Published online Jun 26, 2022. doi: 10.12998/wjcc.v10.i18.6175
Peer-review started: November 3, 2021
First decision: November 19, 2021
Revised: November 25, 2021
Accepted: March 29, 2022
Article in press: March 29, 2022
Published online: June 26, 2022
Processing time: 225 Days and 10 Hours
Clear cell sarcoma-like tumor of the gastrointestinal tract (CCSLGT) is a rare malignant gastrointestinal mesenchymal soft tissue tumor. Its genetic feature is EWSR1 gene rearrangement. Histologically, it is often accompanied by a varying number of CD68-positive osteoclast-like giant cells. CCSLGT mostly occurs in the small intestinal wall of young people and children. In terms of clinical manifestations, there is no significant difference between it and other gastrointestinal tumors, and the diagnosis depends on immunohistochemistry and gene detection.
A 16-year-old man developed dizziness and fatigue 2 mo ago, and 10 d ago showed progressive exacerbation of paroxysmal epigastric pain and stopped flatulence and defecation. Computed tomography showed a soft tissue mass in the distal ileum. After complete resection of the lesion, it was diagnosed by combined immunohistochemical and genetic examination as CCSLGT. After surgery, the patient gradually developed lymph node, liver, lung, bone, left thigh, pleura and adrenal metastasis. The survival time was 4 years and 8 mo.
Whole abdominal computed tomography enhancement is recommended for patients with gastrointestinal symptoms. There is no effective treatment for CCSLGT with multiple metastases via the lymphatic system and bloodstream after surgical resection.
Core Tip: Clear cell sarcoma-like tumor of the gastrointestinal tract (CCSLGT) is a rare malignant mesenchymal tumor with unique morphological, immunophenotypic and molecular genetic characteristics. Its clinical manifestations are unspecific, and the diagnosis depends on immunohistochemistry and gene testing. Positron emission tomography/computed tomography is recommended for patients with CCSLGT, which can provide functional and metabolic information in addition to anatomical information, and effectively reduce missed lesions. Currently, there is no effective treatment for CCSLGT.
- Citation: Huang WP, Li LM, Gao JB. Postoperative multiple metastasis of clear cell sarcoma-like tumor of the gastrointestinal tract in adolescent: A case report. World J Clin Cases 2022; 10(18): 6175-6183
- URL: https://www.wjgnet.com/2307-8960/full/v10/i18/6175.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i18.6175
Clear cell sarcoma-like tumor of the gastrointestinal tract (CCSLGT) is a rare malignant mesenchymal soft tissue tumor that can occur in any part of the gastrointestinal tract, mostly in the small intestinal wall, followed by the stomach, colon and peritoneum[1,2]. Zambrano et al[3] first reported in 2003 that, because of the characteristic appearance of osteoclast-like giant cells in the tumor, they believed that this type of tumor had some but not all of the characteristics of soft tissue clear cell sarcoma. The etiology and pathogenesis of CCSLGT are not clear. Its genetic feature is EWSR1 gene rearrangement, which is seen in 81% of cases[4]. Histologically, CCSLGT shows diffuse sheet-like or irregular nest-like arr
A 16-year-old man had dizziness and fatigue without obvious cause 2 mo ago. After strenuous exercise 15 d ago, dizziness was aggravated, with nausea and tinnitus.
Laboratory test results at a local hospital showed neutrophils (6.35 × 109/L, normal level 1.80 × 109-6.30 × 109/L), erythrocytes (3.91 × 1012/L, normal level 4.80 × 109–5.80 × 109/L), platelets (486 × 1012/L, normal level 100 × 109-300 × 109/L), high sensitivity C-reactive protein (188.0 mg/L, normal level 0.5-10 mg/L), fecal occult blood test positive, and gastroscopy suggested erosive gastritis. No obvious abnormality was found on colonoscopy. The patient was diagnosed with gastrointestinal bleeding and hemorrhagic anemia. The patient presented with tolerable paroxysmal epigastric pain 10 d prior to hospital visit, but the abdominal pain worsened progressively when eating 10 h previously, and he came to our hospital for medical treatment. He had normal spirit and appetite, poor sleep, and normal defecation and urination, and a weight loss of 3 kg.
The patient was in good health.
The patient had no family history of hereditary diseases.
On admission, he had pressure pain in the wall of the upper abdomen, and no rebound tenderness, bowel sounds at 8 beats/min.
Laboratory test results showed elevated fibrinogen (5.72 g/L, normal level 2–4 g/L), but no obvious abnormality was found in tumor markers.
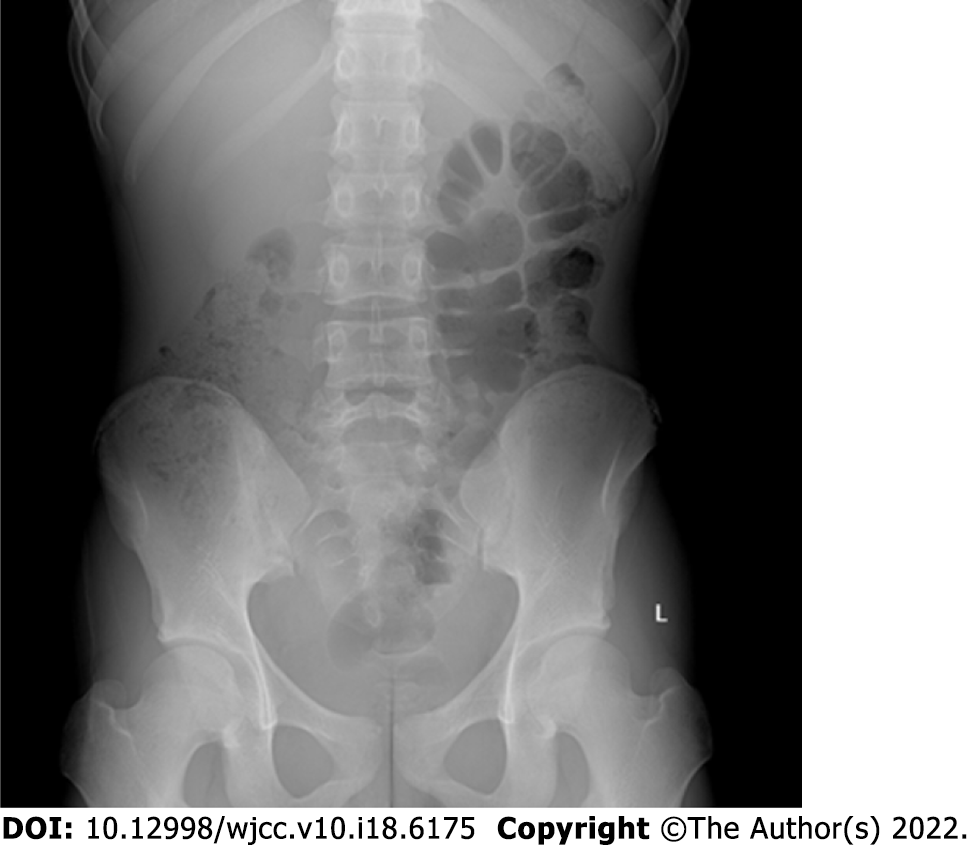
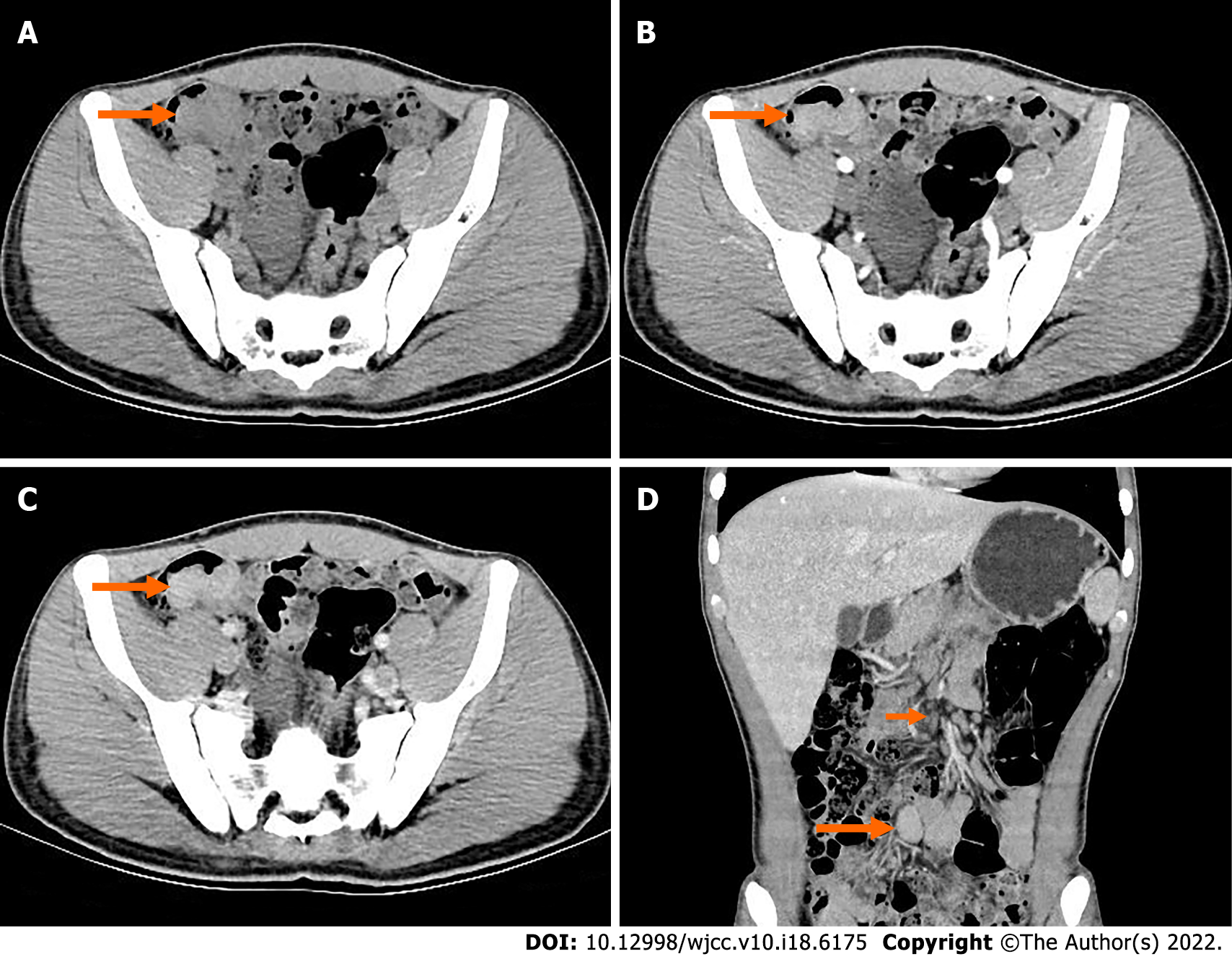
Abdominal digital radiography did not show any dilated loop of bowel to suggest bowel obstruction (Figure 1). Further contrast-enhanced computed tomography (CT) of the abdomen and pelvis showed obvious localized thickening of the ileal wall in the right lower abdomen, with soft tissue density mass, involving the intestinal wall with a length of approximately 4.1 cm, plain scan CT value of approximately 59 HU (Figure 2A), arterial phase CT value of approximately 78 HU (Figure 2B) and venous phase CT value of approximately 97 cm (Figure 2C), showing moderate uniform progressive enha
Combined with pathological morphology, immunohistochemistry and gene detection, the tumor was diagnosed as CCSLGT.
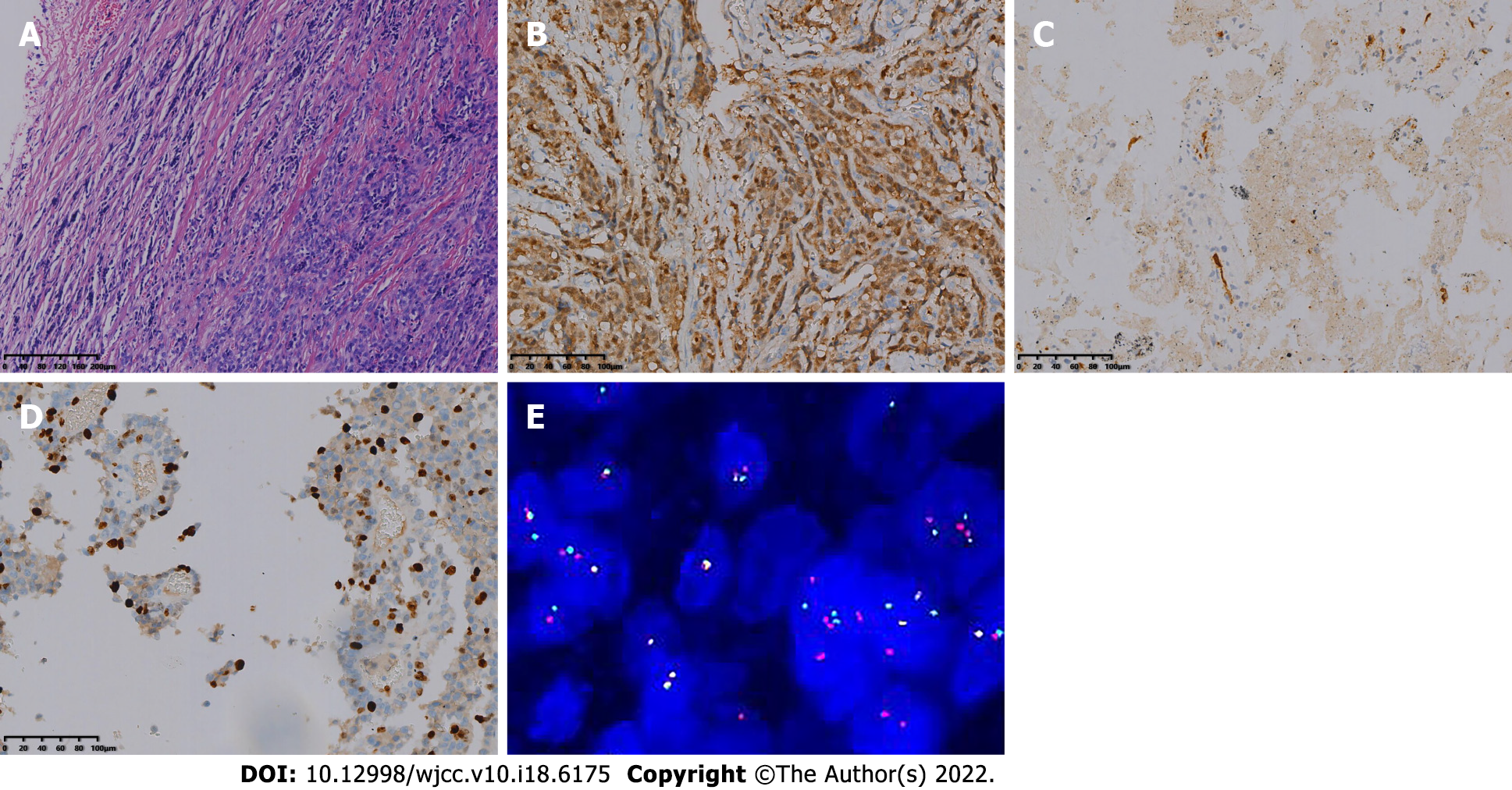
The patient underwent resection of the small intestinal masses. During the operation, a solid and hard protuberant tumor was located in the ileum approximately 15 cm from the ileocecal part. The tumor of about 5 cm × 3.5 cm × 2 cm invaded the whole layer of the intestine; did not invade the surrounding organs; enlarged lymph nodes were seen at the root of the mesentery, with a size about 2 cm × 1 cm; dark red content could be seen in the distal intestine, and old bleeding was considered; and no abnormality was found in the liver and pelvis. The tumor and part of the small intestine were removed at 5 cm from both ends of the lesion, and the mesenteric and perienteric fat lymph nodes were dissected. The postoperative specimens were sent for pathological examination. Under light microscopy, the tumor cells were diffusely arranged and separated by a slender fibrous septum (Figure 3A). Immunohistochemical detection: AE1/AE3 (-), CK7 (focal ±), Smur100 (+) (Figure 3B), MelanA (-), HIB45 (-), CD34 (vascular +) (Figure 3C), CD117 (-), Dog-1 (-), CDX-2 (-), Villin (-), CK8/18 (-), LCA (-), CD10 (-), TFE-3
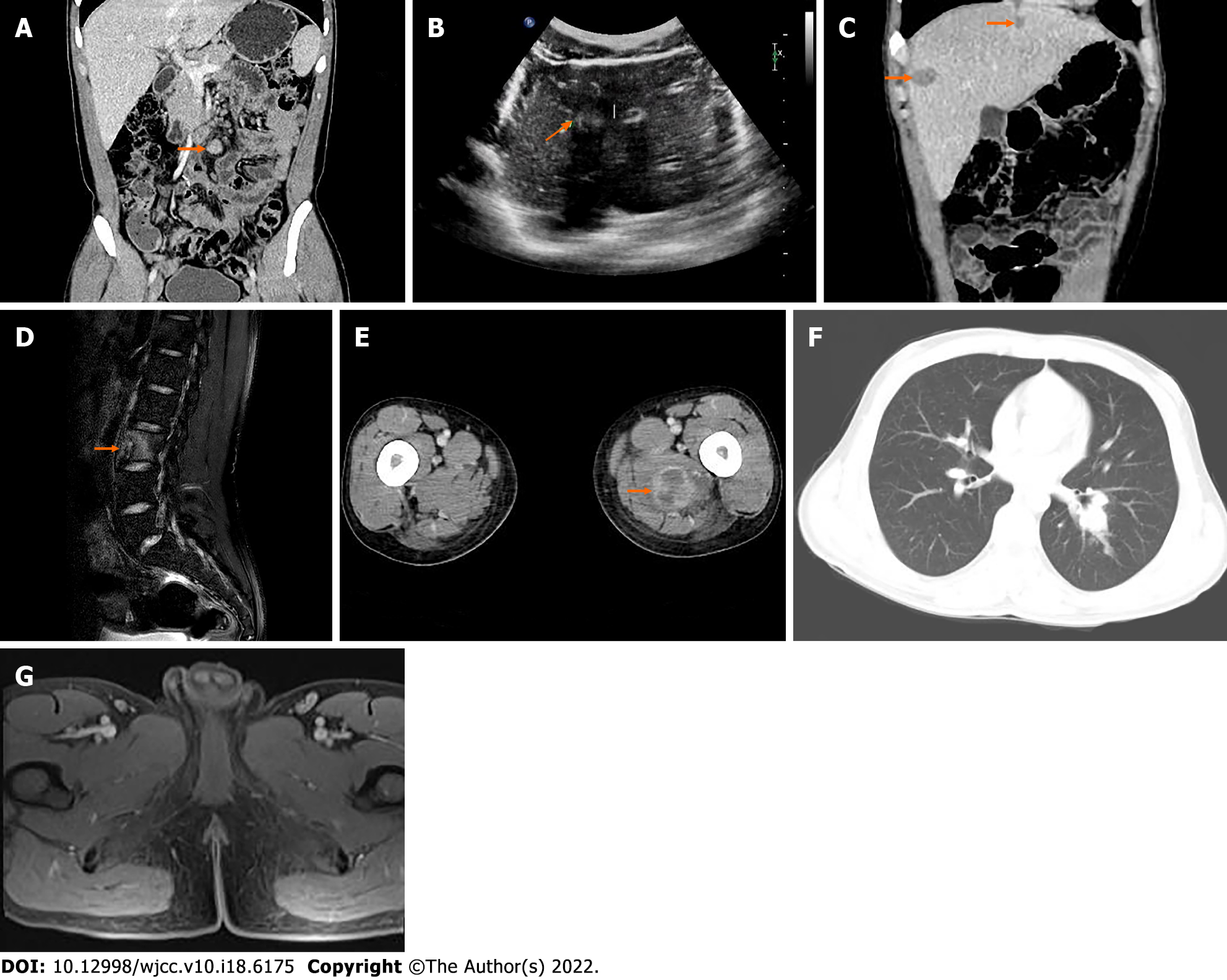
The patient was followed up regularly without adjuvant treatment. After 13 mo, he stopped defecation and flatulence and came to our hospital because of intermittent severe abdominal pain. CT examination showed multiple enlarged lymph nodes at the root of the mesentery (Figure 4A). Clinicians considered metastasis and carried out abdominal exploration. Tumors of 6.0 cm × 4.0 cm × 3.0 cm and 1.5 cm × 1.0 cm × 0.5 cm were found at the root of the retroperitoneal small intestine.
After surgical resection, the specimens were sent for pathological examination, combined with medical history and immunohistochemistry in accordance with CCSLGT mesenteric lymph node metastasis. Ultrasonography (US) 27 mo after the operation showed that the intrahepatic echo was hyperechoic (Figure 4B), and the internal echo was inhomogeneous, with a size of about 1.4 cm × 1.2 cm. There was no obvious blood flow signal on color Doppler flow imaging. Ultrasound-guided percu
Contrast-enhanced US showed multiple areas of solid inhomogeneous hypoechogenicity adjacent to the superior mesenteric artery with clear boundaries. The larger areas were about 3.6 cm × 2.9 cm, and the enhancement was slow and uneven. Solid inhomogeneous areas of hypoechogenicity of 1.3 cm × 1.2 cm and 2.2 cm × 1.9 cm were seen under the capsule of the right anterior lobe and posterior lobe of the liver, respectively. The boundary was unclear, the arterial phase showed circular enhancement, and the enhancement in the portal vein and delayed phases decreased. Ultrasound-guided lymph node biopsy and radiofrequency ablation of enlarged lymph nodes adjacent to the superior mesenteric artery and radiofrequency ablation of liver space were performed. Postoperative pathology combined with medical history and immunohistochemistry were consistent with CCSLGT superior mesenteric artery paramesenteric lymph node metastasis.
At 35 mo after the operation, the patient found a mass on the inside of the left thigh, with hard texture, poor range of motion, traction-like pain in the left thigh, and tension in the posterior lumbar muscles with fluctuating pain, passive posture and limited activity, which lasted for 1 h. The symptoms gradually worsened. Magnetic resonance imaging (MRI) of lumbar vertebrae showed low signal intensity in T12, L1 and L3 vertebrae, slightly high signal intensity in T2-weighted imaging, and high signal intensity in L3 vertebrae on turbo inversion recovery magnitude fat pressing sequence (Figure 4D).
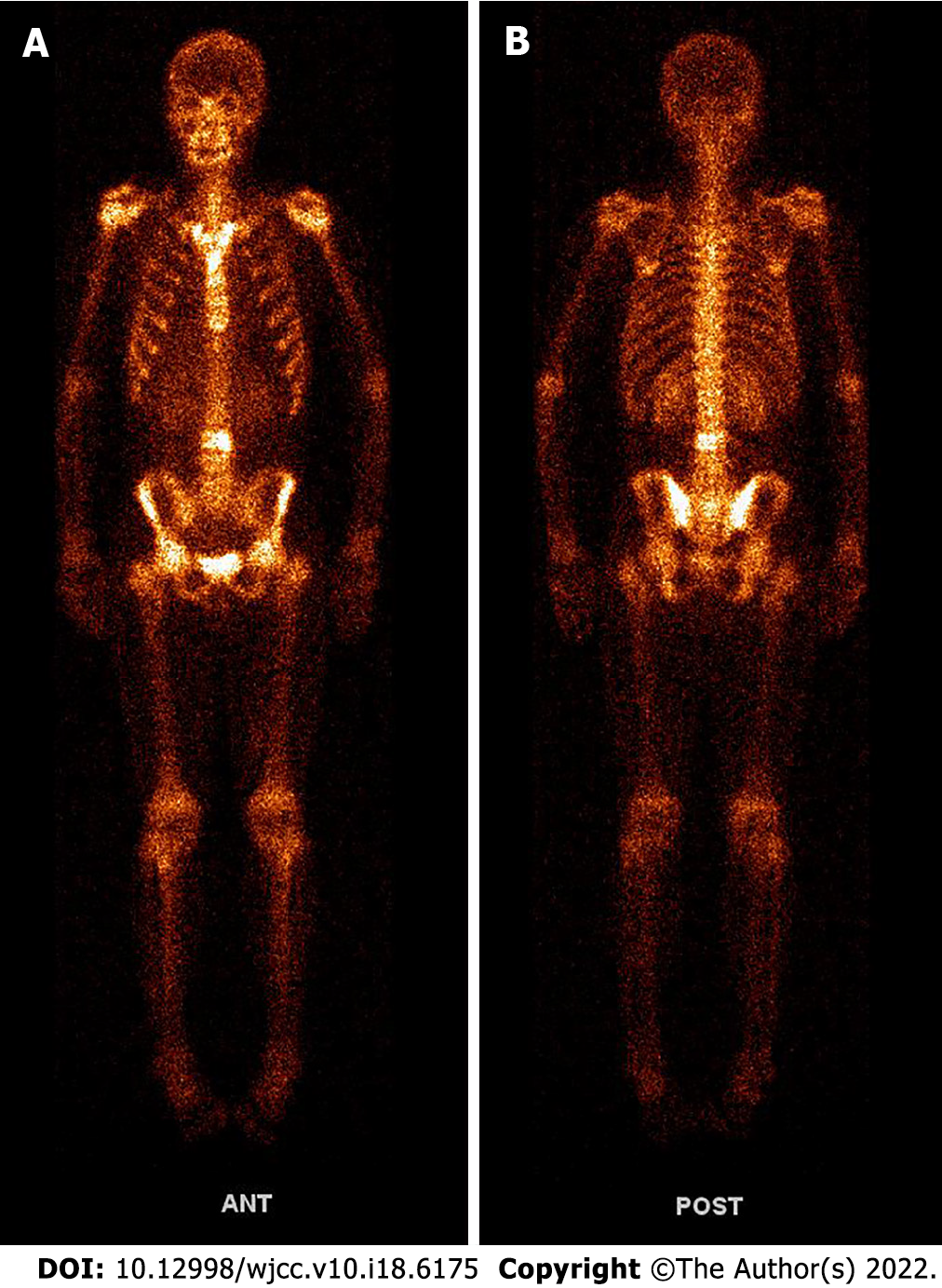
Whole-body 99mTc-methylene diphosphonate bone scintigraphy showed increased radiotracer uptake in L3 vertebral body (Figure 5), and bone metastasis was considered. The patient was treated regularly with intensity-modulated radiotherapy. CT showed more lesions in the liver, unclear cystic-solid, low-density, space-occupying lesion on the inner side of the left thigh, and uneven circular enhancement (Figure 4E). The maximum dimension was about 3.3 cm × 2.9 cm. Ultrasound-guided puncture biopsy and radiofrequency ablation of the mass in the medial thigh and radiofrequency ablation of the liver were performed. Postoperative pathology combined with morphology, immunohistochemistry and previous medical history were consistent with CCSLGT medial metastasis of the left thigh.
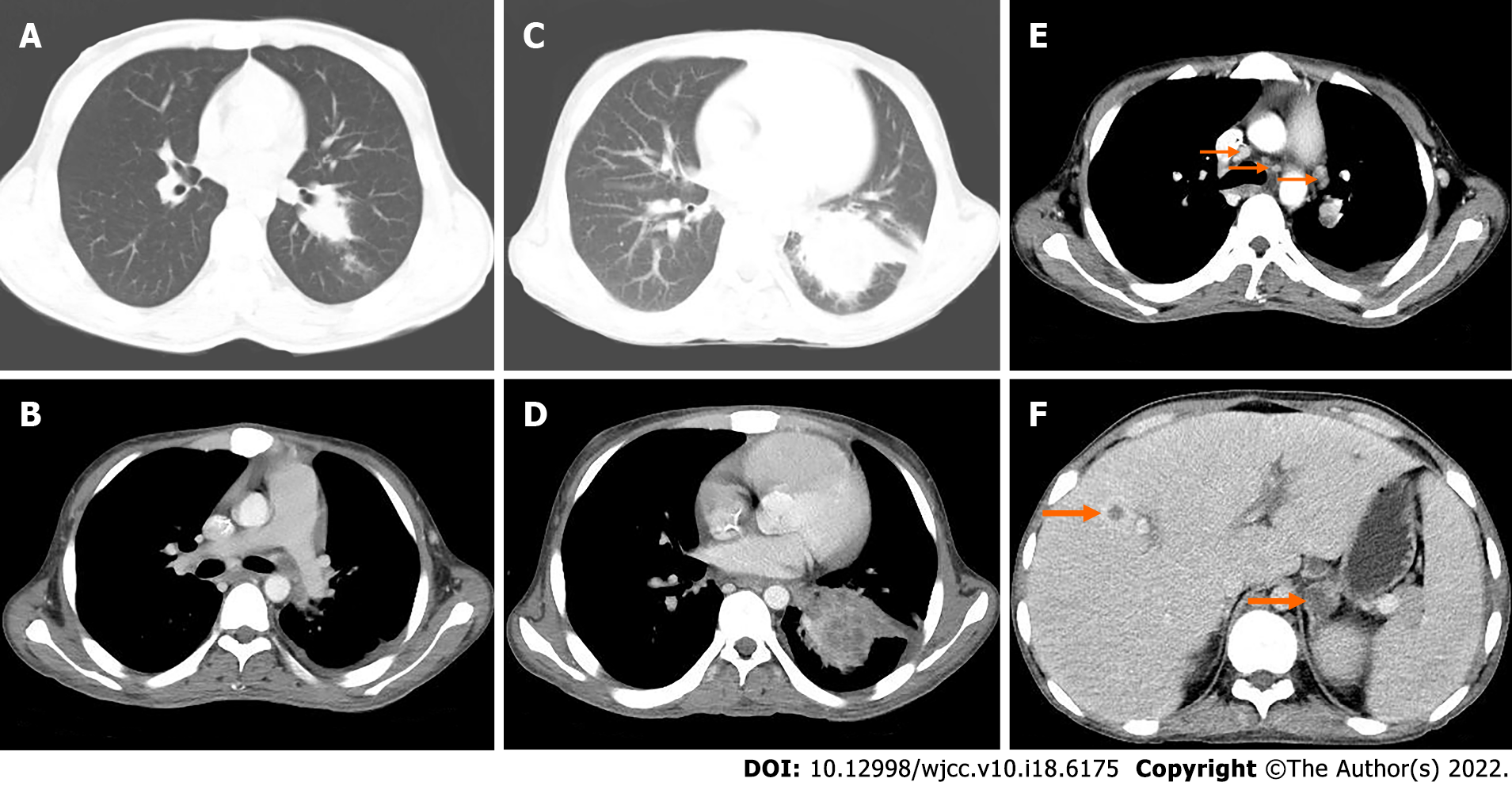
Thirty-nine months after the operation, the patient came to our hospital with recurrent fever, cough and expectoration for > 1 mo. The body temperature fluctuated from 38.0 to 39.5 °C. CT examination showed more multiple small nodules in both lungs than before (Figure 4F).
Forty-three months after the operation, pelvic MRI showed multiple diffuse restricted high signals in the bilateral inguinal area and adjacent iliac vessels, with mild MRI enhancement (Figure 4G). Re-examination by CT showed that the nodule of the left lower lobe was significantly enlarged and the boundary was unclear (Figure 6A). The size of the nodule was 2.5 cm × 3.5 cm, with mild inhomogeneous enhancement, and the left pleural soft tissue nodule showed enhancement (Figure 6B). Bilateral inguinal lymph nodes were enlarged and there was a small amount of fluid density in bilateral pleura and pericardium.
CT examination at 50 mo after the operation showed an irregular mass in the lower lobe of the left lung, which was significantly larger in size than before (Figure 6C) and moderate enhancement (Figure 6D). Other nodules in both lungs were enlarged and increased in number, with multiple mediastinal, left hilar, and axillary lymphadenopathy (Figure 6E). Soft tissue nodules in the left pleura were enlarged, low-density soft tissue masses were seen in the left adrenal gland with a diameter of approximately 2.3 cm, and the boundary was unclear, moderately reinforced in a circular pattern (Figure 6F), with small pleural and pericardial effusion.
At 56 mo after the operation, the patient died of multiple metastases of CCSLGT.
The most common clinical manifestations of CCSLGT are abdominal pain, intestinal obstruction, accompanied by varying degrees of anemia, nausea, vomiting, weight loss and fatigue[2-4]. This patient was in good health, had no family history of tumors, and the symptoms were atypical at the beginning, because the primary tumor of the small intestine was rare. After an occult blood test was positive, the patient was examined by gastroscopy and colonoscopy, but not by enteroscopy. He was diagnosed with erosive gastritis with gastrointestinal bleeding, no small intestinal lesions were found, and antitumor treatment was delayed. Later, the patient developed paroxysmal epigastric pain, stopped flatulence and defecation, and no intestinal obstruction was found by digital radiography. Further CT enhancement found a space-occupying soft tissue mass in the ileum. Therefore, patients with gastrointestinal symptoms should be examined by CT, which is beneficial for the detection of lesions in other parts of the body. The median diameter of CCSLGT is 45 mm, ranging from 15 mm to 135 mm. In general, it is multinodular and can be accompanied by hemorrhage, necrosis or cystic changes[4]. The present case was consistent with reports in the literature.
Histologically, tumor cells infiltrate between the walls of the gastrointestinal tract, often into the mucous membrane and serous layer, forming mucosal ulcers. CCSLGT consists of medium-sized tumor cells arranged in flaky or irregular nest-like structures separated by a slender fibrous diaphragm, pseudopapillary, pseudoglandular and pseudochrysanthemum-shaped structures and myxoid stroma, and occasionally pseudoangioma-like structures. Osteoclast-like multinucleated giant cells are scattered in approximately 50% of the cases[5-7]. Mitotic activity is significantly active in these tumors, accompanied by typical focal necrosis[8]. Stockman et al[7] reviewed and analyzed 16 cases of CCSLGT, and found that the tumor showed neurodifferentiation potential and expressed neuroendocrine markers, indicating that gastrointestinal CCS originated from neuroectodermal precursor cells and may have lost the ability of melanocyte differentiation. Therefore, a new name was proposed, malignant gastrointestinal neuroectodermal tumor.
The imaging findings of CCSLGT have rarely been reported. CT examination of our patient showed that the peripheral wall of the intestinal canal in the distal part of the right lower abdominal ileum was localized and thickened, the density was uniform, and no calcification or cystic necrosis was found. After MPR, the tumor showed strong invasive growth into the intestine, a clear boundary, obvious narrowing of the intestinal cavity and moderate progressive enhancement, which was different from that of traditional sarcomas. It may be associated with the presence of mucoid stroma in the tumor pathological tissue, which makes the contrast medium enter the tumor tissue slowly. Pathologically, the tumor invaded the whole layer of the intestinal wall, and the peri-intestinal fat was clear on CT images, and no invasion was found.
CCSLGT in the small intestine should be differentiated from small intestinal stromal tumor, small intestinal adenocarcinoma and small intestinal lymphoma. Small intestinal stromal tumors often occur in the jejunum, with characteristic expression of CD117. CT shows irregular or circular thickening of the intestinal wall, or manifested as a round or lobulated mass protruding from the intestinal lumen. The enhanced mass or thickened intestinal wall showed mild to moderate enhancement. CT of small intestinal adenocarcinoma shows irregular or circular thickening of the intestinal wall, irregular mucous membrane, or localized soft tissue masses protruding into the intestinal lumen, and mild to moderate enhancement of the mass or thickened intestinal wall. Most small intestinal lymphomas show diffuse uniform thickening of the intestinal wall, the involved intestinal segment is long and some of them show single or multiple polypoid masses protruding into the intestinal cavity, and most of them show mild to moderate homogeneous enhancement.
CCSLGT has highly invasive biological behavior, and local recurrence and distant metastasis readily occur in the later stage. Regional lymph nodes, lungs, bones and liver are the main sites of metastasis[9]. Currently, there is no effective treatment, and the main treatment is surgical resection. Even after treatment, CCSLGT usually relapses in a wide range of metastatic nodules and visceral diseases[10]. After extensive surgical resection, metastasis of mesenteric lymph nodes, liver, lung, bone, medial left thigh, pleura, mediastinum, hilar lymph nodes and adrenal gland gradually appeared in the present patient, indicating that CCSLGT metastasizes through lymph nodes and blood. Although our patient received active antitumor therapy, the survival time was < 5 years and his prognosis was poor. Disappointingly, conventional adriamycin-based chemotherapy for other non-small round cell soft tissue sarcomas is ineffective, and there is no report that postoperative radiotherapy and chemotherapy are helpful; therefore, larger prospective studies are needed to determine the best treatment options[11]. Although there are currently no effective treatments for this highly aggressive tumor, future targeted therapies that inhibit the function of the EWSR1–CREB1 fusion oncogene or its associated downstream pathways may be effective in treating the disease. CT can accurately locate CCSLGT and provide the characteristics of lesion size, shape, internal structure and growth. It is of value in observing invasion of adjacent tissues and organs and lymph nodes, and distant metastasis. Positron emission tomography/CT is recommended for patients with CCSLGT, which can provide functional metabolic information beyond anatomical images and effectively reduce missed lesions.
In summary, Whole abdominal CT enhancement is recommended for patients with gastrointestinal symptoms. CCSLGT is a rare malignant mesenchymal tumor with unique morphological, there is no effective treatment for CCSLGT with systemic multiple metastases via the lymphatic system and bloodstream after surgical resection. immunophenotypic and molecular genetic characteristics. The clinical manifestations are not specific, and the diagnosis depends on immunohistochemistry and gene detection. Currently, there is no effective treatment. Clinicians should consider its highly aggressive biological behavior, follow it closely, and recommend regular postoperative whole-body imaging to reduce missed lesions. The diagnosis of this young patient with CCSLGT was clear. After active antitumor treatment, the metastatic focus in vivo is still progressing, the survival time is short, and the prognosis is poor.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gabazza EC, Japan; Hashimoto K, Japan S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Taminelli L, Zaman K, Gengler C, Peloponissios N, Bouzourene H, Coindre JM, Hostein I, Guillou L. Primary clear cell sarcoma of the ileum: an uncommon and misleading site. Virchows Arch. 2005;447:772-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Huang W, Zhang X, Li D, Chen J, Meng K, Wang Y, Lu Z, Zhou X. Osteoclast-rich tumor of the gastrointestinal tract with features resembling those of clear cell sarcoma of soft parts. Virchows Arch. 2006;448:200-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Zambrano E, Reyes-Mugica M, Franchi A, Rosai J. An osteoclast-rich tumor of the gastrointestinal tract with features resembling clear cell sarcoma of soft parts: reports of 6 cases of a GIST simulator. Int J Surg Pathol. 2003;11:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 131] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Green C, Spagnolo DV, Robbins PD, Fermoyle S, Wong DD. Clear cell sarcoma of the gastrointestinal tract and malignant gastrointestinal neuroectodermal tumour: distinct or related entities? Pathology. 2018;50:490-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Askan G, Kombak FE, Seven IE, Basturk O. Clear Cell Sarcoma-Like Tumor of the Gastrointestinal Tract. J Gastrointest Cancer. 2019;50:651-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Wang J, Thway K. Clear cell sarcoma-like tumor of the gastrointestinal tract: an evolving entity. Arch Pathol Lab Med. 2015;139:407-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 7. | Stockman DL, Miettinen M, Suster S, Spagnolo D, Dominguez-Malagon H, Hornick JL, Adsay V, Chou PM, Amanuel B, Vantuinen P, Zambrano EV. Malignant gastrointestinal neuroectodermal tumor: clinicopathologic, immunohistochemical, ultrastructural, and molecular analysis of 16 cases with a reappraisal of clear cell sarcoma-like tumors of the gastrointestinal tract. Am J Surg Pathol. 2012;36:857-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 8. | Gahanbani Ardakani A, Boyle DJ, Elton C. Gastrointestinal clear cell sarcoma-like tumour of the ascending colon. Ann R Coll Surg Engl. 2016;98:e37-e39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Hisaoka M, Ishida T, Kuo TT, Matsuyama A, Imamura T, Nishida K, Kuroda H, Inayama Y, Oshiro H, Kobayashi H, Nakajima T, Fukuda T, Ae K, Hashimoto H. Clear cell sarcoma of soft tissue: a clinicopathologic, immunohistochemical, and molecular analysis of 33 cases. Am J Surg Pathol. 2008;32:452-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 186] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 10. | Thway K, Judson I, Fisher C. Clear cell sarcoma-like tumor of the gastrointestinal tract, presenting as a second malignancy after childhood hepatoblastoma. Case Rep Med. 2014;2014:984369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Kawai A, Hosono A, Nakayama R, Matsumine A, Matsumoto S, Ueda T, Tsuchiya H, Beppu Y, Morioka H, Yabe H; Japanese Musculoskeletal Oncology Group. Clear cell sarcoma of tendons and aponeuroses: a study of 75 patients. Cancer. 2007;109:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |