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 13. | Araujo Z, Giampietro F, Rivas-Santiago B, Luna-Herrera J, Wide A, Clark W, de Waard JH. Patients exposed to Mycobacterium tuberculosis infection with a prominent IgE response. Arch Med Res. 2012;43:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Zhao Y, Xu J. Imaging features, clinicopathological analysis and diagnostic strategy of IgG4-related hypertrophic pachymeningitis. Ann Palliat Med. 2020;9:2551-2558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Detiger SE, Karim F, Monserez D, Verdijk R, van Hagen M, Paridaens D, van Laar J. IgG4-Related Disease of Skull Base: Case Series of 3 Patients with Headache. World Neurosurg. 2020;134:536-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Tokushige S, Matsuura H, Hideyama T, Tamura K, Maekawa R, Shiio Y. Hypertrophic Pachymeningitis as a Potential Cause of Headache Associated with Temporal Arteritis. Intern Med. 2016;55:523-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Kamisawa T, Okazaki K, Kawa S, Shimosegawa T, Tanaka M; Research Committee for Intractable Pancreatic Disease and Japan Pancreas Society. Japanese consensus guidelines for management of autoimmune pancreatitis: III. Treatment and prognosis of AIP. J Gastroenterol. 2010;45:471-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 18. | Park SY, Gong HS, Kim KM, Kim D, Kim HY, Jeon CH, Ju JH, Lee SS, Park DA, Sung YK, Kim SW. Korean Guideline for the Prevention and Treatment of Glucocorticoid-induced Osteoporosis. J Bone Metab. 2018;25:195-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Buckley L, Guyatt G, Fink HA, Cannon M, Grossman J, Hansen KE, Humphrey MB, Lane NE, Magrey M, Miller M, Morrison L, Rao M, Robinson AB, Saha S, Wolver S, Bannuru RR, Vaysbrot E, Osani M, Turgunbaev M, Miller AS, McAlindon T. 2017 American College of Rheumatology Guideline for the Prevention and Treatment of Glucocorticoid-Induced Osteoporosis. Arthritis Rheumatol. 2017;69:1521-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 353] [Article Influence: 44.1] [Reference Citation Analysis (0)] |