Published online Jun 26, 2022. doi: 10.12998/wjcc.v10.i18.6050
Peer-review started: December 18, 2021
First decision: January 23, 2022
Revised: January 31, 2022
Accepted: April 24, 2022
Article in press: April 24, 2022
Published online: June 26, 2022
Processing time: 180 Days and 20.3 Hours
Gastric antral vascular ectasia (GAVE) has diverse associations and presumed causes, which include liver cirrhosis, chronic kidney disease, and autoimmune disease. This heterogeneity of underlying disorders suggests that the pathogenesis of GAVE may be variable.
To compare the clinical features and long-term outcomes of GAVE according to endoscopic patterns and etiologies.
The medical records and endoscopic images of 23 consecutive patients diagnosed with GAVE by endoscopy at Yeungnam University Hospital from January 2006 to December 2020 were retrospectively reviewed. Patients were allocated to cirrhosis (16 patients) and non-cirrhosis groups (7 patients). GAVE subtypes, as determined by endoscopy, were categorized as punctate (a diffuse, honeycomb-like appear
All GAVE patients with cirrhosis (16/16, 100%) had a punctate pattern by endoscopy, whereas the majority of patients (6/7, 85.7%) without cirrhosis had a striped pattern (P < 0.001). Overt GAVE bleeding (10/23, 43%) was significantly more common in the non-cirrhosis group than in the cirrhosis group (6/7, 85.7% vs 4/16, 25.0%; P = 0.019), and more common in the striped group than in the punctate group (5/6, 83.3% vs 5/17, 29.4%; P = 0.052). However, mean numbers of admissions due to GAVE bleeding and argon plasma coagulation (APC) sessions to address overt bleeding were similar in the cirrhosis and non-cirrhosis groups and in the punctate and striped groups. All patients with GAVE bleeding were successfully treated by APC, and no patient died from GAVE-related blood loss during a median follow-up of 24 mo.
Punctate-type GAVE is strongly associated with liver cirrhosis, and GAVE patients without cirrhosis tend to be more prone to overt bleeding. However, the presence of cirrhosis and endoscopic patterns did not influence long-term clinical courses or outcomes in cases of overt bleeding.
Core Tip: The study shows that punctate (diffuse, honeycomb)-type gastric antral vascular ectasia (GAVE) is strongly associated with liver cirrhosis, whereas striped (linear, watermelon)-type GAVE is strongly associated with non-cirrhotic underlying disease. Additionally, GAVE patients without cirrhosis tended to be more prone to overt bleeding. However, the presence of cirrhosis and endoscopic GAVE patterns did not influence clinical courses or the outcomes of overt bleeding after endoscopic APC treatment. It appears that clinical manifestations are dependent on etiologies, but that etiologies do not influence clinical courses in cases of GAVE bleeding.
- Citation: Kwon HJ, Lee SH, Cho JH. Influences of etiology and endoscopic appearance on the long-term outcomes of gastric antral vascular ectasia. World J Clin Cases 2022; 10(18): 6050-6059
- URL: https://www.wjgnet.com/2307-8960/full/v10/i18/6050.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i18.6050
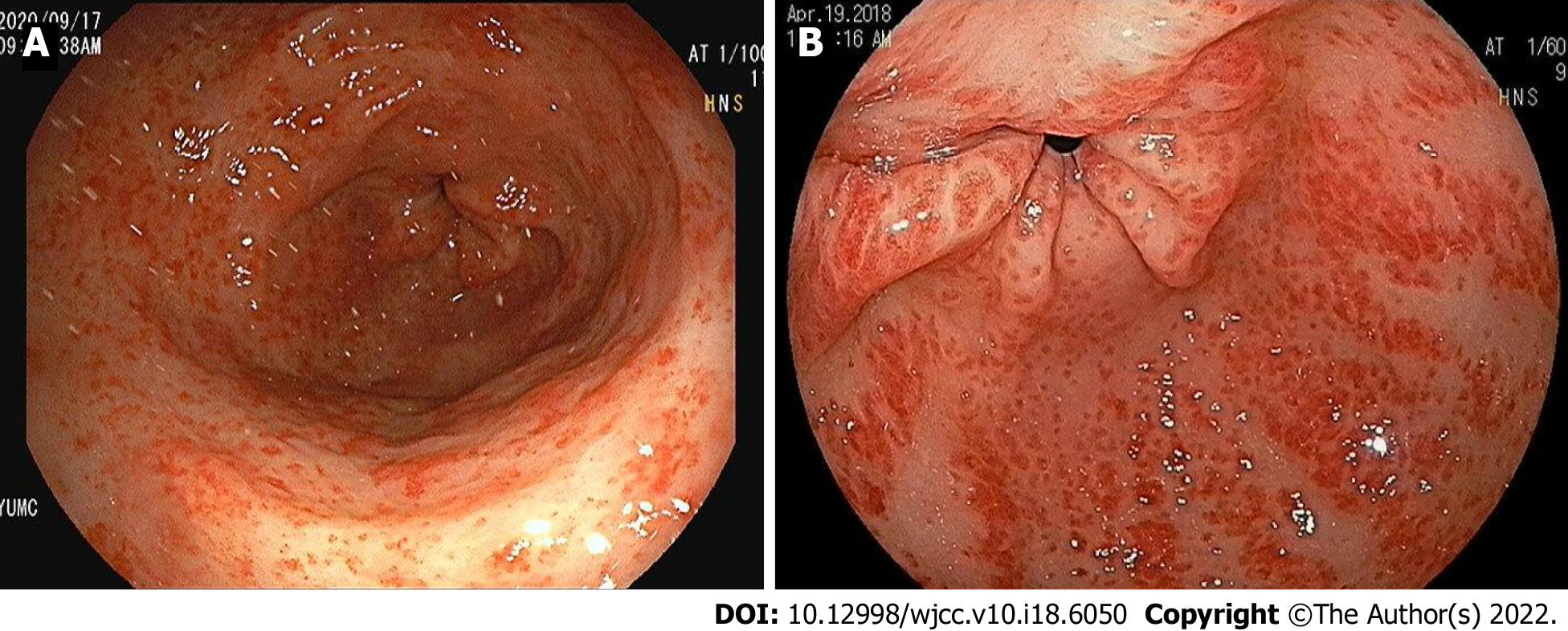
Gastric antral vascular ectasia (GAVE), also known as “watermelon stomach”, is a rare clinical condition related to upper gastrointestinal (GI) bleeding, which varies from chronic occult blood loss requiring serial transfusions to acute hemorrhage[1]. GAVE was first described by Rider et al[2] in 1953 and fully defined by Jabbari et al[3] in 1984, who described patients with hyperemic striae in gastric antrum. GAVE is characterized by a unique endoscopic appearance, which takes two forms, punctate (a diffuse, honeycomb-like appearance) and striped (a linear, watermelon-like appearance). Striped GAVE has the endoscopic appearance of red stripes radiating from antrum and converging at pylorus, whereas the punctate form is characterized by diffuse red spots indicating dilated blood vessels in antrum.
Liver cirrhosis is the most frequent underlying condition associated with GAVE and portal hypertension is seen in approximately 40% of reported cases[4,5], though GAVE may not respond to therapies directed at reducing portal pressure. In addition, GAVE has been associated with other diverse medical conditions including chronic renal failure, diabetes, and autoimmune and connective tissue disorders such as systemic lupus erythematosus, rheumatoid arthritis, and systemic sclerosis[6-10]. Thus, the heterogeneity of underlying diseases observed in GAVE patients suggests its pathogenesis may be variable[11].
Epidemiologic evidence indicating that the clinical outcomes of GAVE patients with or without cirrhosis differ is lacking. Although numerous studies have investigated GAVE and many case reports and series have been reported, little information is available on the effects of its etiologies and gross endoscopic appearances on long-term clinical courses or outcomes, especially in Asians. In this study, we aimed to determine whether the presence of cirrhosis and endoscopic patterns are related to the clinical features and course of GAVE.
This retrospective cohort study was approved by the Institutional Review Board of Yeungnam University Hospital (IRB No. 2021-10-044), which waived the requirement for written informed consent due to the retrospective nature of the study. We reviewed the medical records of 23 consecutive patients diagnosed with GAVE by endoscopy at our institution from January 2006 to December 2020. Baseline demographic data such as gender, etiology of liver disease, and severity of liver disease (as determined using Child-Pugh scores) were collected, as were details of medical comorbidities (liver cirrhosis, chronic renal failure, autoimmune or connective tissue disease, diabetes, and others), medications [proton pump inhibitors (PPIs), non-steroidal anti-inflammatory drugs (NSAIDs), and anticoagulant and antiplatelet use], and follow-up durations. Laboratory findings such as hemoglobin levels, platelet counts, serum urea nitrogen and creatinine, serum albumin, and prothrombin activity were also reviewed. Patients were categorized into two groups based on the presence (16 patients) or absence (7 patients) of cirrhosis. Cirrhosis was diagnosed based on a combination of clinical findings, liver function tests, and computed tomographic or ultrasonographic appearances of liver and spleen. Only patients aged 18 or older were included in the analysis. Median follow-up duration was 24 mo (range 6-84 mo) in the cirrhosis group and 26 mo (range 4-96 mo) in the non-cirrhosis group.
GAVE was diagnosed during upper GI endoscopy by skilled gastroenterologists. Histologic specimens were not required to differentiate GAVE from portal hypertensive gastropathy. GAVE was diagnosed primarily based on endoscopic patterns and in uncertain cases by histology[12]. Endoscopy reports and photographs were reviewed independently by two gastroenterologists (Cho JH and Kwon HJ) unaware of clinical statuses. When these two gastroenterologists disagreed regarding diagnoses, a third independent gastroenterologist (Lee SH) reviewed the information available and made final decisions. Patients were classified into one of two groups according to the two typical endoscopic appearances of GAVE, that is, to a “punctate” (diffuse, honeycomb) group (17 patients) with sharply demarcated red spots diffusely spread within antrum (Figure 1A) or a “striped” (linear, watermelon) type group (6 patients) with prominent flat or raised red stripes radiating longitudinally from pylorus to antrum (Figure 1B)[6]. During upper GI endoscopy, bleeding from GAVE lesions and the presence of other nonbleeding lesions were noted (these included lesions due to portal hypertension in patients with cirrhosis). Overt GI bleeding was defined based on the presence of symptoms, such as hematemesis, melena, or hematochezia, a change in hemodynamics, or gross bleeding by upper GI endoscopy. Median follow-up duration was 23 mo (range 6-84 mo) in the punctate group and 27 mo (range 4-96 mo) in the striped group.
Argon plasma coagulation (APC) treatment for GAVE consisted of electrocoagulation of all angiectatic lesions during an endoscopic session with an APC probe introduced through the working channel of an endoscope. Argon gas flow ranged from 0.8 L/min to 1 L/min, and electric power output from 50 W to 70 W. Angioectasia was managed using a combination of focal pulse and ‘‘spray-painting’’ techniques. During endoscopic sessions, argon and coagulation smoke were regularly aspirated, and coagulum on probes was removed when required. Treatment time per session varied from 20 min to 40 min. All patients received PPI therapy for 3 wk to 4 wk to promote mucosal healing after the procedure. The endpoint of treatment was defined as complete or near complete disappearance of vascular ectasia. In cases of anemia recurrence requiring transfusion of packed red blood cells or cases of overt GI bleeding recurrence, another APC session was performed. The criteria for successful APC treatment were cessation of GI bleeding and the need for transfusion. Numbers of APC sessions per patient were documented and APC treatment success rates were evaluated.
Data were analyzed using SPSS version 20.0 for Windows (SPSS Inc, Chicago, IL). GAVE patients with or without cirrhosis were compared with respect to multiple factors, which included demographics, associated underlying diseases, laboratory values, and clinical outcomes of endoscopic APC treatment. Patients with punctate or striped GAVE were also compared. Continuous variables are expressed as mean ± SD and were compared using the Student’s t test or the Mann-Whitney test. Categorical variables are expressed as absolute numbers and proportions and were compared using Fisher’s exact test. All statistical tests were two-sided, and statistical significance was accepted for P < 0.05.
Over the fifteen-year study period (January 2006 to December 2020), 23 patients were diagnosed with GAVE at our institution. Mean patient age was 59.8 ± 12.6 years (range 41-79) and 14 (60.9%) were male. Indications for initial endoscopy were screening for varices (8 patients), iron deficiency anemia (5), melena (8), or hematemesis (2). The etiology of cirrhosis was viral in 6 patients, alcohol in 6, auto
| Cirrhosis (n = 16) | No cirrhosis (n = 7) | P value | |
| Endoscopic pattern of GAVE, n (%) | < 0.001 | ||
| Punctate (Diffuse) | 16 (100) | 1 (14.3) | |
| Stripe (Linear) | 0 | 6 (85.7) | |
| Age, yr, mean ± SD | 55.4 ± 12.2 | 69.8 ± 6.1 | 0.015 |
| Gender (males/females) | 12/4 | 2/5 | 0.066 |
| BMI, kg/m2, mean ± SD | 23.4 ± 4.0 | 25.0 ± 4.6 | 0.397 |
| Etiology of cirrhosis, n (%) | |||
| Viral | 6 (37.5) | ||
| Alcohol | 6 (37.5) | ||
| Autoimmune hepatitis | 1 (6.3) | NA | NA |
| NASH | 1 (6.3) | ||
| Cryptogenic | 2 (12.5) | ||
| Child Pugh Score, n (%) | |||
| Class A | 5 (31.3) | ||
| Class B | 8 (50.0) | NA | NA |
| Class C | 3 (18.7) | ||
| Other associated diseases, n (%) | |||
| Chronic kidney disease | 2 (12.5) | 5 (71.4) | 0.011 |
| SLE | 0 | 1 (14.3) | 0.304 |
| Hypertension | 3 (18.8) | 2 (28.6) | 0.621 |
| Diabetes mellitus | 5 (31.3) | 3 (42.9) | 0.657 |
| Medication, n (%) | |||
| PPI use | 6 (37.5) | 3 (42.9) | 1.000 |
| Antiplatelet use | 0 | 1 (14.3) | 0.304 |
| Anticoagulant use | 0 | 1 (14.3) | 0.304 |
| Initial laboratory findings, mean ± SD | |||
| Hemoglobin level, g/dL | 7.8 ± 3.4 | 6.2 ± 1.6 | 0.494 |
| Platelet count, k/μL | 103.9 ± 73.0 | 176.8 ± 62.2 | 0.041 |
| Total bilirubin, mg/dL | 1.74 ± 1.35 | 1.15 ± 1.30 | 0.188 |
| Albumin, g/dL | 2.52 ± 0.36 | 3.03 ± 0.60 | 0.048 |
| INR | 1.23 ± 0.27 | 1.09 ± 0.25 | 0.179 |
| BUN, mg/dL, | 23.3 ± 14.0 | 44.9 ± 39.1 | 0.416 |
| Creatinine, mg/dL | 1.12 ± 0.44 | 3.65 ± 2.93 | 0.244 |
The characteristics of patients in the cirrhosis and non-cirrhosis groups at GAVE diagnosis are summarized in Table 1. Mean age was significantly greater in the cirrhosis group (69.8 ± 6.1 vs 55.4 ± 12.2, P = 0.015). Men predominated in the cirrhosis group (12/16, 75.0%) and women in the non-cirrhosis group (5/7, 71.4%). Mean group body mass indices (BMIs) were not significantly different. In the non-cirrhosis group, the most common associated disease was chronic kidney disease (5/7, 71.4%), and this was followed by diabetes mellitus (3/7, 42.9%), and the prevalence of chronic kidney disease was significantly greater in the non-cirrhosis group (5/7, 71.4% vs 2/16, 12.5%; P = 0.011). The frequencies of other associated diseases, except chronic kidney disease, were similar in the two groups. Although no patient received corticosteroids or NSAIDs, 2 patients in the non-cirrhosis group were on antiplatelet or anticoagulant medication, respectively, which did not amount to a significant difference versus the cirrhotic group. As regards initial laboratory findings, platelet count and serum albumin level were significantly lower in the cirrhosis group (103.9 ± 73.0 k/μL vs 176.8 ± 62.2 k/μL, P = 0.041; and 2.52 ± 0.36 g/dL vs 3.03 ± 0.60 g/dL, P = 0.048, respectively). Mean hemoglobin levels were not significantly different in the cirrhosis and non-cirrhosis groups (7.8 ± 3.4 vs 6.2 ± 1.6, respectively, P = 0.494). Total bilirubin and international normalized ratio (INR) were non-significantly greater in the cirrhosis group (1.74 ± 1.35 vs 1.15 ± 1.30, and 1.23 ± 0.27 vs 1.09 ± 0.25; P = 0.188 and 0.179, respectively). Blood urea nitrogen (BUN) and serum creatinine were higher in the non-cirrhosis group (44.9 ± 39.1 vs 23.3 ± 14.0, and 3.65 ± 2.93 vs 1.12 ± 0.44, respectively), but again differences were not significant (NS, P = 0.416 and 0.244, respectively). However, group endoscopic patterns were significantly different; all patients in the cirrhosis group had punctate GAVE, whereas 6/7 patients (85.7%) in the non-cirrhosis group had striped GAVE (P < 0.001).
The characteristics of patients in the punctate and striped groups at diagnosis are summarized in Table 2. Mean age was significantly greater in the striped group (70.8 ± 6.3 vs 56.1 ± 12.0, P = 0.011). Men predominated in the punctate group (13/17, 76.5%), and women predominated in the striped group (5/6, 83.3%). Group BMIs were not significantly different. Sixteen of the 17 patients in the punctate group (16/17, 94.1%) had concurrent cirrhosis, whereas no patient in the striped group (0/6, 0%) had cirrhosis (P < 0.001). In the striped group, the most common associated disease was chronic kidney disease (5/6, 83.3%), which was followed by diabetes mellitus (3/6, 50%), and patients in this group had an increased prevalence of chronic kidney disease (5/6, 83.3% vs 2/17, 11.8%; P = 0.014). The frequencies of other associated diseases were not significantly different in the two groups. As regards initial laboratory findings, serum bilirubin was significantly higher in the punctate group (1.88 ± 1.39 vs 0.64 ± 0.41, P = 0.044), but creatinine was significantly higher in the striped group (4.27 ± 2.79 vs 1.07 ± 0.45, P = 0.034), and BUN was non-significantly higher in the striped group (51.9 ± 39.9 vs 22.5 ± 13.8, P = 0.130). Mean hemoglobin levels were similar in the punctate and striped groups (7.5 ± 3.4 vs 6.6 ± 1.4, P = 0.528), but platelet counts and serum albumin levels were non-significantly lower in the punctate group (114.5 ± 81.5 vs 159.6 ± 51.1, and 2.51 ± 0.39 vs 3.00 ± 0.59; P = 0.168 and 0.070, respectively). INR was non-significantly higher in the punctate group (1.23 ± 0.26 vs 1.08 ± 0.28, respectively, P = 0.130).
| Punctate (n = 17) | Stripe (n = 6) | P value | |
| Age, yr, mean ± SD | 56.1 ± 12.0 | 70.8 ± 6.3 | 0.011 |
| Gender (males/females) | 13/4 | 1/5 | 0.018 |
| BMI, kg/m2, mean ± SD | 23.5 ± 3.9 | 25.2 ± 2.6 | 0.612 |
| Liver cirrhosis, n (%) | 16 (94.1) | 0 | < 0.001 |
| Other associated diseases, n (%) | |||
| Chronic kidney disease | 2 (11.8) | 5 (83.3) | 0.003 |
| SLE | 0 | 1 (16.7) | 0.261 |
| Hypertension | 3 (17.6) | 2 (33.3) | 0.576 |
| Diabetes mellitus | 5 (29.4) | 3 (50.0) | 0.621 |
| Initial laboratory findings, mean ± SD | |||
| Hemoglobin level, g/dL | 7.5 ± 3.4 | 6.6 ± 1.4 | 0.528 |
| Platelet count, k/μL | 114.5 ± 81.5 | 159.6 ± 51.1 | 0.168 |
| Total bilirubin, mg/dL | 1.88 ± 1.39 | 0.64 ± 0.41 | 0.044 |
| Albumin, g/dL | 2.51 ± 0.39 | 3.00 ± 0.59 | 0.070 |
| INR | 1.23 ± 0.26 | 1.08 ± 0.28 | 0.130 |
| BUN, mg/dL, | 22.5 ± 13.8 | 51.9 ± 39.9 | 0.130 |
| Creatinine, mg/dL | 1.07 ± 0.45 | 4.27 ± 2.79 | 0.034 |
Ten of the 23 patients (43.5%) were diagnosed with overt GAVE bleeding, and this was significantly more common in the non-cirrhosis group than in the cirrhosis group (6/7, 85.7% vs 4/16, 25.0%; P = 0.019, Table 3), and more common in the striped group than in the punctate group (5/6, 83.3% vs 5/17, 29.4%; P = 0.052, Table 4). Endoscopic APC treatment was required significantly more frequently in the non-cirrhosis group than in the cirrhosis group (6/7, 85.7% vs 4/16, 25.0%; P = 0.019), and more frequently required in the striped group than in the punctate group (5/6, 83.3% vs 5/17, 29.4%; P = 0.052). However, numbers of admissions due to GAVE bleeding were no different in the cirrhosis and non-cirrhosis groups (3.00 ± 2.45 vs 3.00 ± 2.83, NS) and in the punctate and striped groups (3.00 ± 2.83 vs 3.00 ± 2.45, NS). In addition, no patient in either group needed therapy escalation to surgery. All 10 patients with GAVE bleeding underwent endoscopic APC treatment on a weekly or fortnightly basis, and all responded well to treatment. The mean numbers of APC sessions in patients with GAVE bleeding were similar in the cirrhosis and non-cirrhosis groups (4.50 ± 4.95 vs 4.80 ± 5.08, NS) and in the punctate and striped groups (4.00 ± 4.34 vs 5.25 ± 5.44, NS). Median follow-up durations were 24 mo and 26 mo in cirrhosis and non-cirrhosis groups, respectively, and 23 mo and 27 mo in punctate group and striped groups, respectively (neither intergroup difference was significant). During follow-up, 3 patients in the cirrhosis group and 1 patient in the non-cirrhosis group died of a cause other than GAVE bleeding (hepatocellular carcinoma in 1, hepatic failure in 1, and multiple organic failure in 2). No patient succumbed to GI hemorrhage.
| Cirrhosis (n = 16) | No cirrhosis (n = 7) | P value | |
| Overt bleeding, n (%) | 4 (25.0) | 6 (85.7) | 0.019 |
| APC treatment, n (%) | 4 (25.0) | 6 (85.7) | 0.019 |
| No. of admissions d/t GAVE bleeding, mean ± SD | 3.00 ± 2.45 | 3.00 ± 2.83 | NS |
| No. of APC sessions, mean ± SD | 4.50 ± 4.95 | 4.80 ± 5.08 | NS |
| Success of APC | 4/4 | 6/6 | NS |
| Surgery | 0 | 0 | NS |
| Death due to GAVE bleeding | 0 | 0 | NS |
| Follow-up, month, median (range) | 24 (6-84) | 26 (4-96) | NS |
| Punctate (n = 17) | Stripe (n = 6) | P value | |
| Overt bleeding, n (%) | 5 (29.4) | 5 (83.3) | 0.052 |
| APC treatment, n (%) | 5 (29.4) | 5 (83.3) | 0.052 |
| No. of admissions d/t GAVE bleeding, mean ± SD | 3.00 ± 2.83 | 3.00 ± 2.45 | NS |
| No. of APC sessions, mean ± SD | 4.00 ± 4.34 | 5.25 ± 5.44 | NS |
| Success of APC | 5/5 | 5/5 | NS |
| Surgery | 0 | 0 | NS |
| Death due to GAVE bleeding | 0 | 0 | NS |
| Follow-up, month, median (range) | 23 (6-84) | 27 (4-96) | NS |
The pathogenesis of GAVE is unknown but seems to be related to liver cirrhosis in most cases. Several authors have suggested that portal hypertension is the most influential contributor to the development of GAVE. Tobin et al[7] reported that patients with GAVE-induced gastric bleeding after bone marrow transplantation had hepatic veno-occlusive disease, which supports suggestions that portal hypertension might be a predisposing factor of GAVE. On the other hand, it has been demonstrated that GAVE lesions may develop in patients with cirrhosis independently of portal hypertension[8,11]. Indeed, Spahr et al[8] reported that portal decompression using a transjugular intrahepatic portosystemic shunt or a surgical end-to-side portacaval shunt was ineffective at treating GAVE bleeding. In addition to cirrhosis, several other disorders such as chronic kidney disease[9], chronic valvular, ischemic, or hypertensive heart disease, and a variety of autoimmune diseases[13], including Raynaud’s phenomena, rheumatoid arthritis, primary biliary cirrhosis, and systemic sclerosis (diffuse and limited), have been also associated with the development of GAVE.
The remarkable heterogeneity of diseases associated with GAVE generates a wide array of factors that might cause angioectasia. Several factors have been suggested to play roles in the pathogenesis of GAVE. Charneau et al[14] reported that gastric antral motility was remarkably different in cirrhotic patients with or without GAVE. Others have suggested prostaglandin E2[15], gastrin[16], vasoactive intestinal polypeptide (VIP), and the proliferation of local neuroendocrine cells containing 5-hydroxytryptamine (5-HT)[17] are causative factors. Thus, it appears GAVE is probably caused by vasodilation and impaired motility[17] driven by these neurohumoral factors[15-18]. However, the pathophy
In our study, all 16 patients with cirrhosis had punctate-type GAVE and almost all patients (16/17) with punctate-type GAVE had concurrent cirrhosis. In contrast, 6 of 7 patients without cirrhosis had striped-type GAVE; the other patient had punctate-type GAVE. Thus, in this study punctate-type GAVE appeared to be associated with cirrhosis, and striped-type GAVE was not. Several reports have described linear lesions within antrum in non-cirrhotic patients and diffuse lesions in cirrhotic patients[1,6,21,22], and our results concur with these observations.
We also found that GAVE patients without cirrhosis more frequently exhibited overt bleeding and required APC treatment than those with cirrhosis. Unfortunately, we could not adjust our multivariate analysis for GI bleeding confounders due to the small number of overt GAVE bleeding cases included. Nonetheless, these observations do indicate GAVE with cirrhosis-associated nonvariceal GI bleeding is rare. However, in contrast to our results, in a retrospective study of 30 patients that underwent APC for GAVE bleeding (17 patients had cirrhosis), Lecleire et al[23] reported that patients with cirrhosis more frequently exhibited overt bleeding (65% vs 15%, P = 0.01), whereas patients without cirrhosis more frequently had occult bleeding as revealed by isolated iron deficiency anemia (35% vs 85%, P = 0.01). However, Wang et al[24] in a retrospective case-control study, reported that multivariate regression analysis adjusted for confounders showed the absence of cirrhosis best predicted active bleeding from GAVE with an odds ratio of 5.151 (95% confidence interval: 1.08-24.48, P = 0.039). In this previous study, of 84 GAVE patients with cirrhosis, 27 (32.1%) had overt bleeding, and of 26 patients without cirrhosis, 17 (63.4%) had overt bleeding. The reason for a significant difference between the incidences of overt bleeding according to the presence or absence of cirrhosis in GAVE remains unclear, although it has been shown circulating neurohumoral factor levels, such as those of prostaglandin E, VIP, and 5-HT, differ in GAVE patients with or without cirrhosis[15-18]. Future studies are needed to clarify mechanistic differences between patients with or without cirrhosis in order to determine whether neurohumoral factors are responsible for clinically observed differences.
APC has been the endoscopic treatment of choice for GAVE since its introduction. In general, patients exhibit good initial response and low complication rates, but recurrent bleeding rates are usually high, and rates of 35.0% to 78.9% have been reported[25-27]. In the present study, endoscopic APC was performed in 10 patients with GAVE bleeding (4 patients had cirrhosis) and no complication was recorded after APC treatment. However, as has also been reported, 8 of the 10 patients required additional hospitalization due to GAVE bleeding after APC treatment and only two patients did not (one in the non-cirrhosis group and one in the cirrhosis group). In order to compare the clinical courses of GAVE bleeding cases with or without cirrhosis or with punctate or striped GAVE, we focused on the number of admissions due to GAVE bleeding. Although the proportion of patients with overt GAVE bleeding that received APC treatment was significantly greater among those without cirrhosis than those with cirrhosis, mean numbers of admissions due to GAVE bleeding and of APC sessions in overt bleeding patients were similar in the cirrhosis and non-cirrhosis groups and in the striped and punctate groups. These results suggest vulnerability to bleeding depends on GAVE etiology, but that clinical course after overt bleeding does not.
All patients with GAVE bleeding were successfully treated by APC. No patient needed surgery or died of GAVE-related blood loss, and mortalities during follow-up were similar in the cirrhosis and non-cirrhosis groups and in the punctate and striped groups. These findings suggest that APC is effective and safe for treating GAVE bleeding. Furthermore, the outcomes of endoscopic APC were similar regardless of endoscopic appearance or the presence of cirrhosis, which concurs with the results of a previous study[1]. Based on the above findings, we conclude that the presence of cirrhosis and endoscopic type have no influence on response to endoscopic APC treatment. We cannot explain why regardless of etiology, APC is an effective hemostatic treatment for GAVE. Indeed, the pathophysiologic features of GAVE are undetermined, and thus, open to debate.
Some limitations of the present study warrant consideration. First, some clinical data such as radiologically determined portal vein diameters and liver fibroscan data were missing due to the retrospective design of the study and lack of availability. Second, the sample size was small, especially numbers of bleedings and APC cases, and thus, it was not possible to compare study groups with respect to some variables or perform further multivariate analysis to identify risk factors of GAVE bleeding. Nevertheless, this study is one of the few to compare long-term clinical courses according to the presence or absence of cirrhosis and endoscopic type in a non-Western region.
GAVE patients without cirrhosis tended to be more prone to overt bleeding; however, the long-term clinical courses of GAVE bleeding after endoscopic APC treatment were similar irrespective of the presence of cirrhosis or endoscopic appearance. These findings suggest that the etiologies of GAVE may result in different clinical manifestations, especially bleeding, but do not influence the clinical course of GAVE bleeding. Additional studies are needed to identify the factors that play key roles in the development and clinical course of GAVE and to clarify its pathophysiologic mechanism. Furthermore, although these issues remain to be clarified, we recommend that when GAVE is diagnosed endoscopically, a careful investigation should be undertaken to determine whether liver cirrhosis and other non-cirrhotic disease processes associated with GAVE are present.
Gastric antral vascular ectasia (GAVE) is associated with diverse medical conditions such as liver cirrhosis, chronic kidney disease, and autoimmune disease. This heterogeneity of underlying disorders suggests that the pathogenesis of GAVE may not be uniform.
Many studies have sought to determine whether clinical features differ in GAVE with or without cirrhosis. However, few have examined the effects of its etiologies and endoscopic patterns on long-term clinical courses or outcomes, especially in Asians.
To determine whether etiologies and endoscopic patterns are related to the clinical features and course of GAVE.
A retrospective analysis of 23 consecutive patients diagnosed with GAVE from January 2006 to December 2020 was conducted. Patients were allocated to cirrhosis (16 patients) and non-cirrhosis groups (7 patients), and GAVE subtypes, as determined by endoscopy, were categorized as punctate (a diffuse, honeycomb-like appearance, 17 patients) or striped (a linear, watermelon-like appearance, 6 patients).
Punctate-type GAVE was strongly associated with liver cirrhosis, whereas striped-type GAVE was strongly associated with non-cirrhotic underlying disease. Additionally, GAVE patients without cirrhosis experienced overt bleeding more often and required APC treatment more frequently than those with cirrhosis. However, mean numbers of admissions due to GAVE bleeding and of APC sessions for overt bleeding were similar in the cirrhosis and non-cirrhosis groups and in the striped and punctate groups.
GAVE etiologies may result in different clinical manifestations, especially as regards bleeding. However, etiologies and endoscopic patterns were not found to influence long-term clinical courses or treatment outcomes in cases of overt bleeding.
This study is one of the few to analyze the effects of GAVE etiologies and endoscopic patterns on long-term clinical courses and outcomes. Additional studies are needed to identify those factors that play key roles in the development and clinical course of GAVE and to clarify its pathophysiologic mechanism.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: The Korean Society of Gastroenterology.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Nakeep S, Egypt; Govindarajan KK, India S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Dulai GS, Jensen DM, Kovacs TO, Gralnek IM, Jutabha R. Endoscopic treatment outcomes in watermelon stomach patients with and without portal hypertension. Endoscopy. 2004;36:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | RIDER JA, KLOTZ AP, KIRSNER JB. Gastritis with veno-capillary ectasia as a source of massive gastric hemorrhage. Gastroenterology. 1953;24:118-123. [PubMed] |
| 3. | Jabbari M, Cherry R, Lough JO, Daly DS, Kinnear DG, Goresky CA. Gastric antral vascular ectasia: the watermelon stomach. Gastroenterology. 1984;87:1165-1170. [PubMed] |
| 4. | Egger C, Kreczy A, Kirchmair R, Waldenberger P, Jaschke W, Vogel W. Gastric antral vascular ectasia with portal hypertension: treatment with TIPSS. Am J Gastroenterol. 1997;92:2292-2294. [PubMed] |
| 5. | Fisher NC. Gastric antral vascular ectasia and its relation with portal hypertension. Gut. 2000;46:441-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Sebastian S, O'Morain CA, Buckley MJ. Review article: current therapeutic options for gastric antral vascular ectasia. Aliment Pharmacol Ther. 2003;18:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Tobin RW, Hackman RC, Kimmey MB, Durtschi MB, Hayashi A, Malik R, McDonald MF, McDonald GB. Bleeding from gastric antral vascular ectasia in marrow transplant patients. Gastrointest Endosc. 1996;44:223-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Spahr L, Villeneuve JP, Dufresne MP, Tassé D, Bui B, Willems B, Fenyves D, Pomier-Layrargues G. Gastric antral vascular ectasia in cirrhotic patients: absence of relation with portal hypertension. Gut. 1999;44:739-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 116] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Stefanidis I, Liakopoulos V, Kapsoritakis AN, Ioannidis I, Eleftheriadis T, Mertens PR, Winograd R, Vamvaka E, Psychos AK, Potamianos SP. Gastric antral vascular ectasia (watermelon stomach) in patients with ESRD. Am J Kidney Dis. 2006;47:e77-e82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Elkayam O, Oumanski M, Yaron M, Caspi D. Watermelon stomach following and preceding systemic sclerosis. Semin Arthritis Rheum. 2000;30:127-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Payen JL, Calès P, Voigt JJ, Barbe S, Pilette C, Dubuisson L, Desmorat H, Vinel JP, Kervran A, Chayvialle JA. Severe portal hypertensive gastropathy and antral vascular ectasia are distinct entities in patients with cirrhosis. Gastroenterology. 1995;108:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 129] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Vesoulis Z, Naik N, Maseelall P. Histopathologic changes are not specific for diagnosis of gastric antral vascular ectasia (GAVE) syndrome: a review of the pathogenesis and a comparative image analysis morphometric study of GAVE syndrome and gastric hyperplastic polyps. Am J Clin Pathol. 1998;109:558-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Sargeant IR, Loizou LA, Rampton D, Tulloch M, Bown SG. Laser ablation of upper gastrointestinal vascular ectasias: long term results. Gut. 1993;34:470-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Charneau J, Petit R, Calès P, Dauver A, Boyer J. Antral motility in patients with cirrhosis with or without gastric antral vascular ectasia. Gut. 1995;37:488-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Saperas E, Pigué JM, Perez-Ayuso R, Bombi JA, Bordas JM, Sentis J, Rodés J. Comparison of snare and large forceps biopsies in the histologic diagnosis of gastric vascular ectasia in cirrhosis. Endoscopy. 1989;21:165-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Quintero E, Pique JM, Bombi JA, Bordas JM, Sentis J, Elena M, Bosch J, Rodes J. Gastric mucosal vascular ectasias causing bleeding in cirrhosis. A distinct entity associated with hypergastrinemia and low serum levels of pepsinogen I. Gastroenterology. 1987;93:1054-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 189] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Lowes JR, Rode J. Neuroendocrine cell proliferations in gastric antral vascular ectasia. Gastroenterology. 1989;97:207-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Qureshi K, Al-Osaimi AM. Approach to the management of portal hypertensive gastropathy and gastric antral vascular ectasia. Gastroenterol Clin North Am. 2014;43:835-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Selinger CP, Ang YS. Gastric antral vascular ectasia (GAVE): an update on clinical presentation, pathophysiology and treatment. Digestion. 2008;77:131-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | Beales IL. Watermelon stomach in the CREST syndrome. Postgrad Med J. 1994;70:766-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Ito M, Uchida Y, Kamano S, Kawabata H, Nishioka M. Clinical comparisons between two subsets of gastric antral vascular ectasia. Gastrointest Endosc. 2001;53:764-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Fuccio L, Mussetto A, Laterza L, Eusebi LH, Bazzoli F. Diagnosis and management of gastric antral vascular ectasia. World J Gastrointest Endosc. 2013;5:6-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Lecleire S, Ben-Soussan E, Antonietti M, Goria O, Riachi G, Lerebours E, Ducrotté P. Bleeding gastric vascular ectasia treated by argon plasma coagulation: a comparison between patients with and without cirrhosis. Gastrointest Endosc. 2008;67:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Wang J, Stine JG, Cornella SL, Argo CK, Cohn SM. Patients with Gastric Antral Vascular Ectasia (GAVE) Are at a Higher Risk of Gastrointestinal Bleeding in the Absence of Cirrhosis. J Clin Transl Hepatol. 2015;3:254-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Probst A, Scheubel R, Wienbeck M. Treatment of watermelon stomach (GAVE syndrome) by means of endoscopic argon plasma coagulation (APC): long-term outcome. Z Gastroenterol. 2001;39:447-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Yusoff I, Brennan F, Ormonde D, Laurence B. Argon plasma coagulation for treatment of watermelon stomach. Endoscopy. 2002;34:407-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Roman S, Saurin JC, Dumortier J, Perreira A, Bernard G, Ponchon T. Tolerance and efficacy of argon plasma coagulation for controlling bleeding in patients with typical and atypical manifestations of watermelon stomach. Endoscopy. 2003;35:1024-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |