Published online Jun 26, 2022. doi: 10.12998/wjcc.v10.i18.6184
Peer-review started: November 5, 2021
First decision: February 14, 2022
Revised: February 28, 2022
Accepted: April 22, 2022
Article in press: April 22, 2022
Published online: June 26, 2022
Processing time: 223 Days and 22.3 Hours
Neoadjuvant or perioperative chemotherapy combined with surgery can reduce postoperative recurrence and improve the long-term survival rate of patients with locally advanced resectable gastric carcinoma. Nivolumab combined with chemotherapy has been recommended by the National Comprehensive Cancer Network guidelines as a first-line therapy for advanced gastric carcinoma/ adenocarcinoma of the gastroesophageal junction and serves as the basis for immunotherapy combined with chemotherapy to become a neoadjuvant therapy. Herein, we report a case in which pathologic complete response was achieved by neoadjuvant administration of toripalimab, Herceptin, and docetaxel, oxaliplatin, calcium folinate, and fluorouracil (FLOT) chemotherapy followed by surgery for human epidermal growth factor receptor 2 (HER2)- and programmed death-ligand 1 (PD-L1)-positive locally advanced gastric carcinoma. We hope that this case will shed some light on neoadjuvant therapy for gastric carcinoma.
The patient was diagnosed with locally advanced adenocarcinoma of the cardia. Immunohistochemistry of the baseline tissues suggested that the tissues were HER2- (fluorescent in situ hybridization) and PD-L1-positive (combined positive score = 1). The patient underwent surgery following a four-cycle neoadjuvant therapy comprising Herceptin, toripalimab, and FLOT chemotherapy. The postoperative pathological findings showed mild atypical hyperplasia of the local glands with chronic mucosal inflammation (proximal stomach), no clear residual tumor (tumor regression grade 0), no regional lymph node metastasis, and negative upper and lower cut ends. The levels of tumor markers were reduced to normal levels after re-examination. With good postoperative recovery, the four-cycle preoperative chemotherapy was continued at the same dosage as that previously administered. After the treatment, the patient was monitored every 3 mo with a follow-up of 12 mo (4 times). As of February 27, 2022, he was in a good condition without disease progression. The clinical trial registration number is E2019401.
There are many ongoing studies on neoadjuvant immunotherapy combined with chemotherapy or radiotherapy; however, most of these studies are phase II studies with small cohorts. According to the results of some current studies, these combined regimens have shown promising results in terms of efficacy and safety. However, the clinical efficacy and safety of the neoadjuvant therapies used in these combined regimens need to be confirmed by additional prospective phase III clinical trials, and further exploration of molecular markers for effective populations is required.
Core Tip: We report a case in which pathologic complete response was achieved by neoadjuvant administration of toripalimab, Herceptin, and docetaxel, oxaliplatin, calcium folinate, and fluorouracil chemo
- Citation: Liu R, Wang X, Ji Z, Deng T, Li HL, Zhang YH, Yang YC, Ge SH, Zhang L, Bai M, Ning T, Ba Y. Toripalimab combined with targeted therapy and chemotherapy achieves pathologic complete response in gastric carcinoma: A case report. World J Clin Cases 2022; 10(18): 6184-6191
- URL: https://www.wjgnet.com/2307-8960/full/v10/i18/6184.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i18.6184
Gastric carcinoma has a high incidence in China. Surgery might be a radical cure for gastric carcinoma; however, it is limited to only early-stage gastric carcinoma (stage I). The 5-year survival rate of patients with locally advanced gastric carcinoma (late stage) is 30%–50%[1], even with an extended area of resection and lymph node dissection. Many studies have confirmed that the combination of adjuvant/neoadjuvant chemotherapy/chemoradiotherapy can improve patients’ prognosis, enhance R0 resection rates, reduce distant metastases and recurrence rates, and improve survival rates through tumor downstaging[2-4]. In the European randomized controlled phase III AIO-fluorouracil (FLOT)-4 trial, resectable gastric carcinoma patients received either the FLOT (docetaxel, oxaliplatin, calcium folinate, and fluorouracil) or epirubicin, cisplatin, and fluorouracil (ECF) regimen before and after surgery. The results showed that the FLOT regimen had better efficacy, higher R0 resection rate, better disease-free survival (DFS), and better overall survival (OS) than the ECF regimen, which laid the foundation for the FLOT regimen to become a new standard perioperative therapy for advanced gastric carcinoma. Therefore, a regimen that combines surgery with neoadjuvant or perioperative chem
The KEYNOTE-059[5] and ATTRACTION-02[6] trials have suggested that programmed death-1 (PD-1) inhibitors are effective for advanced gastric carcinoma/adenocarcinoma of the gastroesophageal junction. The United States Food and Drug Administration (FDA) and the National Medical Products Administration have approved the indications for pembrolizumab in patients with a programmed death-ligand 1 (PD-L1) combined positive score (CPS) ≥ 1 and nivolumab in the third-line and posterior-line treatment of advanced gastric carcinoma. The CheckMate-649[7] and ATTRACTION-04[8] trials have revealed that nivolumab plus chemotherapy has significantly better efficacy than chemotherapy alone in the first-line treatment of advanced gastric carcinoma/adenocarcinoma. In China, as the first approved immunotherapy targeting PD-1, toripalimab (JS001) induces the endocytosis of PD-1, reduces the expression of PD-1 on the membrane surface, and relieves the immunosuppression of T cells, thereby achieving strong antitumor effects. In 2020, the American Society of Clinical Oncology reported the clinical response and biomarker analysis of first-line toripalimab combined with standard chemotherapy for solid tumors in a phase II cohort study[9]. The study found that the objective response rate (ORR) was 54.5%, the disease control rate was 84.8%, and the duration of response was 8.3 mo. Moreover, in the randomized controlled phase III KEYNOTE-585[10] trial, which is currently enrolling patients, therapy-naive patients with locally advanced gastric carcinoma/adenocarcinoma of the gastroesophageal junction in the experimental group will receive pembrolizumab combined with neoadjuvant chemotherapy before surgery and pembrolizumab combined with adjuvant chemotherapy after surgery, whereas those in the control group will receive placebo combined with chemotherapy.
Human epidermal growth factor receptor 2 (HER2), also known as erythroblastic oncogene B2 (ERBB2), is a proto-oncogenic protein encoded by the ERBB2 gene on human chromosome 17. Tyrosine kinase receptor that binds to the membrane is a protein product of this gene. This receptor can promote cell proliferation and inhibit apoptosis, leading to neoplasm formation[11]. HER2 overexpression or amplification is found in 13%–22% of patients with gastric carcinoma or adenocarcinoma of the esophagogastric junction[12]. Immunohistochemistry (IHC) staining and fluorescent in situ hybridization (FISH) are recommended by the guidelines for the detection of HER2 overexpression in patients with advanced gastric adenocarcinoma. In 2010, trastuzumab was approved by the FDA as a first-line drug in combination chemotherapy for HER2-positive gastric carcinoma. In another study[13], preliminary results were obtained for combined immunotherapy, trastuzumab, and chemotherapy for gastric carcinoma/esophageal cancer/adenocarcinoma of the esophagogastric junction. The study found that the 6-mo progression-free survival (PFS) rate was 75%, the ORR was 91%, the median PFS was 13 mo, and the median OS was 27.3 mo. The above data were better than the previous data for HER2-positive advanced gastric carcinoma.
In this study, we report a case in which pathologic complete response (pCR) was achieved by neoadjuvant toripalimab, Herceptin, and FLOT chemotherapy followed by surgery for HER2- and PD-L1-positive locally advanced gastric carcinoma. We hope to provide more evidence for neoadjuvant therapies in gastric carcinoma patients by reporting this case.
A 63-year-old male patient experienced dysphagia, poor appetite, night sweats, and fatigue on July 2, 2020, and sought medical attention at the Hulunbuir People’s Hospital in Inner Mongolia, China.
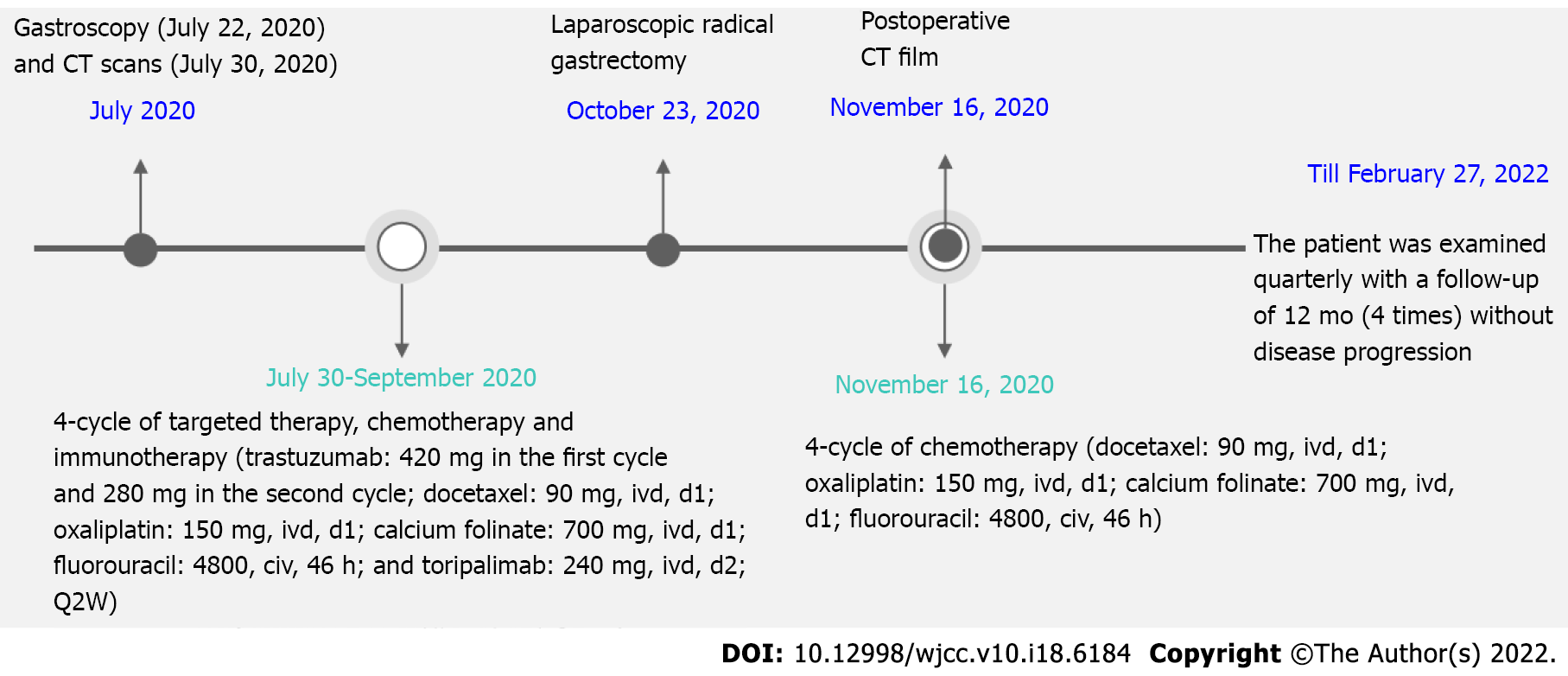
On July 22, 2020, gastroscopy revealed protuberant lesions in the cardia and fundus; bite biopsy revealed adenocarcinoma of the cardia (Figure 1A); IHC revealed HER2 positivity (2+, FISH was recommended); in situ hybridization revealed EBER (−); and FISH revealed HER2 positivity (Figure 1B).
The patient has been suffering from hepatitis B virus (HBV) infection for more than 30 years, without history of hypertension, coronary heart disease, diabetes, or tuberculosis.
The patient’s family history is not applicable.
The tissues obtained from the bite biopsy were tested for PD-1, revealing CPS positivity (CPS = 1) (Figure 1C) and TPS negativity.
On July 22, 2020, gastroscopy revealed protuberant lesions in the cardia and fundus; bite biopsy revealed adenocarcinoma of the cardia; IHC revealed HER-2 positivity (2+, FISH was recommended); in situ hybridization revealed EBER (−); and FISH revealed HER2 positivity. Next-generation sequencing (NGS) revealed tumor protein p53 (TP53) c.329G>C p.Arg110Pro (abundance 33.82%), and ERBB2 copy number amplification (n = 4.5).
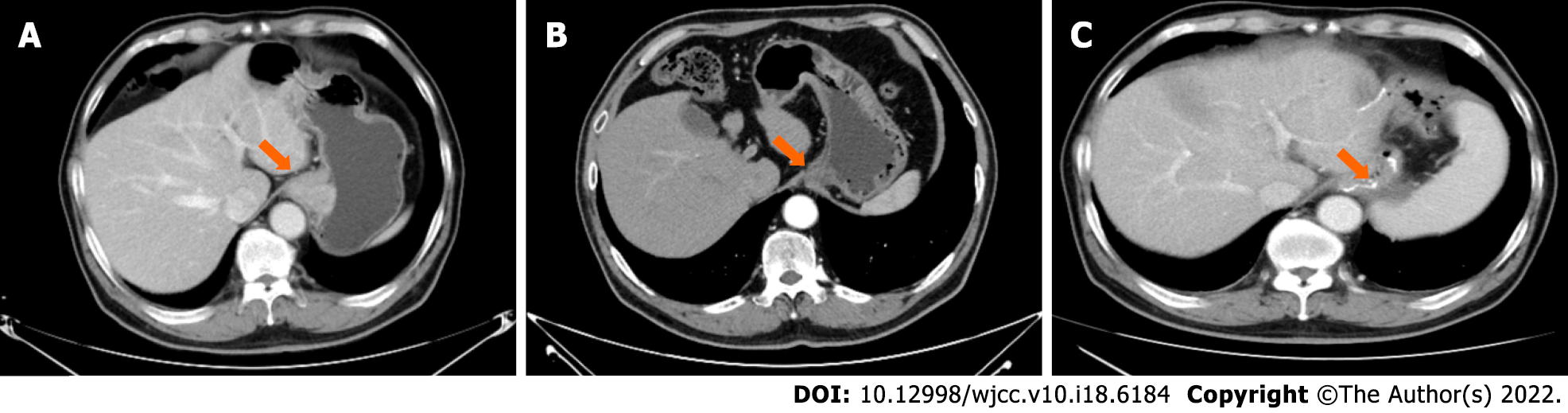
On July 30, 2020, a computed tomography (CT) scan was performed (Figure 2A).
(1) Thickened wall of the cardia and adjacent lesser curvature of the stomach, suggestive of carcinoma of the cardia, and invaded fundus and multiple lymph nodes in the hepatogastric ligament region, for which clinical and endoscopic examination needed to be performed; (2) Multiple cysts in the liver; (3) Cyst of the right kidney; (4) Slightly thickened left adrenal gland, with follow-up visits recommended; (5) Prostatic calcification; (6) Subpleural ground-glass opacity in the right lung and scattered nodules and granules on the pleura of both lungs and under the interlobar pleura, with follow-up visits recommended; and (7) A dot-like compact shadow on the 5th right rib, for which follow-up visits were recommended.
From July 30, 2020 to September 2020, four-cycle targeted therapy, chemotherapy, and immunotherapy were administered. The specific regimen was as follows: Trastuzumab: 420 mg in the first cycle and 280 mg in the second cycle; docetaxel: 90 mg, ivd, d1; oxaliplatin: 150 mg, ivd, d1; calcium folinate: 700 mg, ivd, d1; fluorouracil: 4800, civ, 46 h; and toripalimab: 240 mg, ivd, d2; Q2W. Grade I gastrointestinal reaction occurred and improved after symptomatic treatment.
The imaging findings (CT on October 15, 2020, compared with that on July 30, 2020) after the four cycles of therapy were as follows (Figure 2B): The wall thickness of the cardia and adjacent lesser curvature was less than that before therapy; lymph nodes in the hepatogastric ligament region were reduced in size; and subpleural infiltration in the right lower lobe was more absorbed. No other significant changes were noted. Upper gastrointestinal tract radiography revealed carcinoma of the cardia.
Under general anesthesia, the patient underwent laparoscopic radical D2 gastrectomy for gastric carcinoma on October 23, 2020. Surgical findings revealed a neoplasm at the fundus of the stomach from the cardia, which presented as a 4 cm × 2 cm ulcer with local serosal invasion. No significantly enlarged lymph nodes were found around the stomach. The lesions presented post-chemotherapy scar-like changes. Multiple small lymph nodes were noted around the stomach, most of which were post-chemotherapy changes. The postoperative pathological findings revealed focal (proximal stomach) mild atypical glandular hyperplasia with chronic mucosal inflammation, no clear residual tumor (tumor regression grade 0), no regional lymph node metastasis, and negative upper and lower cut ends. The grading was as follows: Station 1 0/9, Station 2 0/6, Station 3A 0/12, Station 3B 0/2, Station 4SA soft tissue (−), Station 4SB 0/1 and soft tissue (−), Station 4D 0/2, Station 5 soft tissue (−), Station 6 0/1, Station 7 soft tissue (−), Station 8 soft tissue (−), Station 9 0/4, Station 12A soft tissue (−), Station 19 0/1, and Station 20 0/2. No circulating tumor microemboli or circulating tumor cells were detected. A retest of tumor markers showed a return to normal levels.
With good postoperative recovery, the postoperative CT film (performed on November 16, 2020, Figure 2C) was stored, and the four-cycle chemotherapy regimen was continued at the same dosage as that administered previously. Until February 27, 2022, the patient was examined quarterly for 12 mo (4 times), and he was in a good condition without disease progression. The timeline of this case report is indicated in Figure 3.
In a study of combined immunotherapy and trastuzumab treatment for HER2-positive gastric carcinoma[13], 25 patients received immunotherapy and targeted therapy as the initial treatment and chemotherapy in the second cycle, whereas 12 patients received immunotherapy, targeted therapy, and chemotherapy as the initial treatment. In the initial treatment, no significant difference was observed in PFS and 12-mo OS between the 25-patient and 12-patient groups. While in our case, the patient did not receive chemotherapy in the initial treatment; more specifically, the patient received Herceptin and toripalimab in the first cycle and chemotherapy in the second cycle. Previous studies have found no difference in survival between groups receiving chemotherapy and groups not receiving chemotherapy in the initial treatment. Our study suggested that, for HER2-positive gastric carcinoma patients, it is worthy to further evaluate whether the first-line “de-chemotherapy” can be carried out with a large cohort sample.
It is believed that the basis for immunotherapy to benefit HER2-positive patients is that trastuzumab induces antibody-dependent cell-mediated cytotoxicity, improves the presentation of tumor antigens, and paves the way for immune reaction of tumors[14]. Clinical data also show that HER2-positive breast cancer has more types of tumor-infiltrating lymphocytes than average, which demonstrates the importance of trastuzumab in immunity induction[15]. In addition, studies have confirmed that trastuzumab can increase the expression level of PD-L1 in immune cells of patients with breast cancer[16]. Combined with the encouraging clinical outcome of this patient, it is promising to investigate the systemic immune responses in depth, for instance, the lymphocyte infiltration, immune marker dynamics, and functional cytokine secretion. Moreover, this patient has been infected with HBV for many years, which may have an impact on his immune system. Large cohorts are necessary to draw conclusions regarding this aspect if feasible.
There are many ongoing studies of neoadjuvant immunotherapy combined with chemotherapy or radiotherapy; however, most of these studies are phase II studies with small cohorts. According to some of the results reported thus far, these combined regimens have shown promising efficacy and safety[17-19] (Table 1). Many studies have shown that chemotherapy can: (1) Boost the release of damage-associated molecular patterns from tumor cells and improve tumor cell immunogenicity; and (2) Elevate the levels of major histocompatibility complex molecules and enhance tumor antigen presentation[20]. Additionally, chemotherapy promotes the expression of PD-1/PD-L1 through a variety of signaling pathways. Therefore, in this case, chemotherapy and immunotherapy were applied for the patient before the surgical removal. For gastric carcinoma, first-line immunotherapy combined with chem
| Regimen | Neoplasm | Number of participants | pCR rate | MPR | Ref. |
| Camrelizumab plus FOLFOX | Locally advanced gastric carcinoma and adenocarcinoma of the esophagogastric junction | 26 | 2 (9%) | 8 (36%) | [17] |
| Sintilimab plus FLOT | Gastric and adenocarcinoma of the esophagogastric junction | 17 | 3/17 (17.6%) | 10/17 (58.8%) | [18] |
| Sintilimab plus CapeOX | Locally advanced resectable gastric/adenocarcinoma of the esophagogastric junction | 26 | 6/26 (23.1%) | 14/26 (53.8%) | [19] |
Apart from the above aspects, TP53 is a crucial tumor suppressor gene, and TP53 mutation occurs at an incidence of approximately 45% in gastric carcinoma[21]. The efficacy of immunotherapy varies with the TP53 mutation. According to the retrospective meta-analysis mentioned above, TP53 mutation was negatively correlated with the patients’ OS with either colon cancer or gastric carcinoma who received immunotherapy but was positively correlated with the efficacy of immunotherapy for lung cancer[21]. In this study, TP53 c.329G>C p.Arg110Pro mutation was detected using NGS in the baseline tissues of the patient. Therefore, additional prospective cohort studies are required to conclude and explore the correlation between TP53 mutation and the efficacy of immunotherapy for gastric carcinoma.
Here, we demonstrated that in a patient with HER2-positive locally advanced gastric carcinoma, there was scope for resection; therefore, a regimen composed of Herceptin, chemotherapy, and immunotherapy was carefully selected to achieve higher efficacy and better surgical resection. The patient was administered with the perioperative regimen comprising Herceptin, FLOT, and toripalimab. The postoperative pathological findings revealed that this regimen led to complete tumor response and the levels of tumor biomarkers returned to normal. Furthermore, no circulating tumor cell was detected and no significant immune-related adverse effects were noted, demonstrating that this regimen had sufficient efficacy and safety. The four-cycle chemotherapy was continued postoperatively and completed in the patient, in line with the principle of “effective treatment should be continued if the symptoms are relieved”. This patient is currently in the quarterly follow-up period. A previous study suggested that the ability to achieve postoperative pCR in patients with neoadjuvant therapy is positively correlated with longer durations of survival[22]. Thus far, all tumors have been removed from this patient using this regimen, and we hope that this regimen will lead to long-time survival benefits.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Arigami T, Japan; Lal A, United States; Marickar F, India S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR
| 1. | Sehdev A, Catenacci DV. Perioperative therapy for locally advanced gastroesophageal cancer: current controversies and consensus of care. J Hematol Oncol. 2013;6:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, MAGIC Trial Participants. Perioperative chemotherapy vs surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4609] [Article Influence: 242.6] [Reference Citation Analysis (0)] |
| 3. | Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B, Genève J, Lasser P, Rougier P. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1216] [Cited by in RCA: 1504] [Article Influence: 107.4] [Reference Citation Analysis (0)] |
| 4. | Schuhmacher C, Gretschel S, Lordick F, Reichardt P, Hohenberger W, Eisenberger CF, Haag C, Mauer ME, Hasan B, Welch J, Ott K, Hoelscher A, Schneider PM, Bechstein W, Wilke H, Lutz MP, Nordlinger B, Van Cutsem E, Siewert JR, Schlag PM. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol. 2010;28:5210-5218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 533] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 5. | Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges JP, Garrido M, Golan T, Mandala M, Wainberg ZA, Catenacci DV, Ohtsu A, Shitara K, Geva R, Bleeker J, Ko AH, Ku G, Philip P, Enzinger PC, Bang YJ, Levitan D, Wang J, Rosales M, Dalal RP, Yoon HH. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018;4:e180013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1413] [Cited by in RCA: 1461] [Article Influence: 208.7] [Reference Citation Analysis (0)] |
| 6. | Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Chen LT. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461-2471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1283] [Cited by in RCA: 1715] [Article Influence: 214.4] [Reference Citation Analysis (0)] |
| 7. | Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, Liu T, Schenker M, Yanez P, Tehfe M, Kowalyszyn R, Karamouzis MV, Bruges R, Zander T, Pazo-Cid R, Hitre E, Feeney K, Cleary JM, Poulart V, Cullen D, Lei M, Xiao H, Kondo K, Li M, Ajani JA. First-line nivolumab plus chemotherapy vs chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1962] [Cited by in RCA: 1902] [Article Influence: 475.5] [Reference Citation Analysis (1)] |
| 8. | Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC, Chung HC, Lee KW, Omori T, Shitara K, Sakuramoto S, Chung IJ, Yamaguchi K, Kato K, Sym SJ, Kadowaki S, Tsuji K, Chen JS, Bai LY, Oh SY, Choda Y, Yasui H, Takeuchi K, Hirashima Y, Hagihara S, Boku N. Nivolumab plus chemotherapy vs placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23:234-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 488] [Article Influence: 162.7] [Reference Citation Analysis (0)] |
| 9. | Ren C, Wei XL, Xu N, Shen L, Dai GH, Yuan XL, Chen Y, Yang SJ, Shi JH, Hu XC, Lin XY, Zhang QY, Feng JF, Ba Y, Liu YP, Liu W, Shu YQ, Xu RH, Shanghai Junshi Biosciences. Clinical response and biomarker analysis of a phase II basket trial of toripalimab, a PD-1 mAb in combination with standard chemotherapy as a first-line treatment for patients with solid tumors. 2020 ASCO Annual Meeting I. 2020;38:15. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Bang YJ, Van Cutsem E, Fuchs CS, Ohtsu A, Tabernero J, Ilson DH, Hyung WJ, Strong VE, Goetze TO, Yoshikawa T, Tang LH, Hwang PMT, Webb N, Adelberg D, Shitara K. KEYNOTE-585: Phase III study of perioperative chemotherapy with or without pembrolizumab for gastric cancer. Future Oncol. 2019;15:943-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 128] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 11. | Ménard S, Pupa SM, Campiglio M, Tagliabue E. Biologic and therapeutic role of HER2 in cancer. Oncogene. 2003;22:6570-6578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 298] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 12. | Grillo F, Fassan M, Sarocchi F, Fiocca R, Mastracci L. HER2 heterogeneity in gastric/gastroesophageal cancers: From benchside to practice. World J Gastroenterol. 2016;22:5879-5887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 108] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 13. | Janjigian YY, Maron SB, Chatila WK, Millang B, Chavan SS, Alterman C, Chou JF, Segal MF, Simmons MZ, Momtaz P, Shcherba M, Ku GY, Zervoudakis A, Won ES, Kelsen DP, Ilson DH, Nagy RJ, Lanman RB, Ptashkin RN, Donoghue MTA, Capanu M, Taylor BS, Solit DB, Schultz N, Hechtman JF. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21:821-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 265] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 14. | Melero I, Berman DM, Aznar MA, Korman AJ, Pérez Gracia JL, Haanen J. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat Rev Cancer. 2015;15:457-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 523] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 15. | Savas P, Loi S. Investigating the positive relationship between tumor-infiltrating lymphocytes and trastuzumab therapy. Immunotherapy. 2014;6:803-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Chaganty BKR, Qiu S, Gest A, Lu Y, Ivan C, Calin GA, Weiner LM, Fan Z. Trastuzumab upregulates PD-L1 as a potential mechanism of trastuzumab resistance through engagement of immune effector cells and stimulation of IFNγ secretion. Cancer Lett. 2018;430:47-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 144] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 17. | Liu Y, Han G, Li H. Camrelizumab combined with FOLFOX as neoadjuvant therapy for resectable locally advanced gastric and gastroesophageal junction adenocarcinoma. J Clin Oncol. 2020;38 suppl 15:4536. |
| 18. | Li N, Li Z, Fu Q, Zhang B, Luo S. Phase II study of sintilimab combined with FLOT regimen for neoadjuvant treatment of gastric or gastroesophageal junction (GEJ) adenocarcinoma. J Clin Oncol. 2021;39 suppl 3:216. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Jiang H, Yu X, Kong M, Ma Z, Zhou D, Wang W, Wang H, Li N, He K. Sintilimab plus oxaliplatin/capecitabine (CapeOx) as neoadjuvant therapy in patients with locally advanced, resectable gastric (G)/esophagogastric junction (GEJ) adenocarcinoma. J Clin Oncol. 2021;39 suppl 3:211. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Heinhuis KM, Ros W, Kok M, Steeghs N, Beijnen JH, Schellens JHM. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann Oncol. 2019;30:219-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 389] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 21. | Li L, Li M, Wang X. Cancer type-dependent correlations between TP53 mutations and antitumor immunity. DNA Repair (Amst). 2020;88:102785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 22. | Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science. 2020;367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 704] [Article Influence: 140.8] [Reference Citation Analysis (0)] |