Published online Jun 26, 2022. doi: 10.12998/wjcc.v10.i18.6069
Peer-review started: November 22, 2021
First decision: February 7, 2022
Revised: March 13, 2022
Accepted: April 15, 2022
Article in press: April 15, 2022
Published online: June 26, 2022
Processing time: 207 Days and 0.4 Hours
Icotinib could have potential effect and tolerability when used sequentially with chemotherapy for advanced epidermal growth factor receptor (EGFR)-mutated non-small cell lung cancer (NSCLC).
To evaluate the efficacy and safety of chemotherapy followed by icotinib maintenance therapy as first-line treatment for advanced EGFR-mutated NSCLC.
This multicenter, open-label, pilot randomized controlled trial enrolled 68 EGFR-mutated stage IIIB/IV NSCLC patients randomized 2:3 to the icotinib alone and chemotherapy + icotinib groups.
The median progression-free survival in the icotinib alone and chemotherapy + icotinib groups was 8.0 mo (95%CI: 3.84-11.63) and 13.4 mo (95%CI: 10.18-16.33), respectively (P = 0.0249). No significant differences were found in the curative effect when considering different cycles of chemotherapy or chemotherapy regimen (all P > 0.05).
A sequential combination of chemotherapy and EGFR-tyrosine kinase inhibitor is feasible for stage IV EGFR-mutated NSCLC patients.
Core Tip: The combination of chemotherapy and epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor (TKI) (concomitant or intercalated) generally showed improved efficacy compared with EGFR-TKI alone as the first-line treatment for advanced non-small cell lung cancer (NSCLC). This study aimed to evaluate the efficacy and safety of chemotherapy followed by icotinib maintenance therapy as first-line treatment for advanced EGFR-mutated NSCLC. Sixty-eight advanced NSCLC patients were randomized 2:3 to icotinib-alone or chemotherapy plus icotinib. The chemotherapy plus icotinib group showed higher progression-free survival than the icotinib alone group. Our study suggested that the sequential combination of chemotherapy and EGFR-tyrosine kinase inhibitor is feasible for stage IV EGFR-mutated NSCLC patients.
- Citation: Sun SJ, Han JD, Liu W, Wu ZY, Zhao X, Yan X, Jiao SC, Fang J. Sequential chemotherapy and icotinib as first-line treatment for advanced epidermal growth factor receptor-mutated non-small cell lung cancer. World J Clin Cases 2022; 10(18): 6069-6081
- URL: https://www.wjgnet.com/2307-8960/full/v10/i18/6069.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i18.6069
Globally, lung cancer is the malignancy with the highest incidence and mortality. In 2018, 2.1 million new lung cancers and 1.8 million deaths were reported, with an annual age-standardized incidence rate of 22.5 per 100000 individuals and an age-standardized yearly mortality rate of 18.6 per 100000 individuals[1]. Non-small cell lung cancers (NSCLCs) represent the greatest number (85%-90%) of malignant lung tumors[2], and almost half of NSCLCs are adenocarcinomas. Adenocarcinomas display activating mutations in the epithelial growth factor receptor (EGFR) gene, making such cancers candidates for EGFR tyrosine kinase inhibitor (EGFR-TKI) therapy[3-5]. In Asians, individuals harboring EGFR mutations account for 51.4% of adenocarcinoma NSCLCs[3-5]. Currently, EGFR-TKIs are the guideline-recommended first-line treatment for advanced NSCLC with EGFR mutations[5].
Despite the improvement in progression-free survival (PFS) by EGFR-TKIs, acquired resistance inevitably develops after about 10 mo of treatment[3,6]. Due to the complexity of the EGFR-TKI resistance mechanisms[6-8], a combined treatment approach could be used to prevent or delay resistance development[7]. One of the combination therapies of interest and most frequently explored is chemotherapy + TKI. In clinical trials, a combination of chemotherapy and EGFR-TKI (concomitant or intercalated) generally showed improved efficacy compared with EGFR-TKI alone as the first-line treatment for advanced NSCLC[9-13]. Nevertheless, the best combinational strategy remains controversial.
In preclinical studies, compared with concurrent administration of gefitinib alone, the sequential administration of pemetrexed or paclitaxel with gefitinib exerted stronger anti-tumor activity by enhancing cell cytotoxicity[14-18]. Sequential chemotherapy followed by maintenance EGFR-TKI therapy may be a potential strategy, as suggested by recent clinical trials[19-21]. Icotinib was suggested to have potential effects and tolerability when used sequentially with chemotherapy[22-24]. Therefore, the present pilot study aimed to evaluate the efficacy and safety of different sequential combinations of chemotherapy (varying cycle number and chemotherapeutic agents), followed by icotinib maintenance vs icotinib alone as a first-line treatment for advanced EGFR-mutated NSCLC. The results might help improve the treatment strategies for these patients.
This multicenter, open-label, pilot randomized controlled trial (RCT) was conducted in four centers in China between November 2012 and July 2015. The study was carried out according to the principles of the Declaration of Helsinki and the guidelines of the Good Clinical Practice of the International Council for Harmonization. The trial was approved by the ethics committees of General Hospital of People's Liberation Army. All patients signed an informed consent form before any study procedure.
The inclusion criteria were: (1) Age 18-72 years; (2) patients with treatment-naïve advanced lung cancer having EGFR-sensitive mutation confirmed by pathological examinations; (3) stage IIIB or IV lung cancer; (4) Eastern Cooperative Oncology Group (ECOG) score of 0-2; (5) normal cardiac, liver, and renal functions, and routine blood test results; (6) expected survival > 3 mo; (7) negative urine pregnancy test within 7 d before screening for women of child-bearing age, and agreement to apply effective contraception measures to prevent pregnancy during and within 3 mo after the study for fertile men and women; and (8) signed informed consent forms. The exclusion criteria were: (1) Brain metastases; (2) active infection (according to the judgment of investigators); (3) major organ failure, such as decompensated cardiopulmonary failure; (4) newly developed myocardial infarction or cerebral infarction within 3 mo; (5) presence of a second malignant tumor (except for cured cervical cancer or skin cancer); (6) interstitial lung disease; or (7) pregnant or breastfeeding women.
Trial registration: ClinicalTrials.gov, NCT01665417. Registered on August 12, 2012, https://clinicaltrials.gov/ct2/show/NCT01665417.
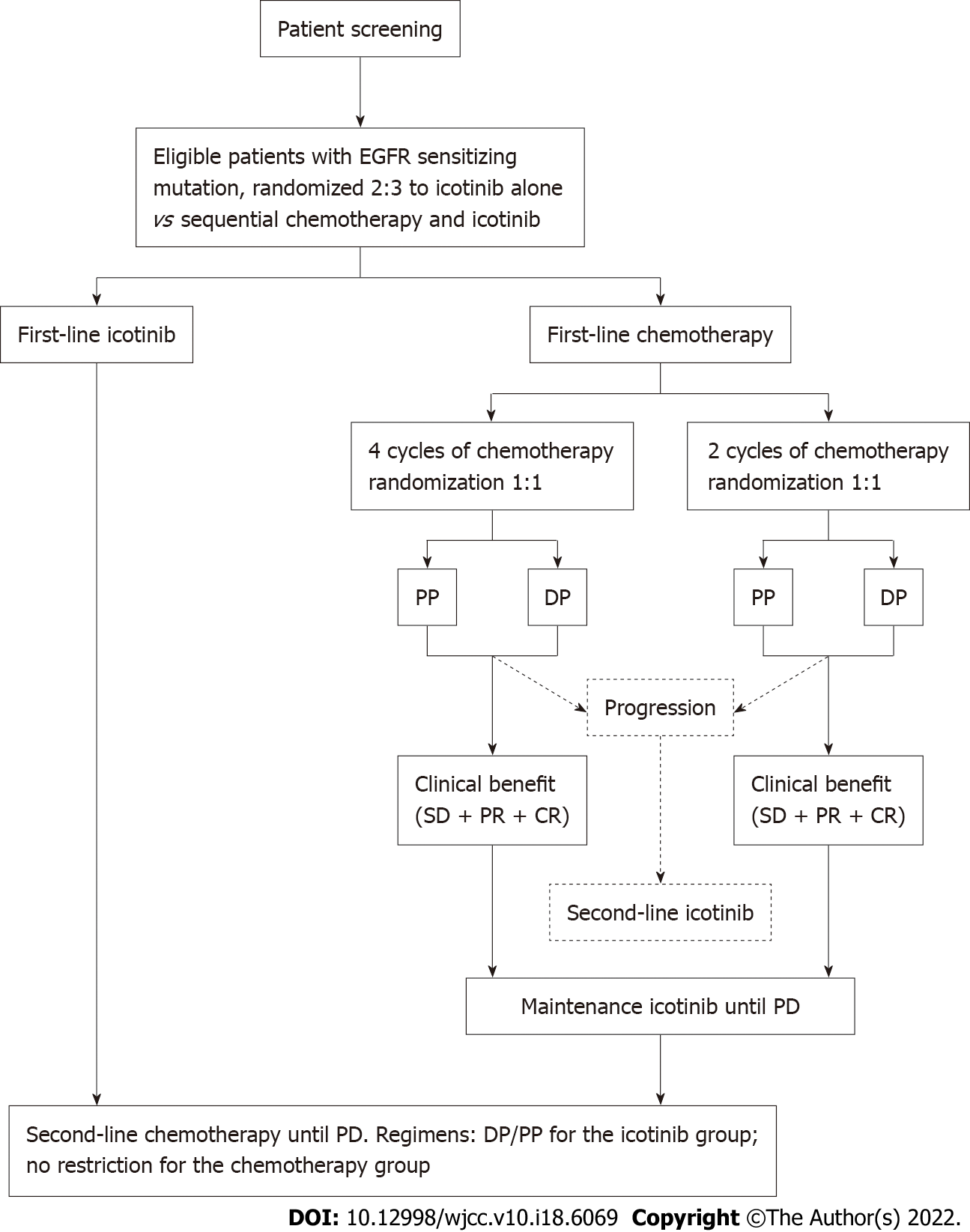
This study involved three randomizations. The patients were first randomized 2:3 to icotinib-alone vs chemotherapy + icotinib. The patients in the chemotherapy group were then randomized 1:1 to two vs four cycles of chemotherapy and further randomized 1:1 to pemetrexed and cisplatin (PP) vs docetaxel and cisplatin (DP) (Figure 1). All randomizations were carried out using a central randomization system designed by an independent biostatistician. The stratification factors after randomization included clinical stage (IIIB vs IV), type of EGFR mutation (exon 19 mutation vs exon 21 mutation), ECOG score (0-1 vs 2), and smoking status (non-smokers vs mild smokers vs regular smokers). This study was a pilot study, and the patients, treating physicians, and data assessors could not be blind to treatment allocation due to the nature of the treatments.
Icotinib was provided by Betta Pharmaceutical Co., Ltd. (Zhejiang, China). Two or four cycles of PP (pemetrexed disodium 500 mg/m2 iv d1, cisplatin 75 mg/m2 iv d1, q3w) or DP (docetaxel 75 mg/m2 iv d1, cisplatin 75 mg/m2 iv d1, q3w) were administered to the patients assigned to the first-line chemotherapy + icotinib treatment. Icotinib hydrochloride (oral, 125 mg, tid) was used as maintenance therapy or second-line therapy until disease progression or the occurrence of severe toxicity for patients with clinical benefits or progressive disease after chemotherapy. Second-line chemotherapy after disease progression on icotinib was the crossover of the first-line chemotherapy. The chemotherapy regimen after DP/PP treatment had no restriction.
For the patients assigned to first-line icotinib treatment, 125 mg icotinib was administered orally three times per day until disease progression or the occurrence of severe toxicity. For second-line treatment, the patients received the PP (pemetrexed disodium 500 mg/m2 iv d1, cisplatin 75 mg/m2 iv d1, q3w) or DP (docetaxel 75 mg/m2 iv d1, cisplatin 75 mg/m2 iv d1, q3w) chemotherapy regimen, at the discretion of the treating physician.
For patients on first-line chemotherapy, the tumor response was assessed after every two cycles of chemotherapy. During icotinib maintenance therapy, treatment efficacy assessment was performed 4 wk after treatment initiation and then every 6 wk until disease progression. For patients on first-line icotinib therapy, tumor response was assessed 4 wk after treatment initiation and then every 6 wk until disease progression. The tumors were assessed by plain and enhanced pulmonary computed tomography (CT) scanning, abdominal ultrasound examination, CT scanning or magnetic resonance imaging (MRI), ultrasound examination of superficial lymph nodes, brain MRI (if necessary), and emission CT (if necessary). The response to treatment was classified as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) according to RECIST 1.1[25]. The safety evaluation was performed using physical examinations and laboratory examinations (hematological and blood biochemical examinations). All adverse events were recorded from the informed consent until 30 d after the last dose of the study drug. The severity of the adverse events was assessed and documented according to the National Cancer Institute-Common Toxicity Criteria 3.0. The investigators judged the relationship between the adverse events and treatment.
The study's primary endpoint was PFS, defined as the date of the start of treatment to the date of PD (per RECIST 1.1) or death, whichever occurred first. The secondary endpoint was overall survival (OS), defined as the time from the start of treatment to death. Other efficacy endpoints included overall response rate (ORR) and disease control rate (DCR). The ORR was defined as the proportion of patients achieving CR or PR, and the DCR was defined as the proportion of patients achieving CR, PR, or SD.
All analyses were performed using SAS 9.2 (SAS Institute, Inc., NC, United States). The efficacy analysis was performed in the full analysis set, defined as all randomized patients who received at least one dose of the study drug. The safety set included all randomized patients who received at least one dose of the study drug. Continuous data are presented as means ± SD and medians (ranges). Categorical data are presented as numbers (percentages). PFS and OS were analyzed using the Kaplan-Meier method and the log-rank test. The ORR and DCR were summarized as percentages and Clopper-Pearson 95%CIs. Two-sided P values of < 0.05 were considered statistically significant.
Between November 2012 and July 2015, 68 participants were recruited: 24 in the icotinib-alone group and 44 in the chemotherapy + icotinib group. The participants who received single-dose treatment (22 in the icotinib-alone group and 36 in the chemotherapy + icotinib group) were included in the analysis. All participants were randomized, and treatment was initiated. The characteristics of the participants are shown in Table 1. All patients except one had stage IV NSCLC.
| Icotinib | Chemotherapy + icotinib | Chemotherapy + icotinib, n = 34 | ||||
| 2DP | 2PP | 4DP | 4PP | |||
| n = 22 | n = 34 | n = 11 | n = 10 | n = 4 | n = 9 | |
| Age (yr) | 57.0 ± 7.4 | 52.7 ± 11.05 | 52.9 ± 11.5 | 49.8 ± 9.2 | 49.8 ± 13.7 | 57.1 ± 11.7 |
| Sex | ||||||
| Male | 11 (50.0) | 9 (26.5) | 2 (18.2) | 2 (20.0) | 2 (50.0) | 3 (33.3) |
| Female | 11 (50.0) | 25 (73.5) | 9 (81.8) | 8 (80.0) | 2 (50.0) | 6 (66.7) |
| Stage | ||||||
| IIIB | 0 | 1 (2.9) | 0 | 0 | 0 | 1 (11.1) |
| IV | 22 (100.0) | 33 (97.1) | 11 (100.0) | 10 (100.0) | 4 (100.0) | 8 (88.9) |
| EGFR mutation | ||||||
| 19 Del | 12 (54.5) | 19 (55.9) | 5 (45.5) | 5 (50.0) | 4 (100.0) | 5 (55.6) |
| 21 L858R | 7 (31.8) | 14 (41.2) | 5 (45.5) | 5 (50.0) | 0 | 4 (44.4) |
| Other | 3 (13.6) | 1 (2.9) | 1 (9.1) | 0 | 0 | 0 |
| Smoking | ||||||
| Yes | 6 (27.3) | 4 (11.8) | 1 (9.1) | 1 (10.0) | 1 (25.0) | 1 (11.1) |
| No | 15 (68.2) | 30 (88.2) | 10 (90.9) | 9 (90.0) | 3 (75.0) | 8 (88.9) |
| Quit smoking | 1 (4.5) | 0 | 0 | 0 | 0 | 0 |
| ECOG PS | ||||||
| 0 | 5 (22.7) | 6 (17.6) | 1 (9.1) | 2 (20.0) | 0 | 3 (33.3) |
| 1 | 13 (59.1) | 25 (73.5) | 7 (63.6) | 8 (80.0) | 4 (100.0) | 6 (66.7) |
| 2 | 2 (9.1) | 0 | 0 | 0 | 0 | 0 |
| Other | 2 (9.1) | 3 (8.8) | 3 (27.3) | 0 | 0 | 0 |
Table 2 shows the responses to treatment. No participants achieved CR. In the icotinib-alone group, the ORR was 54.5% (95%CI: 32.2-75.6) and the DCR was 90.9% (95%CI: 70.8-98.9) compared with 44.1% (95%CI: 27.2-62.1) and 97.1% (95%CI: 84.7-99.9), respectively, in the chemotherapy + icotinib group.
| n | PR (95%CI) | SD (95%CI) | PD (95%CI) | NE (95%CI) | ORR (95%CI) | DCR (95%CI) | |
| Icotinib | 22 | 54.5 (32.2-75.6) | 36.4 (17.2-59.3) | 4.5 (0.1-22.8) | 4.5 (0.1-22.8) | 54.5 (32.2-75.6) | 90.9 (70.8-98.9) |
| Chemotherapy + icotinib | 34 | 44.1 (27.2-62.1) | 52.9 (35.1-70.2) | 2.9 (0.1-15.3) | 44.1 (27.2-62.1) | 97.1 (84.7-99.9) | |
| 2-cycle chemo | 21 | 47.6 (25.7-70.2) | 52.4 (29.8-74.3) | 47.6 (25.7-70.2) | 100.0 (83.9-100.0) | ||
| 2DP | 11 | 36.4 (10.9-69.2) | 63.6 (30.8-89.1) | 36.4 (10.9-69.2) | 100.0 (71.5-100.0) | ||
| 2PP | 10 | 60.0 (26.2-87.8) | 40.0 (12.2-73.8) | 60.0 (26.2-87.8) | 100.0 (69.2-100.0) | ||
| 4-cycle chemo | 13 | 38.5 (13.9-68.4) | 53.8 (25.1-80.8) | 7.7 (0.2-36.0) | 38.5 (13.9-68.4) | 92.3 (64.0-99.8) | |
| 4DP | 4 | 50.0 (6.8-93.2) | 50.0 (6.8-93.2) | 50.0 (6.8-93.2) | 100.0 (39.8-100.0) | ||
| 4PP | 9 | 33.3 (7.5-70.1) | 55.6 (21.2-86.3) | 11.1 (0.3-48.2) | 33.3 (7.5-70.1) | 88.9 (51.8-99.7) | |
| DP | 15 | 40.0 (16.3-67.7) | 60.0 (32.3-83.7) | 40.0 (16.3-67.7) | 100.0 (78.2- 100.0) | ||
| PP | 19 | 47.4 (24.4-71.1) | 47.4 (24.4-71.1) | 5.3 (0.1-26.0) | 47.4 (24.4-71.1) | 94.7 (74.0-99.9) | |
| Total | 56 | 48.2 (34.7-62.0) | 46.4 (33.0-60.3) | 3.6 (0.4-12.3) | 1.8 (0.0-9.6) | 48.2 (34.7-62.0) | 94.6 (85.1-98.9) |
When considering the number of chemotherapy cycles, the ORR was 47.6% (95%CI: 25.7-70.2) and the DCR was 100.0% (95%CI: 83.9-100.0) for two cycles, and the ORR was 38.5% (95%CI: 13.9-68.4) and the DCR was 92.3% (95%CI: 64.0-99.8) for four cycles. When considering the chemotherapy types, the ORR was 40.0% (95%CI: 16.3-67.7) and the DCR was 100.0% (95%CI: 78.2-100.0) for DP, and the ORR was 47.4% (95%CI: 24.4-71.1) and the DCR was 94.7% (95%CI: 74.0-99.9) for PP. When considering each chemotherapy regimen, the ORR was 33.3%-60.0%, and the DCR was 88.9%-100%.
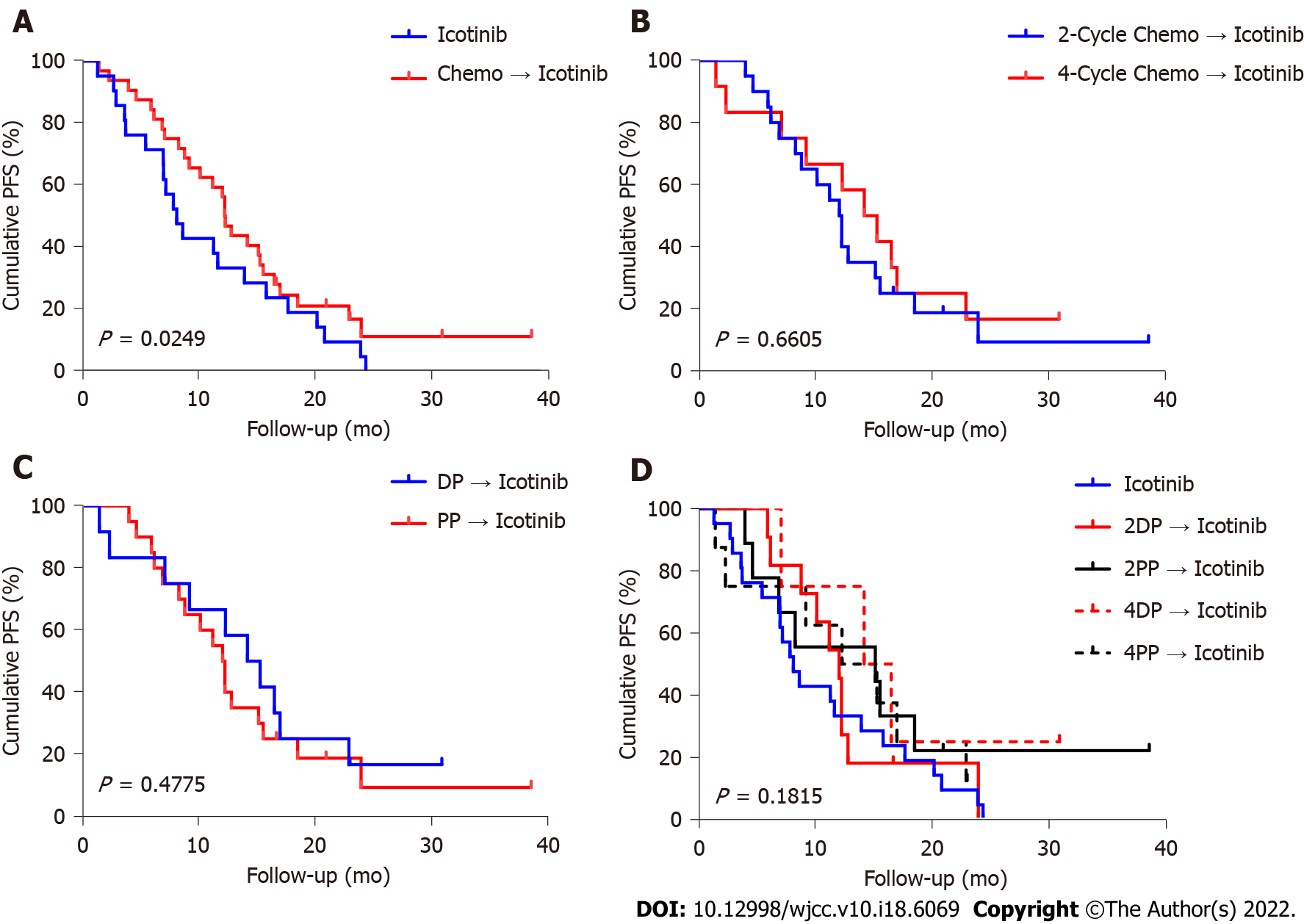
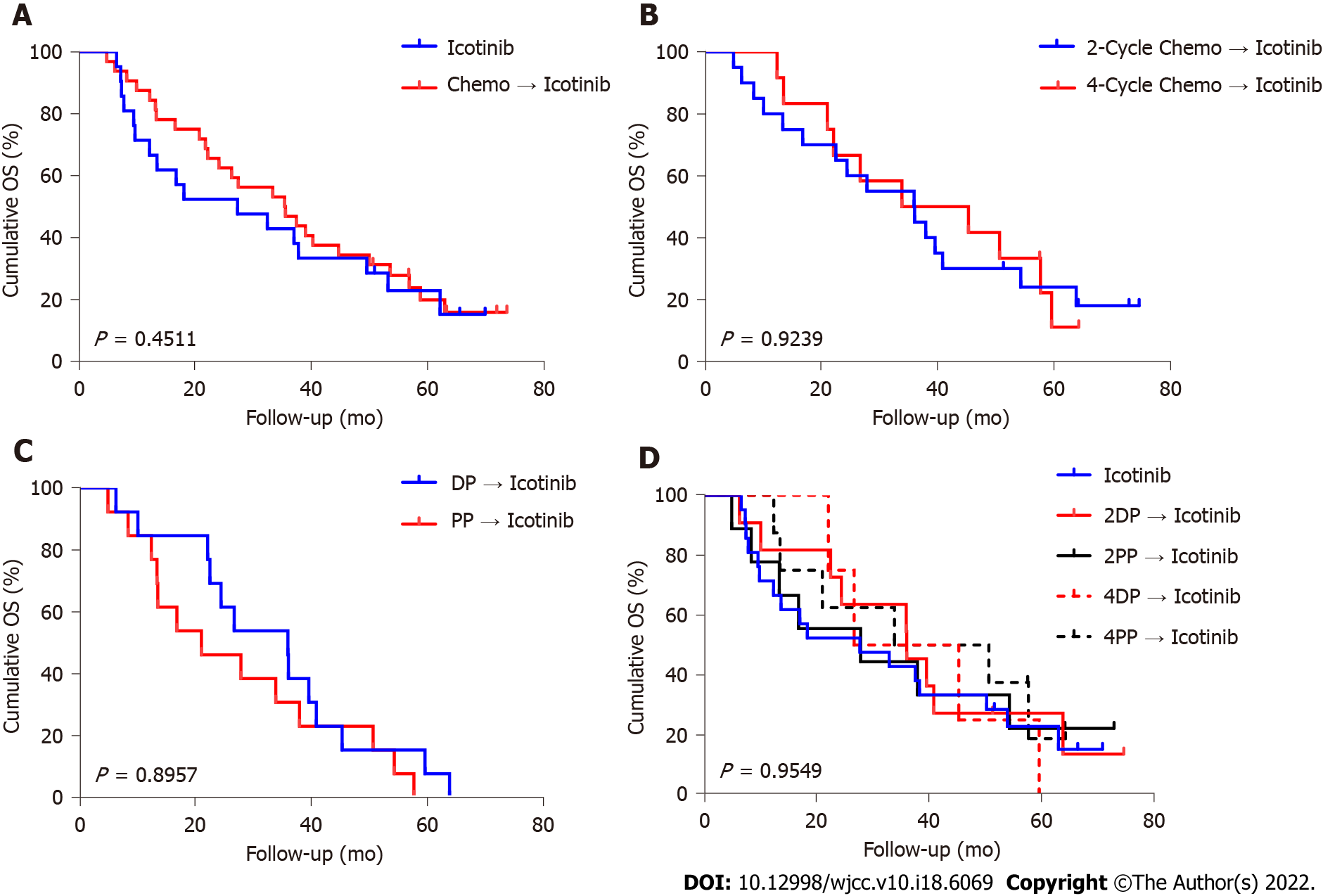
In the icotinib group, the median follow-up was 23.1 (range, 2.5-71.9) mo. The median follow-up in the chemotherapy + icotinib group was 36.0 (range, 5.1-75.7) mo. Figures 2 and 3 present the PFS and OS, respectively. The median PFS in the icotinib-alone and chemotherapy + icotinib groups was 8.0 mo (95%CI: 3.8-11.6) vs 13.4 mo (95%CI: 10.2-16.3), respectively (P = 0.0249). The median OS was 23.1 (95%CI: 9.7-50.3) vs 36.0 mo (95%CI: 22.2-45.4), respectively (P = 0.4511). The median PFS of the participants who received two and four chemotherapy cycles was 12.1 mo vs 15.1 mo, and the median OS was 36.1 mo vs 33.9 mo, with no significant differences (PFS, P = 0.6605; OS, P = 0.9239). The PFS after two cycles of DP, two cycles of PP, four cycles of DP, and four cycles of PP was 11.9, 15.2, 15.2, and 15.1 mo, respectively; the median OS was 36.1, 28.0, 36.1, and 33.9 mo, respectively. No significant difference was observed among the different treatment regimens (PFS, P = 0.1815; OS, P = 0.9549).
Table 3 presents the treatment received after icotinib-based therapy. The treatment profile was similar in the two groups.
| Icotinib | Chemotherapy + icotinib | Chemotherapy + icotinib, n = 34 | ||||
| 2DP | 2PP | 4DP | 4PP | |||
| n = 22 | n = 34 | n = 11 | n = 10 | n = 4 | n = 9 | |
| Chemotherapy | 13 (59.1) | 16 (47.1) | 6 (54.6) | 4 (40.0) | 2 (50.0) | 4 (44.4) |
| Osimertinib | 10 (45.5) | 17 (50.0) | 6 (54.6) | 4 (40.0) | 2 (50.0) | 5 (55.6) |
| Other TKI | 2 (9.1) | 3 (8.8) | 1 (9.1) | 0 | 0 | 2 (22.2) |
| Radiotherapy | 3 (13.6) | 7 (20.6) | 1 (9.1) | 3 (30.0) | 2 (50.0) | 2 (22.2) |
| Other | 3 (13.6) | 5 (14.7) | 1 (9.1) | 2 (20.0) | 0 | 2 (22.2) |
The rates of all-grade treatment-related adverse events (TRAEs) were lower in the icotinib-alone group compared with the chemotherapy + icotinib group, and included rash (40.9% vs 55.9%), gastrointestinal system disorders (0.0% vs 82.4%), alanine transaminase elevation (27.3% vs 41.2%), aspartate aminotransferase elevation (13.6% vs 29.4%), leukopenia (0.0% vs 64.7%), and thrombocytopenia (0.0% vs 11.8%). Grade 3-4 TRAEs were not observed in the icotinib-alone group. However, grade 3-4 gastrointestinal system disorders (5.9%) and leukopenia (8.8%) were recorded in the chemotherapy + icotinib group (Table 4).
| All-grade TRAE | Grade 3-4 TRAE | |||
| Icotinib (n = 22) | Chemotherapy + icotinib (n = 34) | Icotinib (n = 22) | Chemotherapy + icotinib (n = 34) | |
| Rash | 9 (40.9) | 19 (55.9) | 0 (0.0) | 0 (0.0) |
| Gastrointestinal system disorders | 0 (0.0) | 28 (82.4) | 0 (0.0) | 2 (5.9) |
| Alanine transaminase elevation | 6 (27.3) | 14 (41.2) | 0 (0.0) | 0 (0.0) |
| Aspartate aminotransferase elevation | 3 (13.6) | 10 (29.4) | 0 (0.0) | 0 (0.0) |
| Leukopenia | 0 (0.0) | 22 (64.7) | 0 (0.0) | 3 (8.8) |
| Thrombocytopenia | 0 (0.0) | 4 (11.8) | 0 (0.0) | 0 (0.0) |
Sequential chemotherapy followed by maintenance TKI may be a potential strategy for advanced NSCLC with EGFR mutation. However, the optimal regimen remains to be determined. In this study, icotinib was selected because of the potential effect and tolerability of sequential chemotherapy and icotinib[22-24]. The present study indicated that the sequential combination of chemotherapy followed by icotinib improved PFS by 5.4 mo compared with icotinib alone as the first-line therapy of NSCLC. In addition, no differences were observed between two and four cycles of chemotherapy and between PP and DP. Therefore, for patients with advanced NSCLC with EGFR mutation, a sequential combination of chemotherapy and an EGFR-TKI is feasible. Considering the chemotherapy toxicity, the efficacy of a two-cycle chemotherapy regimen was comparable to that of a four-cycle chemotherapy regimen.
In the present study, no significant differences were observed in OS (36 mo vs 23.1 mo) and PFS (8.0 mo vs 13.4 mo), which was probably due to the small sample size or the fact of crossover of the treatment group upon disease progression. Considering the synergistic effect of EGFR-TKIs and chemotherapy in the elimination of tumor cells, as reported by some preclinical studies, gefitinib and erlotinib were combined with two chemotherapy regimens (cisplatin + gemcitabine; carboplatin + paclitaxel), thus launching four large phase III clinical studies, including INTACT 1 and 2 and TRIBUTE[26,27]. These studies showed no significant difference between chemotherapy and combined treatment groups (PFS and OS), which might be because the participants were not selected according to their EGFR mutation status[28]. A retrospective analysis of the OPTIMAL study on EGFR mutation (exon 19 deletion or exon 21 L858R mutation) showed that the OS of patients treated with chemotherapy alone was significantly lower than that of patients who received TKI and sequential chemotherapy [median OS: 11.2 vs 29.7 mo, HR = 2.97 (1.74-5.07)]. Although it was a retrospective analysis, it also suggested that sequential treatment with TKI and chemotherapy for selected patients with EGFR mutation could prolong patient OS[29]. However, a phase II clinical study in Japan, NEJ00, reported that in NSCLC patients with EGFR mutation, the combined therapy of gefitinib, pemetrexed, and carboplatin was significantly superior to chemotherapy followed by targeted therapy[30]. Among the 80 enrolled patients, 41 received concurrent combination therapy, while 39 also had sequential therapy. The median PFS was 18.3 mo vs 15.3 mo [HR = 0.71 (0.42-1.2), P = 0.02], and median OS was 41.9 mo vs 30.7 mo [HR = 0.51 (0.26-0.99), P = 0.042], respectively. The response rates in the two groups were similar (87.8% and 84.6%, respectively). Furthermore, phase II clinical studies conducted in China reported similar results for gefitinib combined with pemetrexed-based chemotherapy[31]. Based on the results of NEJ005, the phase III clinical study NEJ009 further confirmed that the efficacy of gefitinib combined with carboplatin and pemetrexed was superior to that of single-drug gefitinib treatment[32], which showed that the PFS was 20.9 mo (18.0-24.2) vs 11.2 mo (9.0-13.4) [HR = 0.43 (0.39-0.62), P < 0.001], and more importantly, OS was 52.2 mo vs 38.8 mo (HR = 0.69, P = 0.013). Subsequently, CTRI/2016/08/007149, conducted in India and almost completely similar to NEJ009, further confirmed that the efficacy of gefitinib combined with carboplatin and pemetrexed was significantly superior to that of gefitinib alone[33]. It also demonstrated that the PFS of gefitinib combined with pemetrexed-based chemotherapy was longer than 16 mo and longer than 20.9 mo in NEJ009, which was a much longer PFS than achieved by gefitinib alone. In particular, two phase III clinical trials, NEJ009 and CTRI/2016/08/007149, confirmed the benefits of OS in the combination treatment group. The studies mentioned above mainly focused on targeting, a synchronous combination of chemotherapy, or alternating sequential combination of targeting and chemotherapy. However, evidence on the use of sequential therapy based on chemotherapy followed by the target drug in EGFR-mutant patients is lacking. Studies at the molecular level confirmed that sequential chemotherapy with the EGFR-TKI erlotinib after docetaxel could enhance the M-phase stagnation of tumor cell division and growth, resulting in cell apoptosis. They suggested a synergistic effect between molecular targeted therapy and appropriate sequential chemotherapy. These experimental results indicated that the use of chemotherapy first to induce tumor cell stagnation and apoptosis in the M phase, followed by EGFR-TKIs to enhance this effect[34], would result in sequential therapy having a superposition effect, which might be used as a feasible option. Similar to the present study, Han et al[13] compared gefitinib + pemetrexed + carboplatin vs gefitinib alone vs pemetrexed + carboplatin and reported a higher ORR with the TKI + chemotherapy combination than for TKI alone or chemotherapy alone (82.5% vs 65.9% vs 32.5%), with similar trends in PFS and OS. Similar results were also reported by Wen et al[35] and Yan et al[36]. Another RCT focused on first-line chemotherapy and TKI sequential treatment in patients with advanced non-squamous NSCLC[37,38]. PFS and OS were similar in the pemetrexed + cisplatin + gefitinib and gefitinib monotherapy groups in the ITT population and EGFR-mutated subgroup, but the sample size in the EGFR-mutated subgroup was too small to draw a firm conclusion. The combination therapy may outperform the monotherapy ORR as chemotherapy and TKIs do not affect the cancer cells using the same mechanisms (i.e., hitting the cells in multiple ways), and intratumor heterogeneity may be present (i.e., using multiple drugs increases the likelihood of killing cells resistant to one of the drugs used). The immune system can also be activated[9,34,39,40]. Nevertheless, the PFS in the sequential treatment group in the present study was superior to that in the TKI-alone therapy group. The reason for the inconsistent results in these two studies might be that the number of patients with EGFR mutation in either study was small, affecting the consistency of the study results. Of note, the recent results of the FLAURA trial showed that first-line osimertinib achieved better OS and PFS than the comparator EGFR-TKIs[41], and sequential osimertinib with chemotherapy as a first-line option should be investigated. Due to the TRAE profile of osimertinib, the sequential use of chemotherapy and osimertinib could decrease the occurrence of TRAEs in the first-line treatment of NSCLC. Furthermore, the combination of EGFR-TKIs with vascular endothelial growth factor inhibitors could be a potential strategic option[42] and should also be examined.
In the present study, four cycles of chemotherapy were not better in terms of ORR, DCR, PFS, and OS compared with two cycles. Two cycles might be enough to eliminate tumor cells sensitive to chemotherapy and activate the immune system, while four cycles might lead to adverse events and decreases in blood immune cells[43]. In addition, fewer cycles could help reduce the physical, psychological, and economic burden of chemotherapy[43]. The rate of grade ≥ 3 TRAEs was 14.3% in the two-cycle subgroup and 15.4% in the four-cycle subgroup. Hence, the present study suggests similar efficacy and safety for the two- and four-cycle regimens, which could be supported by a meta-analysis that suggested no added benefit of six cycles of first-line chemotherapy compared with three and four cycles[43]. However, this study was not powered to compare two- vs four-cycle regimens, and additional studies are necessary to examine this point.
There are many therapeutic options in lung cancer, including chemotherapy, targeted therapy, and immunotherapy[2,44-48]. Icotinib is a promising targeted therapy for EGFR-mutated NSCLC[18,22-24]. The present study selected the combination of icotinib (or other EGFR-TKIs) and chemotherapy since it is the most studied combination in NSCLC, with apparent benefits in response and survival[9,18,24,34-36,49]. Still, the combination of EGFR-TKIs and immunotherapy could be a promising option for NSCLC[50-52], but some evidence suggests that immunotherapy is not effective in patients with EGFR-mutated NSCLC, probably because of the specific tumor microenvironment[52,53]. Indeed, early trials showed that immunotherapy monotherapy was inferior to EGFR-TKIs in EGFR-mutated NSCLC[52,53]. Subsequent studies showed that the combination of immunotherapy with EGFR-TKIs in EGFR-mutated NSCLC resulted in high rates of serious AEs (33.3%-71.4% of grade 3-4 AEs)[54-56]. Therefore, additional studies are necessary before being able to use immunotherapy with EGFR-TKIs in patients with EGFR-mutated NSCLC.
Roviello et al[57] reported that EGFR-TKIs led to good outcomes in older adults with EGFR-mutated NSCLC. We agree that EGFR-TKIs could be a valuable and less toxic treatment option for older adults who often have difficulties with chemotherapy. Unfortunately, in the present study, the sample size was too small to be able to examine the influence of age on the treatment outcomes. Furthermore, as per the inclusion criteria, no patients > 72 years old were enrolled. Nevertheless, examining treatment options specifically in older adults is indeed a future direction for research.
This study had some limitations. This study was an exploratory study with a small sample size, and the analysis of OS had limited power. In addition, it was restricted to Chinese patients. It was an investigator-initiated trial. Only icotinib was provided, and the patients had to pay for the chemotherapy. This could have influenced recruitment. Although the trial was open to stage IIIB-IV patients, only one stage IIIB participant was actually recruited, mostly limiting the conclusions to stage IV patients. Due to the limited generalizability, the efficacy of sequential chemotherapy followed by TKI in the Caucasian population requires further investigation. Whether the results could also be generalized to non-stage IV patients remains to be examined.
For patients with stage IV NSCLC and EGFR mutation, sequential chemotherapy followed by TKI maintenance therapy is feasible. No significant differences were found in terms of the influence of the different number of chemotherapy cycles or different chemotherapy drugs on the curative effect, suggesting that fewer chemotherapy cycles could result in the same therapeutic effect in these specific patients.
In 2018, 2.1 million new lung cancers and 1.8 million deaths were reported, and non-small cell lung cancers (NSCLCs) represent the greatest number (85%-90%) of malignant lung tumors. In Asians, 51.4% of epidermal growth factor receptor (EGFR)-mutated NSCLC was reported and EGFR-tyrosine kinase inhibitors (TKIs) have proved to be an effective treatment for this population.
Drug resistance always occurs after 10 mo of EGFR-TKIs treatment, and combination therapy could be an alternative to solve this difficulty. However, the most adequate combinational strategy remains controversial.
Some clinical studies have reported that sequential chemotherapy followed by maintenance EGFR-TKIs might be a potential strategy compared with EGFR-TKIs monotherapy. The efficacy and tolerability of icotinib has been demonstrated in many studies. Therefore, this pilot randomized controlled trial (RCT) aims to evaluate the efficacy and safety of combination therapy compared with monotherapy.
This multicenter, open-label, pilot RCT enrolled 68 EGFR-mutated stage IIIB/IV NSCLC patients randomized 2:3 to the icotinib-alone and chemotherapy + icotinib groups.
A statistically significant difference was observed between the icotinib-alone and chemotherapy + icotinib groups regarding median progression-free survival (P = 0.0249). No statistically significant difference was found between two and four cycles of chemotherapy which means that the sequential combination of chemotherapy and EGFR-TKIs is feasible. Sequential chemotherapy followed by maintenance EGFR-TKIs might be a potential strategy for EGFR-mutated NSCLC patients; however, the optimal regimen remains to be determined.
The sequential combination of chemotherapy and EGFR-TKIs could be a feasible strategy for stage IV EGFR-mutated NSCLC patients. It is suggested that 2-cycle sequential combination chemotherapy could have similar effectiveness to that of 4-cycle sequential combination chemotherapy in these patients.
Future studies should involve a large population from multiple centers around the world to further validate the efficacy and safety of sequential treatment in EGFR-mutated NSCLC patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chiu CC, Taiwan; Roudi R, United States; Singh I, United States S-Editor: Zhang H L-Editor: Webster JR P-Editor: Zhang H
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55842] [Article Influence: 7977.4] [Reference Citation Analysis (132)] |
| 2. | Novello S, Barlesi F, Califano R, Cufer T, Ekman S, Levra MG, Kerr K, Popat S, Reck M, Senan S, Simo GV, Vansteenkiste J, Peters S; ESMO Guidelines Committee. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v1-v27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 654] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 3. | Cabanero M, Sangha R, Sheffield BS, Sukhai M, Pakkal M, Kamel-Reid S, Karsan A, Ionescu D, Juergens RA, Butts C, Tsao MS. Management of EGFR-mutated non-small-cell lung cancer: practical implications from a clinical and pathology perspective. Curr Oncol. 2017;24:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol. 2011;6:49-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 625] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 5. | Singer J, Irmisch A, Ruscheweyh HJ, Singer F, Toussaint NC, Levesque MP, Stekhoven DJ, Beerenwinkel N. Bioinformatics for precision oncology. Brief Bioinform. 2019;20:778-788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Morgillo F, Della Corte CM, Fasano M, Ciardiello F. Mechanisms of resistance to EGFR-targeted drugs: lung cancer. ESMO Open. 2016;1:e000060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 324] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 7. | Maione P, Sacco PC, Casaluce F, Sgambato A, Santabarbara G, Rossi A, Gridelli C. Overcoming Resistance to EGFR Inhibitors in NSCLC. Rev Recent Clin Trials. 2016;11:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Del Re M, Crucitta S, Gianfilippo G, Passaro A, Petrini I, Restante G, Michelucci A, Fogli S, de Marinis F, Porta C, Chella A, Danesi R. Understanding the Mechanisms of Resistance in EGFR-Positive NSCLC: From Tissue to Liquid Biopsy to Guide Treatment Strategy. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 9. | Nan X, Xie C, Yu X, Liu J. EGFR TKI as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer. Oncotarget. 2017;8:75712-75726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 10. | Decoster L, Giron P, Mignon S, De Grève J. The evolving first-line treatment of advanced non-small cell lung cancer harbouring epidermal growth factor receptor mutations. Transl Lung Cancer Res. 2018;7:S134-S137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Sebastian M, Schmittel A, Reck M. First-line treatment of EGFR-mutated nonsmall cell lung cancer: critical review on study methodology. Eur Respir Rev. 2014;23:92-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Zhang C, Leighl NB, Wu YL, Zhong WZ. Emerging therapies for non-small cell lung cancer. J Hematol Oncol. 2019;12:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 13. | Han B, Jin B, Chu T, Niu Y, Dong Y, Xu J, Gu A, Zhong H, Wang H, Zhang X, Shi C, Zhang Y, Zhang W, Lou Y, Zhu L, Pei J. Combination of chemotherapy and gefitinib as first-line treatment for patients with advanced lung adenocarcinoma and sensitive EGFR mutations: A randomized controlled trial. Int J Cancer. 2017;141:1249-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 14. | Wu M, Yuan Y, Pan YY, Zhang Y. Combined gefitinib and pemetrexed overcome the acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Mol Med Rep. 2014;10:931-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Cheng H, An SJ, Dong S, Zhang YF, Zhang XC, Chen ZH, Jian-Su, Wu YL. Molecular mechanism of the schedule-dependent synergistic interaction in EGFR-mutant non-small cell lung cancer cell lines treated with paclitaxel and gefitinib. J Hematol Oncol. 2011;4:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Li T, Ling YH, Goldman ID, Perez-Soler R. Schedule-dependent cytotoxic synergism of pemetrexed and erlotinib in human non-small cell lung cancer cells. Clin Cancer Res. 2007;13:3413-3422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 170] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 17. | Giovannetti E, Lemos C, Tekle C, Smid K, Nannizzi S, Rodriguez JA, Ricciardi S, Danesi R, Giaccone G, Peters GJ. Molecular mechanisms underlying the synergistic interaction of erlotinib, an epidermal growth factor receptor tyrosine kinase inhibitor, with the multitargeted antifolate pemetrexed in non-small-cell lung cancer cells. Mol Pharmacol. 2008;73:1290-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 18. | Feng X, Zhang Y, Li T, Li Y. Sequentially administrated of pemetrexed with icotinib/erlotinib in lung adenocarcinoma cell lines in vitro. Oncotarget. 2017;8:114292-114299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Kubo E, Yamamoto N, Nokihara H, Fujiwara Y, Horinouchi H, Kanda S, Goto Y, Ohe Y. Randomized phase II study of sequential carboplatin plus paclitaxel and gefitinib in chemotherapy-naïve patients with advanced or metastatic non-small-cell lung cancer: Long-term follow-up results. Mol Clin Oncol. 2017;6:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Choi YJ, Lee DH, Choi CM, Lee JS, Lee SJ, Ahn JH, Kim SW. Randomized phase II study of paclitaxel/carboplatin intercalated with gefitinib compared to paclitaxel/carboplatin alone for chemotherapy-naïve non-small cell lung cancer in a clinically selected population excluding patients with non-smoking adenocarcinoma or mutated EGFR. BMC Cancer. 2015;15:763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Patil VM, Noronha V, Joshi A, Choughule AB, Bhattacharjee A, Kumar R, Goud S, More S, Ramaswamy A, Karpe A, Pande N, Chandrasekharan A, Goel A, Talreja V, Mahajan A, Janu A, Purandare N, Prabhash K. Phase III study of gefitinib or pemetrexed with carboplatin in EGFR-mutated advanced lung adenocarcinoma. ESMO Open. 2017;2:e000168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 22. | Wang MC, Liang X, Liu ZY, Cui J, Liu Y, Jing L, Jiang LL, Ma JQ, Han LL, Guo QQ, Yang CC, Wang J, Wu T, Nan KJ, Yao Y. In vitro synergistic antitumor efficacy of sequentially combined chemotherapy/icotinib in nonsmall cell lung cancer cell lines. Oncol Rep. 2015;33:239-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Zhang Y, Wang J, Yu Z, Ge H, Zhang LW, Feng LX. Outcomes of concurrent versus sequential icotinib therapy and chemotherapy in advanced non-small cell lung cancer with sensitive EGFR mutations. Clin Transl Sci. 2021;14:890-897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Zheng Y, Fang W, Deng J, Zhao P, Xu N, Zhou J. Sequential treatment of icotinib after first-line pemetrexed in advanced lung adenocarcinoma with unknown EGFR gene status. J Thorac Dis. 2014;6:958-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 25. | Nishino M, Jackman DM, Hatabu H, Yeap BY, Cioffredi LA, Yap JT, Jänne PA, Johnson BE, Van den Abbeele AD. New Response Evaluation Criteria in Solid Tumors (RECIST) guidelines for advanced non-small cell lung cancer: comparison with original RECIST and impact on assessment of tumor response to targeted therapy. AJR Am J Roentgenol. 2010;195:W221-W228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 159] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 26. | Herbst RS, Prager D, Hermann R, Fehrenbacher L, Johnson BE, Sandler A, Kris MG, Tran HT, Klein P, Li X, Ramies D, Johnson DH, Miller VA; TRIBUTE Investigator Group. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5892-5899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1120] [Cited by in RCA: 1113] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 27. | Johnson DH. Targeted therapies in combination with chemotherapy in non-small cell lung cancer. Clin Cancer Res. 2006;12:4451s-4457s. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Davies AM, Ho C, Lara PN Jr, Mack P, Gumerlock PH, Gandara DR. Pharmacodynamic separation of epidermal growth factor receptor tyrosine kinase inhibitors and chemotherapy in non-small-cell lung cancer. Clin Lung Cancer. 2006;7:385-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 29. | Zhou C, Wu YL, Liu X, Wang CL, Chen GY, Feng JF, Zhang SC, Wang J, Zhou SW, Ren SX, Lu S, Zhang L, Hu CP, Luo Y, Chen L, Ye M, Huang JN, Zhi XY, Zhang YP, Xiu QY. Overall survival (OS) results from OPTIMAL (CTONG0802), a phase III trial of erlotinib (E) vs carboplatin plus gemcitabine (GC) as first-line treatment for Chinese patients with EGFR mutation-positive advanced non-small cell lung cancer (NSCLC). J Clin Oncol. 2012;30:7520. [RCA] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Sugawara S, Oizumi S, Minato K, Harada T, Inoue A, Fujita Y, Maemondo M, Yoshizawa H, Ito K, Gemma A, Nishitsuji M, Harada M, Isobe H, Kinoshita I, Morita S, Kobayashi K, Hagiwara K, Kurihara M, Nukiwa T. Randomized phase II study of concurrent versus sequential alternating gefitinib and chemotherapy in previously untreated non-small cell lung cancer with sensitive EGFR mutations: NEJ005/TCOG0902. Ann Oncol. 2015;26:888-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 31. | Yang JC, Cheng Y, Murakami H, Yang PC, He J, Nakagawa K, Kang JH, Kim JH, Hozak RR, Nguyen TS, Zhang WL, Enatsu S, Puri T, Orlando M. A Randomized Phase 2 Study of Gefitinib With or Without Pemetrexed as First-line Treatment in Nonsquamous NSCLC With EGFR Mutation: Final Overall Survival and Biomarker Analysis. J Thorac Oncol. 2020;15:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 32. | Nakamura A, Inoue A, Morita S, Hosomi Y, Kato T, Fukuhara T, Gemma A, Takahashi K, Fujita Y, Harada T, Minato K, Takamura K, Kobayashi K, Nukiwa T. Phase III study comparing gefitinib monotherapy (G) to combination therapy with gefitinib, carboplatin, and pemetrexed (GCP) for untreated patients (pts) with advanced non-small cell lung cancer (NSCLC) with EGFR mutations (NEJ009). J Clin Oncol. 2018;36:9005. [RCA] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 33. | Noronha V, Patil VM, Joshi A, Menon N, Chougule A, Mahajan A, Janu A, Purandare N, Kumar R, More S, Goud S, Kadam N, Daware N, Bhattacharjee A, Shah S, Yadav A, Trivedi V, Behel V, Dutt A, Banavali SD, Prabhash K. Gefitinib Versus Gefitinib Plus Pemetrexed and Carboplatin Chemotherapy in EGFR-Mutated Lung Cancer. J Clin Oncol. 2020;38:124-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 296] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 34. | Zhang L, Qi Y, Xing K, Qian S, Zhang P, Wu X. A novel strategy of EGFRTKI combined chemotherapy in the treatment of human lung cancer with EGFRsensitive mutation. Oncol Rep. 2018;40:1046-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Wen M, Xia J, Sun Y, Wang X, Fu X, Zhang Y, Zhang Z, Zhou Y, Li X. Combination of EGFR-TKIs with chemotherapy versus chemotherapy or EGFR-TKIs alone in advanced NSCLC patients with EGFR mutation. Biologics. 2018;12:183-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | Yan X, Wang H, Li P, Zhang G, Zhang M, Yang J, Zhang X, Zheng X, Ma Z. Efficacy of first-line treatment with epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) alone or in combination with chemotherapy for advanced non-small cell lung cancer (NSCLC) with low-abundance mutation. Lung Cancer. 2019;128:6-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Yang JC, Kang JH, Mok T, Ahn MJ, Srimuninnimit V, Lin CC, Kim DW, Tsai CM, Barraclough H, Altug S, Orlando M, Park K. First-line pemetrexed plus cisplatin followed by gefitinib maintenance therapy versus gefitinib monotherapy in East Asian patients with locally advanced or metastatic non-squamous non-small cell lung cancer: a randomised, phase 3 trial. Eur J Cancer. 2014;50:2219-2230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Yang JC, Srimuninnimit V, Ahn MJ, Lin CC, Kim SW, Tsai CM, Mok T, Orlando M, Puri T, Wang X, Park K. First-Line Pemetrexed plus Cisplatin followed by Gefitinib Maintenance Therapy versus Gefitinib Monotherapy in East Asian Never-Smoker Patients with Locally Advanced or Metastatic Nonsquamous Non-Small Cell Lung Cancer: Final Overall Survival Results from a Randomized Phase 3 Study. J Thorac Oncol. 2016;11:370-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Patil PD, Shepherd F, Johnson DH. A Career in Lung Cancer: Pushing Beyond Chemotherapy. Am Soc Clin Oncol Educ Book. 2019;39:583-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Lim ZF, Ma PC. Emerging insights of tumor heterogeneity and drug resistance mechanisms in lung cancer targeted therapy. J Hematol Oncol. 2019;12:134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 362] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 41. | Cheng Y, He Y, Li W, Zhang HL, Zhou Q, Wang B, Liu C, Walding A, Saggese M, Huang X, Fan M, Wang J, Ramalingam SS. Osimertinib Versus Comparator EGFR TKI as First-Line Treatment for EGFR-Mutated Advanced NSCLC: FLAURA China, A Randomized Study. Target Oncol. 2021;16:165-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 104] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 42. | Horinouchi H. To combine or not to combine: anti-vascular endothelial growth factor therapies in EGFR mutation positive non-small cell lung cancer. Ann Transl Med. 2020;8:554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 43. | Rossi A, Chiodini P, Sun JM, O'Brien ME, von Plessen C, Barata F, Park K, Popat S, Bergman B, Parente B, Gallo C, Gridelli C, Perrone F, Di Maio M. Six versus fewer planned cycles of first-line platinum-based chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol. 2014;15:1254-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 44. | Tartarone A, Roviello G, Lerose R, Roudi R, Aieta M, Zoppoli P. Anti-PD-1 versus anti-PD-L1 therapy in patients with pretreated advanced non-small-cell lung cancer: a meta-analysis. Future Oncol. 2019;15:2423-2433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 45. | Mohsenzadegan M, Peng RW, Roudi R. Dendritic cell/cytokine-induced killer cell-based immunotherapy in lung cancer: What we know and future landscape. J Cell Physiol. 2020;235:74-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 46. | Petrelli F, Ferrara R, Signorelli D, Ghidini A, Proto C, Roudi R, Sabet MN, Facelli S, Garassino MC, Luciani A, Roviello G. Immune checkpoint inhibitors and chemotherapy in first-line NSCLC: a meta-analysis. Immunotherapy. 2021;13:621-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 47. | Petrelli F, Ghidini A, Luciani A. Topotecan or other agents as second-line therapy for relapsed small-cell lung cancer: A meta-analysis of randomized studies. Mol Clin Oncol. 2021;15:218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 48. | NCCN Clincal Practice Guidelines in Oncology (NCCN Guidelines). Non-Small Cell Lung Cancer. Version 2.2020. Fort Washington: National Comprehensive Cancer Network, 2019. |
| 49. | Rebuzzi SE, Alfieri R, La Monica S, Minari R, Petronini PG, Tiseo M. Combination of EGFR-TKIs and chemotherapy in advanced EGFR mutated NSCLC: Review of the literature and future perspectives. Crit Rev Oncol Hematol. 2020;146:102820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 50. | Jin R, Zhao J, Xia L, Li Q, Li W, Peng L, Xia Y. Application of immune checkpoint inhibitors in EGFR-mutant non-small-cell lung cancer: from bed to bench. Ther Adv Med Oncol. 2020;12:1758835920930333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 51. | Ito T, Nagashima H, Akiyama M, Utsumi Y, Sato H, Chiba S, Sugai M, Ube K, Mori Y, Watanabe K, Fukuhara T, Maemondo M. Treatment with immune checkpoint inhibitors after EGFR-TKIs in EGFR-mutated lung cancer. Thorac Cancer. 2022;13:386-393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | Wiest N, Majeed U, Seegobin K, Zhao Y, Lou Y, Manochakian R. Role of Immune Checkpoint Inhibitor Therapy in Advanced EGFR-Mutant Non-Small Cell Lung Cancer. Front Oncol. 2021;11:751209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 53. | Qiao M, Jiang T, Liu X, Mao S, Zhou F, Li X, Zhao C, Chen X, Su C, Ren S, Zhou C. Immune Checkpoint Inhibitors in EGFR-Mutated NSCLC: Dusk or Dawn? J Thorac Oncol. 2021;16:1267-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 54. | Creelan BC, Yeh TC, Kim SW, Nogami N, Kim DW, Chow LQM, Kanda S, Taylor R, Tang W, Tang M, Angell HK, Roudier MP, Marotti M, Gibbons DL. A Phase 1 study of gefitinib combined with durvalumab in EGFR TKI-naive patients with EGFR mutation-positive locally advanced/metastatic non-small-cell lung cancer. Br J Cancer. 2021;124:383-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 55. | Yang JC, Gadgeel SM, Sequist LV, Wu CL, Papadimitrakopoulou VA, Su WC, Fiore J, Saraf S, Raftopoulos H, Patnaik A. Pembrolizumab in Combination With Erlotinib or Gefitinib as First-Line Therapy for Advanced NSCLC With Sensitizing EGFR Mutation. J Thorac Oncol. 2019;14:553-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 132] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 56. | Ma BBY, Rudin CM, Cervantes A, Dowlati A. Preliminary Safety and Clinical Activity of Erlotinib Plus Atezolizumab From a Phase Ib Study in Advanced NSCLC. Ann Oncol. 2016;27:ix139-ix156. [RCA] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 57. | Roviello G, Zanotti L, Cappelletti MR, Gobbi A, Dester M, Paganini G, Pacifico C, Generali D, Roudi R. Are EGFR tyrosine kinase inhibitors effective in elderly patients with EGFR-mutated non-small cell lung cancer? Clin Exp Med. 2018;18:15-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |