Published online Jun 26, 2022. doi: 10.12998/wjcc.v10.i18.6163
Peer-review started: October 28, 2021
First decision: March 7, 2022
Revised: March 16, 2022
Accepted: April 22, 2022
Article in press: April 22, 2022
Published online: June 26, 2022
Processing time: 231 Days and 22.1 Hours
Strongyloidiasis is usually a chronic infection but it can develop into a fatal disease in immunosuppressed patients.
A 68-year-old male with rheumatoid arthritis was treated with a variety of immunosuppressants for the past 3 years. Recently, the patient presented with a partial small-bowel obstruction, petechia, coughing and peripheral neuropathy. The diagnosis was difficult to clarify in other hospitals. Our hospital found Strongyloides stercoralis larvae with active movement in the routine stool and sputum smears. The diagnosis of disseminated strongyloidiasis was established. Ivermectin combined with albendazole was used for treatment. The patient responded to therapy and was discharged.
This case underscores the importance of comprehensive differential diagnosis in immunocompromised patients.
Core Tip: Strongyloidiasis is usually a chronic infection but it can develop into a fatal disease in immunosuppressed patients. Here, we present a case of an immunocompromised patient with disseminated strongyloidiasis that was ignored by other hospitals. We discuss the challenges of diagnosis and the treatment. Since the disease was widespread, ivermectin combined with albendazole was used for treatment. This case underscores the importance of comprehensive differential diagnosis in immunocompromised patients.
- Citation: Zheng JH, Xue LY. Disseminated strongyloidiasis in a patient with rheumatoid arthritis: A case report. World J Clin Cases 2022; 10(18): 6163-6167
- URL: https://www.wjgnet.com/2307-8960/full/v10/i18/6163.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i18.6163
Strongyloidiasis is a disease caused by the human pathogenic parasitic roundworm Strongyloides stercoralis (S. stercoralis). Most larvae are excreted in the stool but re-infection or self-infection can occur when the mature larvae burrow into the intestinal wall or the anal tissue. S. stercoralis infections can become chronic and even fatal in immunosuppressed patients[1]. This report describes the clinical features of disseminated strongyloidiasis in an immunosuppressed patient as well as the diagnosis and treatment.
A 68-year-old male with repeated multi-joint pain for 3 years, abdominal pain and abdominal distension for 2 mo and progressive difficulty in swallowing, coughing, hoarseness and dysphonia for 1 wk.
The patient with rheumatoid arthritis was treated successively using a variety of immunosuppressants (methylprednisolone, tocilizumab, adalimumab, rituximab) for the past 3 years. Recently, the patient received treatment in several hospitals for a partial small-bowel obstruction of unknown origin which reoccurred repeatedly after treatment. As the patient’s condition worsened, new symptoms appeared including petechia, progressive difficulty in swallowing, coughing, hoarseness and dysphonia. A neurologist considered peripheral neuropathy because electromyography indicated peripheral nerve axonal damage. High-dose intravenous immunoglobulin therapy (2 g/kg over 5 d) was not effective, so plasmapheresis was recommended. At the same time, a parasite was detected in the stool, however, the species was neither identified nor treated. Due to the progress of bulbar palsy, the patient was referred to our hospital.
Diabetic history: Diabetic history for several years, maximum 18 mmol/L. Taking insulin medication, blood sugar is unsatisfactory for control.
The patient had no specific personal and family history.
Upon admission, he displayed weight loss, stable vital signs, hoarseness, dysarthria, wet rales audible in both lungs, weak bowel sounds, muscle strength grade 3 in all limbs and diminished tendon reflexes.
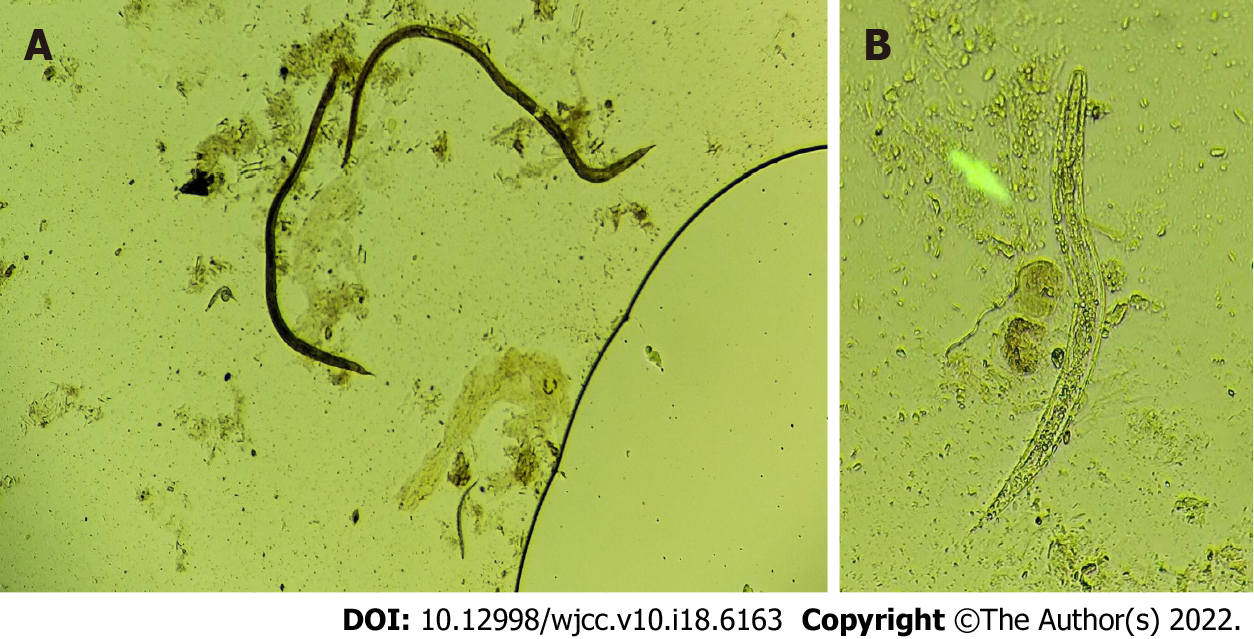
His biochemistry panel was as follows: K of 3.4 mmol/L, Na of 129 mmol/L, Ca of 1.88 mmol/L, and albumin of 25 g/L. Stool-Rt and sputum smears tested positive for S. stercoralis larvae with active movement (Figure 1).
A chest CT showed bilateral infiltrates indicating pneumonia. Echocardiography showed impaired movement of the left ventricular myocardium (EF 42%).
The diagnosis of disseminated strongyloidiasis was established.
After 1 wk of treatment with albendazole 400 mg tid and other supportive treatments, the sputum smear was still positive. The addition of ivermectin 0.2 mg/kg/d × 2 d every 2 wk was then given. On day 4 of ivermectin treatment, the sputum smear and stool tested negative for intestinal parasites. After 2 wk of comprehensive treatment, the patient's mental state gradually improved and muscle strength of the limbs recovered. After 6 wk of hospitalization, his abdominal pain and all previously mentioned symptoms except for the joint pain had dissipated. The patient was discharged and given a small dose of methylprednisolone + methotrexate + celecoxib to control the rheumatoid arthritis and relieve the joint pain. We used albendazole for 4 wk total and ivermectin for a total of 6 wk[2].
At 3 mo after discharge, a follow-up chest CT and electromyography showed lung and cardiac function had recovered.
Strongyloidiasis is a zoonotic intestinal parasitosis caused by S. stercoralis. It is estimated that 30–100 million people are infected worldwide with this parasite[3]. Most infected individuals are asymptomatic or present with intermittent symptoms[4]. Immunosuppressed patients can develop hyperinfection syndrome and disseminated nematode disease which have high mortality rates[5-7]. Strongyloidiasis has been reported following concomitant tocilizumab and methylprednisolone treatment[8]. Some case reports suggest that paralytic ileus may be caused by massive intestinal infestation with S. stercoralis[9].
Our case has two important clinical features. First, the patient had a history of immunosuppression and subsequently developed clinical symptoms (e.g., intestinal obstruction, pneumonia and petechia). The patient’s heart was also affected. Previous hospitals detected the presence of parasites but focused instead on the neurological manifestations. We confirmed the presence of S. stercoralis larvae in the patient’s stool and sputum[10]. Second, the patient presented with choking and hoarseness at the time of diagnosis. Head, neck, mediastinal MRI, cerebrospinal fluid and other examinations found no evidence of neurological invasion. Therefore, we considered two possibilities: (1) Nutritional deficiencies in vitamin B1, vitamin B12 and folic acid due to long periods of fasting, causing malabsorption and intestinal obstruction which can lead to peripheral neuropathy[11]; and (2) Neurotoxic biological agents (e.g., TNF inhibitors, anti-IL-6 receptor antibody), which can cause peripheral neuropathy in approximately 42% of cases[12].
The Centers for Disease Control and Prevention and World Health Organization recommend ivermectin as the first choice for strongyloidiasis. In endemic areas, a combination of albendazole and ivermectin is recommended[13], and Moxidectin has also been tried as a treatment[14]. Repeated or extended dosing is preferred until worms are no longer detected[15]. Considering that the patient was still taking low-dose methylprednisolone and methotrexate tablets for rheumatoid arthritis, we adopted a multi-dose and long course of treatment. At the 3 mo follow-up, no recurrence of the disease was detected, so the treatment was effective.
This case highlights important considerations for patients receiving immunosuppressive therapy. It is necessary to improve medical workers’ awareness of strongyloidiasis to avoid delays in diagnosis and ensure adequate management of infected patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ahmed OM, Egypt S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Khieu V, Srey S, Schär F, Muth S, Marti H, Odermatt P. Strongyloides stercoralis is a cause of abdominal pain, diarrhea and urticaria in rural Cambodia. BMC Res Notes. 2013;6:200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 2. | Hofmann D, Smit C, Sayasone S, Pfister M, Keiser J. Optimizing moxidectin dosing for Strongyloides stercoralis infections: Insights from pharmacometric modeling. Clin Transl Sci. 2022;15:700-708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Schär F, Trostdorf U, Giardina F, Khieu V, Muth S, Marti H, Vounatsou P, Odermatt P. Strongyloides stercoralis: Global Distribution and Risk Factors. PLoS Negl Trop Dis. 2013;7:e2288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 418] [Cited by in RCA: 477] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 4. | Olsen A, van Lieshout L, Marti H, Polderman T, Polman K, Steinmann P, Stothard R, Thybo S, Verweij JJ, Magnussen P. Strongyloidiasis--the most neglected of the neglected tropical diseases? Trans R Soc Trop Med Hyg. 2009;103:967-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 376] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 5. | Marcos LA, Terashima A, Canales M, Gotuzzo E. Update on strongyloidiasis in the immunocompromised host. Curr Infect Dis Rep. 2011;13:35-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 6. | Comelli A, Mangioni D, Scaramella L, Maraschini A, Gaudino C, Folli C, Ceriotti F, Triulzi F, Canetta C, Gori A, Bandera A. Strongyloides stercoralis central nervous system dissemination in a migrant misdiagnosed with eosinophilic granulomatosis with polyangiitis. J Travel Med. 2022;29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Mah J, Lieu A, Holmes E, Vaughan S. A case of disseminated strongyloidiasis after multiple courses of immunosuppression. CMAJ. 2022;194:E89-E92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Lier AJ, Tuan JJ, Davis MW, Paulson N, McManus D, Campbell S, Peaper DR, Topal JE. Case Report: Disseminated Strongyloidiasis in a Patient with COVID-19. Am J Trop Med Hyg. 2020;103:1590-1592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 9. | Yoshida H, Endo H, Tanaka S, Ishikawa A, Kondo H, Nakamura T. Recurrent paralytic ileus associated with strongyloidiasis in a patient with systemic lupus erythematosus. Mod Rheumatol. 2006;16:44-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Buonfrate D, Fittipaldo A, Vlieghe E, Bottieau E. Clinical and laboratory features of Strongyloides stercoralis infection at diagnosis and after treatment: a systematic review and meta-analysis. Clin Microbiol Infect. 2021;27:1621-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Morís G. Inflammatory bowel disease: an increased risk factor for neurologic complications. World J Gastroenterol. 2014;20:1228-1237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Spagnoli C, Pisani F, Di Mario F, Leandro G, Gaiani F, De' Angelis GL, Fusco C. Peripheral neuropathy and gastroenterologic disorders: an overview on an underrecognized association. Acta Biomed. 2018;89:22-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Henriquez-Camacho C, Gotuzzo E, Echevarria J, White AC Jr, Terashima A, Samalvides F, Pérez-Molina JA, Plana MN. Ivermectin versus albendazole or thiabendazole for Strongyloides stercoralis infection. Cochrane Database Syst Rev. 2016;CD007745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 106] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 14. | Bisoffi Z, Buonfrate D. Moxidectin: an ally to ivermectin for treating Strongyloides stercoralis? Lancet Infect Dis. 2021;21:1060-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Mejia R, Nutman TB. Screening, prevention, and treatment for hyperinfection syndrome and disseminated infections caused by Strongyloides stercoralis. Curr Opin Infect Dis. 2012;25:458-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 228] [Article Influence: 17.5] [Reference Citation Analysis (0)] |