Published online Jun 26, 2022. doi: 10.12998/wjcc.v10.i18.6009
Peer-review started: November 2, 2021
First decision: April 7, 2022
Revised: April 19, 2022
Accepted: April 30, 2022
Article in press: April 30, 2022
Published online: June 26, 2022
Processing time: 226 Days and 13 Hours
Although sclerosing adenopathy of the prostate is a very rare benign disease, an effective differential diagnosis is required. Here, we report the clinicopathological and immunohistochemical morphological features of 12 cases of sclerosing adenopathy of the prostate to improve understanding of the disease.
To investigate the clinicopathological features, diagnosis, and immunohistochemical phenotypes that distinguish prostate sclerosing adenopathy from other conditions.
The clinical data, laboratory tests, pathological morphology, and immunohistochemical phenotypes of 12 cases of prostatic sclerosing adenopathy were retrospectively analyzed, and the relevant literature was reviewed.
All patients were elderly men (mean age, 71.7 years; 62–83 years). Eleven of them had hematuria, urinary frequency, urinary urgency, difficulty in urination, and serum total prostate-specific antigen values within the normal range. One patient had increased blood pressure. Enlarged prostates with single to multiple calcifying foci were observed. Moreover, prostate tissue hyperplastic changes were observed in all patients. Small follicular hyperplastic nodules without an obvious envelope, with a growth pattern mimicking the infiltration pattern of "prostate adenocarcinoma" were noted. Basal cells expressed AR, CKH, P63, and CK5/6, and myoepithelial markers, such as calponin, S100, and smooth muscle actin. No recurrence or exacerbation of the lesions was observed, except for one case of death due to bladder cancer.
Prostatic sclerosing adenopathy is highly misdiagnosed as prostate adenocarcinoma or other tumor-like lesions. Therefore, it should attract the attention of clinicopathologic researchers.
Core Tip: Sclerosing prostatic adenopathy is a rare pseudoadenocarcinoma proliferative lesion with a unique histomorphology and immunohistochemical phenotype. Compared to the common prostate adenocarcinoma, the incidence of sclerosing prostatic adenopathy is low, and we are under-recognized and have a high rate of misdiagnosis. Meanwhile, there are no large samples of data available to clinicopathologic to date because it is a rare lesion. To further our understanding of prostatic sclerosing adenopathy, this study aimed to investigate the histopathological morphology and immunohistochemical phenotype of this very rare prostate lesion and to further explore its associated biological significance.
- Citation: Feng RL, Tao YP, Tan ZY, Fu S, Wang HF. Prostate sclerosing adenopathy: A clinicopathological and immunohistochemical study of twelve patients. World J Clin Cases 2022; 10(18): 6009-6020
- URL: https://www.wjgnet.com/2307-8960/full/v10/i18/6009.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i18.6009
Sclerosing adenopathy of the prostate is a rare pseudomalignant proliferative lesion with a distinct histomorphological and immunohistochemical phenotype that often resembles acinar adenocarcinoma but may also resemble sarcomatoid carcinoma[1]. It can manifest as single or multiple lesions, in which good small glands and scattered single-cell components can be seen, forming the impression of cell-like features. These lesions are benign pseudotumor hyperplasia lesions that are very similar to those of small alveolar prostate adenocarcinoma and are often misdiagnosed as prostate cancer by primary pathologists due to their characteristic interstitial spindle cell proliferation and small glandular architecture. They are observed in 2% of the transurethral prostatectomy or prostatectomy specimens and are rare in small samples derived from prostate needle biopsy[2-4]. The glandular components show nuclear atypia, including nuclear enlargement and prominent nucleoli. However, sclerosing adenopathy tends to be a more focal lesion than sarcomatoid carcinoma, which is thought to arise from the myoepithelial cells surrounding the prostate, high molecular weight cytokeratins. Moreover, the lesions are often positive for muscle-specific actin staining. Compared with that prostate adenocarcinoma, sclerosing adenopathy has a lower incidence and a higher misdiagnosis rate. Furthermore, since it is a rare lesion, to date, no large samples have been available for clinical pathology experiments. In clinical treatment, surgical resection is still the best treatment option for sclerosing adenopathy of the prostate. To further understand this condition, we aimed to investigate the histopathological morphology and immunohistochemical phenotype of this very rare prostate lesion and to further explore its associated biological significance and underlying mechanisms.
Thirteen patients with prostate sclerosing adenopathy that visited the Second Affiliated Hospital of Kunming Medical University from January 2015 to November 2021 were enrolled and followed up. Their clinical history, imaging data, and disease were analyzed and further confirmed via the relevant immunohistochemical analysis. Twelve specimens were routinely fixed using 4% neutral formaldehyde, paraffin-embedded, and tissue-sectioned to a thickness of 4 μm, followed by HE staining and immunohistochemical analysis. The HE-stained slides were reviewed by three pathologists to confirm the diagnosis. We reviewed the gross specimen characteristics and read the immunohistochemical product instructions, the expiration dates of the antibodies, the localization of the positive antibodies, and the staining intensity interpretation criteria in detail. The required immunohistochemistry AR, CKH, P63, CD56, CK5/6, calponin, S100, P504S, prostate-specific antigen (PSA), PSAP, P53, and Ki-67 markers were obtained from the Fujian Meixin Company, China, and the procedures were carried out strictly according to the instructions, and positive and negative controls were routinely set up.
The immunohistochemistry results were interpreted by two senior pathologists using a double-blind reading method in strict accordance with the immunohistochemistry interpretation criteria. In case of disagreement between the two pathologists, the reading was interpreted by a third senior pathologist. A standard reading was performed using a semi-quantitative counting method by randomly selecting 10 representative fields at high magnification (400× field of view) and calculating the cell positivity rate (the number of positive tumor cells divided by the total number of cells) × 100%, for which a cell positivity rate below and above 10% was considered as negative and positive, respectively. The staining results were based on the percentage of stained cells: When < 25%, in the 25%–50% range, in the 50%–75% range, and > 75%, the result was negative (-), weakly positive (+), moderately positive (++), and strongly positive (+++), respectively. AR, P63, P53, and Ki-67 were found in the cytosol, and CKH, CD56, CK5/6, calponin, S100, P504S, PSA, and PSAP were found in the cytoplasm or cytosol, and precise color localization was considered positive.
None of the 12 patients in this study had received prior radiotherapy, chemotherapy, immunotherapy, or drug therapy. Complete clinical and pathological data were obtained for all 12 patients, and intraoperative histological specimens were obtained. The study was approved by the Ethics Committee of the Second Affiliated Hospital of Kunming Medical University (approval number: trial-PJ-2021-142). Since the study was performed using medical records and biological specimens obtained from previous clinical consultations, all conditions were met to exempt patients from informed consent.
A total of 7674 surgically resected prostate specimens were collected between January 2015 and November 2021, including 625 radical prostatectomy specimens, 1155 prostate puncture specimens, and 5894 transurethral electrodesiccation prostate specimens. A re-read of the 7674 prostate specimens revealed 12 cases of sclerosing adenopathy (0.2%), 2 of which were found in radical prostatectomy specimens, and 10 were found in transurethral resection specimens, with no lesions found in the prostate puncture specimens. The clinical data, laboratory tests, pathological morphology, and immunohistochemical phenotype of these 12 cases of prostatic sclerosing adenopathy were analyzed and are summarized in Table 1.
| Case | Age of onset (yr) | Clinical symptoms | Difficulty urinating | PSA check value (ng/mL) | Imaging diagnosis | Surgical findings | Surgical approach | Follow up |
| 1 | 76 | Difficulty urinating | Without | 2.43 | Benign prostatic hyperplasia | The lobes on both sides of the prostate protrude and protrude into the bladder, and the urethral cavity is narrowed | Transurethral plasma resection of the prostate | Survival and good, normal rectal examination, 73 mo |
| 2 | 62 | Hematuria | Bladder papilloma | 5.21 | Bladder cancer, invasion of the prostate | Irregular hyperplasia of the right side of the bladder, invading the adjacent prostate | Total cystectomy and double-layer ureterostomy | Died from bladder cancer, 27 mo |
| 3 | 63 | Frequent urination, difficulty urinating | Cholecystectomy; history of hypertension | 1.73 | Prostate cancer | The lobes on both sides of the prostate proliferate and protrude into the bladder | Transurethral plasma resection of the prostate | Survival and good, normal rectal examination, 69 mo |
| 4 | 68 | Gross hematuria | History of gastrectomy | 3.36 | Bladder Cancer | Prostatic hyperplasia, multiple neoplastic new organisms are seen in the bladder triangle and right wall | Total cystectomy and intestinal replacement for new bladder | Alive, 46 mo |
| 5 | 67 | Frequent urination, urgency | Without | 1.34 | Benign prostatic hyperplasia | Irregular hyperplasia of the lobes on both sides of the prostate | Transurethral plasma resection of the prostate | Survival and good, normal rectal examination, 24 mo |
| 6 | 83 | Frequent urination, difficulty urinating | History of hypertension, diabetes | 3.81 | Benign prostatic hyperplasia | Irregular hyperplasia of the lobes on both sides of the prostate | Transurethral plasma resection of the prostate | Survival and good, normal rectal examination, 21 mo |
| 7 | 67 | High blood pressure | History of prostatitis, appendix surgery | 2,76 | Prostatic hyperplasia with calcification | Enlargement of the right side wall of the prostate | Transurethral plasma resection of the prostate | Survival and good, normal rectal examination, 2 mo |
| 8 | 83 | Hematuria with frequent urination and urgency | Right inguinal hernia repair | 4.91 | Prostatic hyperplasia with calcification | Significant enlargement of the bilateral and middle lobes of the prostate | Transurethral resection of the prostate | Survival and good, 3 mo |
| 9 | 72 | Frequent urination, urgency | History of hypertension, diabetes | 2.21 | Prostatic hyperplasia with calcification | The lobes on both sides of the prostate proliferate and protrude into the bladder | Transurethral resection of the prostate | Survival and good, normal rectal examination, 6 mo |
| 10 | 68 | Difficulty urinating | Without | 2.39 | Benign prostatic hyperplasia | Irregular hyperplasia of the lobes on both sides of the prostate | Transurethral resection of the prostate | Survival and good, normal rectal examination, 13 mo |
| 11 | 81 | Frequent urination, urgency | Bladder papilloma | 3.31 | Benign prostatic hyperplasia | Irregular hyperplasia of the lobes on both sides of the prostate | Transurethral resection of the prostate | Survival and good, normal rectal examination, 19 mo |
| 12 | 71 | Difficulty urinating | Without | 3.07 | Prostatic hyperplasia with calcification | Irregular hyperplasia of the lobes on both sides of the prostate | Transurethral resection of the prostate | Survival and good, normal rectal examination, 24 mo |
All 12 patients were elderly men with an average age of 71.7 years (age range, 62–83 years). In terms of clinical symptoms, 11 patients presented varying degrees of hematuria, urinary frequency, urinary urgency, and difficulty in urination, and one patient had increased blood pressure. After admission to the hospital, the laboratory tests revealed that 11 of the 12 patients had serum total prostate-specific antigen (PSA) values within the normal reference range, and only one patient had a slightly higher than normal value. Rectal finger examination showed hyperplastic changes in all the prostate tissues, with varying degrees of prostatic hyperplasia visible intraoperatively, mainly in both lobes and on the right wall. Among the 12 cases, transurethral resection of the prostate was performed in 10 of them, and total cystectomy and bowel replacement with total cystectomy and double ureterostomy for bladder cancer was performed in two cases, respectively.
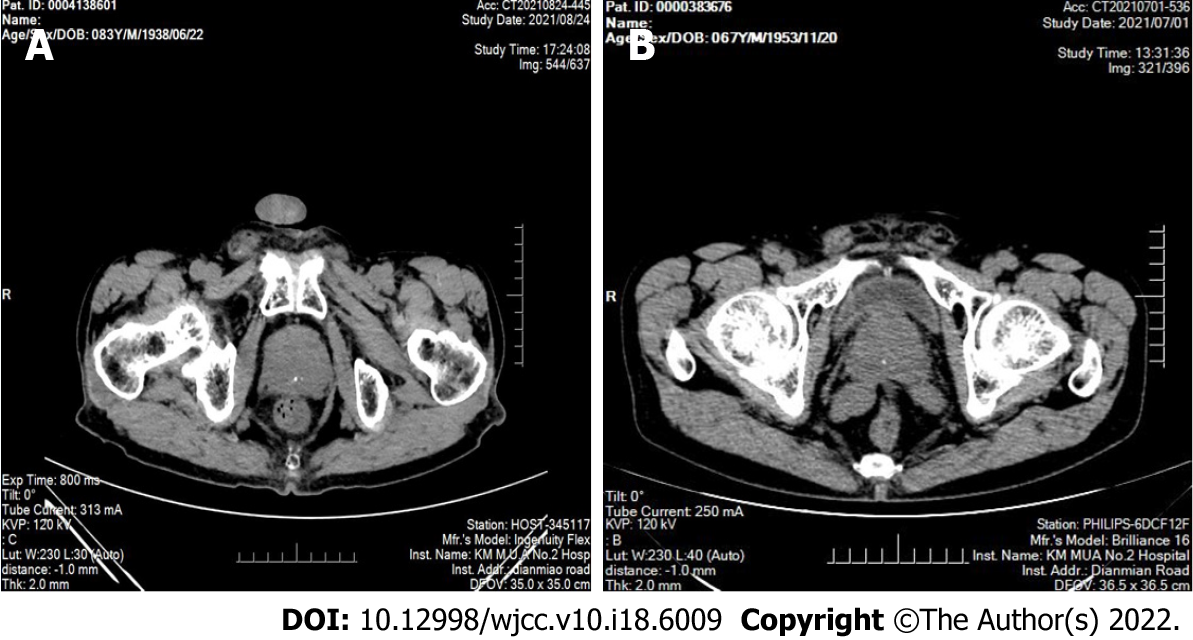
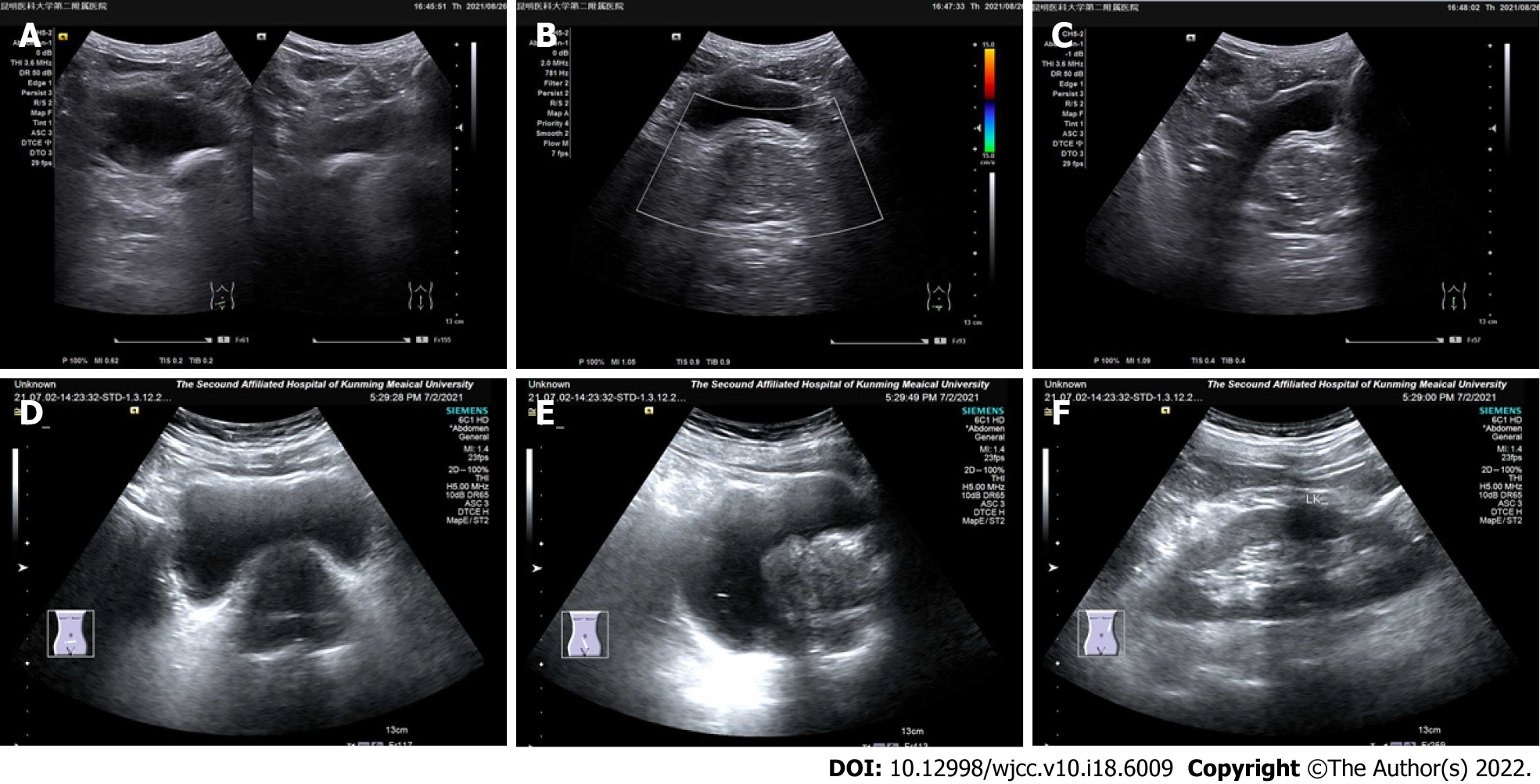
In the 12 patients, the computed tomography examination results showed an enlarged prostate with single to multiple calcified foci and, in three cases, localized protrusion into the bladder trigone with uneven enhancement (Figure 1). The preoperative ultrasonography results showed an enlarged prostate morphology, an enlarged inner gland, a thinning of the outer gland under pressure, and inhomogeneous parenchyma with one to multiple strong echogenic spots in all the 12 patients (Figure 2, Table 2).
| Case | prostate size | Ultrasound image performance |
| 1 | 5.8 cm × 5.0 cm × 5.8 cm | Full shape, regular margins, normal ratio of internal and external glands, uneven echo, and sonographic image of benign prostatic hyperplasia |
| 2 | 3.5 cm × 4.1 cm × 3.2 cm | The shape is normal, the edges are regular, the ratio of internal and external glands is normal, and there is a strong echogenic spot in the parenchyma, with a long diameter of about 0.2 cm |
| 3 | 4.7 cm × 4.5 cm × 3.6 cm | The volume increases, the shape is plump, the internal glands are enlarged, the external glands are compressed and thinned, the parenchyma echoes uniformly, and multiple hyperechoic spots are detected in the parenchyma |
| 4 | 4.3 cm × 4.3 cm × 4.4 cm | The volume increases, the shape is plump, the internal glands are enlarged, the external glands are compressed and thinned, the parenchymal echo is uneven, and a strong echogenic spot is detected in the parenchyma, with a long diameter of about 0.5 cm |
| 5 | 5.5 cm × 4.0 cm × 3.7 cm | Enlarged volume, plump shape, enlarged internal glands, thin external glands under compression, uniform parenchymal echo, and sonographic image of benign prostatic hyperplasia |
| 6 | 5.1 cm × 3.7 cm × 3.2 cm | Enlarged volume, plump shape, enlarged internal glands, thin external glands under compression, uniform parenchymal echo, and sonographic image of benign prostatic hyperplasia |
| 7 | 4.1 cm × 5.5 cm × 4.7 cm | Full-bodied, enlarged internal glands, thin external glands under pressure, uneven parenchymal echo, and multiple hyperechoic spots within the parenchyma |
| 8 | 5.7 cm × 5.4 cm × 4.6 cm | The volume increased, the shape was plump, the edges were still regular, the internal glands were enlarged, the external glands were compressed and thinned, and a strong echogenic spot was detected in the parenchyma, with a long diameter of about 0.1cm |
| 9 | 5.3 cm × 5.1 cm × 3.2 cm | The volume increased, the shape was plump, the edges were still regular, the internal glands were enlarged, the external glands were compressed and thinned, and a strong echogenic spot was detected in the parenchyma, with a long diameter of about 0.6 cm |
| 10 | 4.9 cm × 4.3 cm × 3.2 cm | Enlarged volume, plump shape, enlarged internal glands, thin external glands under compression, uniform parenchymal echo, and sonographic image of benign prostatic hyperplasia |
| 11 | 5.5 cm × 4.0 cm × 3.7 cm | Enlarged volume, plump shape, enlarged internal glands, thin external glands under compression, uniform parenchymal echo, and sonographic image of benign prostatic hyperplasia |
| 12 | 5.5 cm × 4.6 cm × 4.1 cm | The volume increased, the shape was plump, the edges were still regular, the internal glands were enlarged, the external glands were compressed and thinned, and a strong echogenic spot was detected in the parenchyma, with a long diameter of about 0.4 cm |
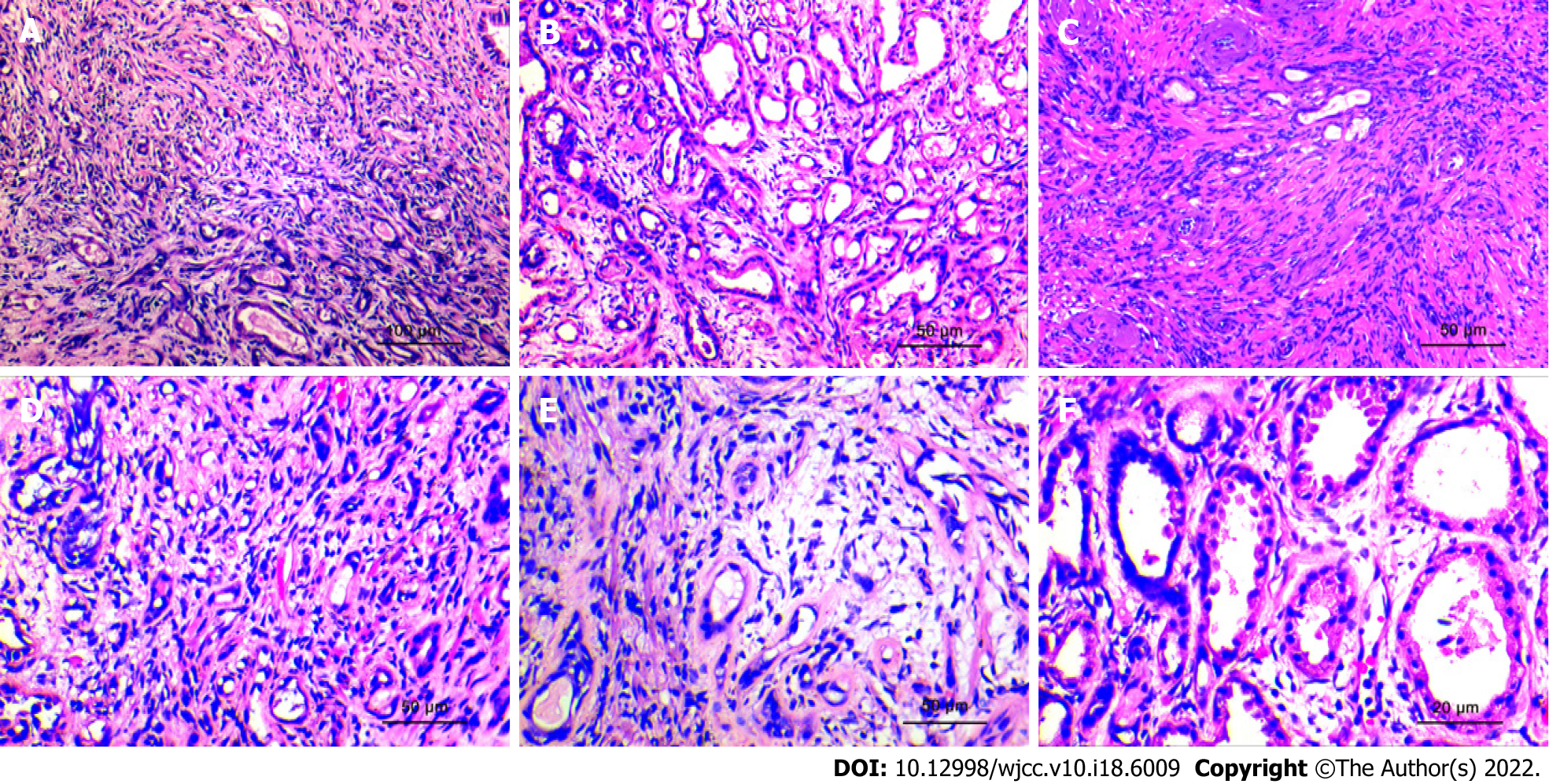
Specimen analysis showed that 10 cases presented a fragmented prostate tissue, two cases presented the complete cystoprostate tissue, which was grayish-red, along with a tissue multi-sectional cut that was tough. No obvious nodules or masses were seen. The microscopic observation results revealed that all 12 cases of prostate sclerosing adenopathy presented modularly distributed lesions indicative of benign prostatic hyperplasia. The lesions only had a few millimeters, had a nodular shape with varying degrees of small alveolar hyperplasia, without an obvious envelope, and with a growth pattern that mimicked the infiltration pattern of "prostate adenocarcinoma" (Figure 3A). The hyperplastic mesenchyme extruded the gland into ductal (Figure 3B), lacunar, and linear (Figure 3C) forms, and the glandular epithelium was covered with eosinophilic spike-like cells. Some of the glands were extruded to form vacuoles, and the increased number of vacuolated cells could form a structure similar to that of printed ring cells (Figure 3D). The interstitium was collagenous or mucinous and consisted of cells with a mild spindle-like morphology (Figure 3E). Observation at high magnification showed an eosinophilic cytoplasm with nuclei located at the base and small nucleoli but no nuclear division (Figure 3F).
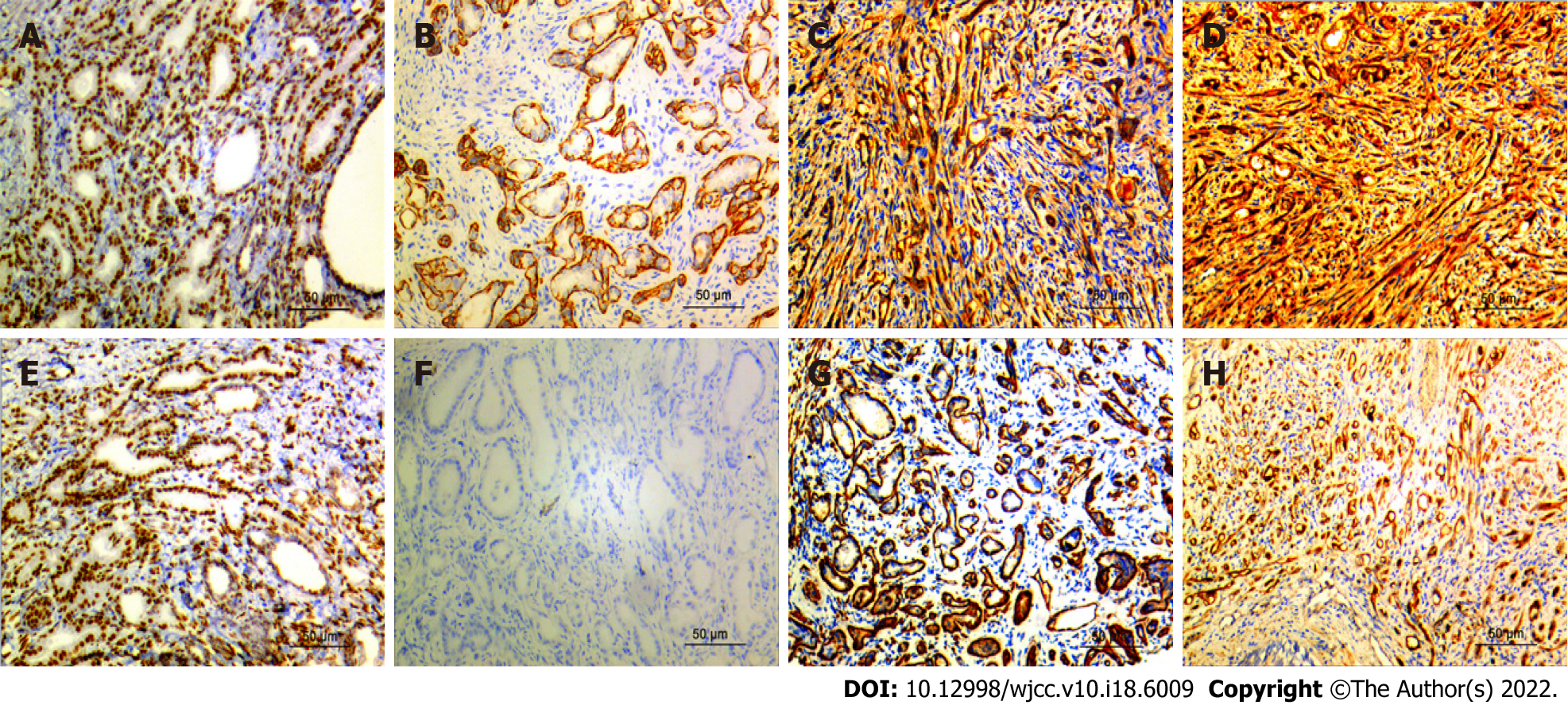
The basal cells of all the 12 cases of prostatic sclerosing adenopathy expressed molecules such as AR (Figure 4A), CKH (Figure 4D), P63 (Figure 4E), and CK5/6 (Figure 4C) to varying degrees, and also myoepithelial markers such as calponin (Figure 4B), S100 (Figure 4H), and smooth muscle actin (SMA) (Figure 4G). Nine of the 12 cases did not express P504S (Figure 4F), and the remaining three cases showed only a weakly positive expression of P504S. In addition, the prostate-specific markers PSA and PSAP were also expressed, P53 was not expressed, and the Ki67 proliferation index was in the 1%–3% range (Table 3).
| Case | AR | CK5/6 | P63 | CKH | S100 | SMA | Calponin | P504S |
| 1 | + | + | ++ | +++ | + | + | - | + |
| 2 | +++ | +++ | + | + | ++ | ++ | + | - |
| 3 | ++ | + | + | + | ++ | + | ++ | + |
| 4 | - | ++ | ++ | +++ | + | + | ++ | - |
| 5 | - | ++ | + | ++ | - | ++ | + | - |
| 6 | + | + | ++ | + | + | + | + | - |
| 7 | ++ | ++ | + | + | ++ | ++ | + | + |
| 8 | + | ++ | ++ | +++ | + | + | - | - |
| 9 | ++ | ++ | + | - | + | + | - | ++ |
| 10 | ++ | + | + | + | + | ++ | - | ++ |
| 11 | + | ++ | ++ | + | + | + | - | + |
| 12 | ++ | + | + | ++ | + | ++ | - | ++ |
The mean follow-up time was 27.6 mo (3–73 mo), and 11 of the 12 patients survived well with normal rectal examinations and no recurrence or exacerbation of the disease. One patient died 26 mo after surgery due to bladder cancer.
In 1983, Chen and Schiff reported a prostate lesion that they considered most notably similar to an adenomatous tumor[5]. Therefore, it was named sclerosing adenopathy[6]. Sclerosing adenopathy of the prostate, first reported in 1987 by Young et al[7], was previously known as an adenomatous prostate tumor, prostatic pseudoadenoma, and fibroepithelial nodules[8-10]. It was originally used to express the similarity of the lesions to testicular adenomatous tumors but was abandoned due to the recognition that the lesions were of prostatic origin based on the immunoreactivity of PSA in acinar cells. It was subsequently found that sclerosing adenopathy is superficially similar to the breast lesion of the same name, is composed of prostate epithelial cells rather than mesothelial cells, and is characterized by glandular hyperplasia of varying sizes in the intercellular substance[11-14]. Sclerosing adenopathy is well characterized both immunohistochemically and ultrastructurally, and there are currently several case reports and small series of this lesion[15,16].
Sclerosing adenopathy is a rare benign proliferative lesion of the small alveoli without a markedly sclerotic stroma that occurs most occasionally after transurethral resection of the prostate, simple prostatectomy, or radical prostatectomy based on benign prostatic hyperplasia. It is detected by pathologists but occurs very rarely[17,18]. The histopathological pattern of sclerosing lymphadenopathy of the prostate is very similar to that of mammary sclerosing lymphadenopathy; however, unlike mammary sclerosing lymphadenopathy, prostatic sclerosing lymphadenopathy results in small lesions, usually with only a few millimeters[19]. It can result in single or multiple lesions, and it mostly occurs in the peripheral area of the prostate. It is difficult to detect the lesion via rectal ultrasound, and it cannot be touched via digital rectal examination. In addition, the serum PSA value is not high; thus, it is very difficult to measure it in clinical practice. In this group of 12 cases of prostate sclerosing lymphadenopathy, ultrasonography and rectal examination showed no abnormalities, and the serum PSA value did not increase. With the continuous improvement of ultrasound-guided technology, the probability of finding prostate sclerosing lymphadenopathy in biopsy tissue samples will increase; however, the possibility of it being misdiagnosed as prostate cancer will also increase. Research analysis found that 2% of the cases with this condition were diagnosed as prostate cancer. T1a stage prostate cancer was confirmed to be prostate sclerosing adenopathy via reexamination[20]. Therefore, surgeon pathologists should consider prostate biopsy specimens as of great importance to avoid overdiagnosis of sclerosing adenopathy as prostate cancer.
Sclerosing adenopathy of the prostate has clear demarcation, is associated with small and concentrated lesions, nodular growth, no obvious capsule, and an infiltrative growth trend to the surrounding normal prostate tissue, similar to the growth pattern of prostate adenocarcinoma, but in the scope of growth-restricted lesions[21]. On the surface, it has a worrying appearance, with morphological distortions that can form stripes, threads, clusters, nests, and even single-cell arrangements. When the gland overproliferates, it may expand into a cyst, in which the amyloid material disappears and is replaced by a crystalline or myxoid material that is suggestive of prostate cancer[9,12]. In addition, several authors have described the lesion as presenting mild atypia and even small nucleoli, but generally no apparent large nucleoli. When individual cases are accompanied by moderate heterogeneity, heterogeneity, such as large and deep nuclear staining and prominent nucleoli, they are likely misdiagnosed as prostate adenocarcinoma. There is no evidence of the underlying malignancy of this lesion, although follow-up in our cases and others documented in the literature is admittedly limited.
In daily clinical practice, immunohistochemical markers are relied upon to help determine when lesions are morphologically atypical or when cellular heterogeneity occurs. Moreover, prostate basal cells are not considered myoepithelial cells[22], contrary to the notion of the myoepithelial origin of salivary gland myoepithelial cells, since they do not have actin filaments. S100 protein and smooth muscle actin are not expressed in normal prostate basal cells[23,24]; in contrast, the presence of the S100 protein and SMA immunoreactivity in basal cells of sclerosing adenopathy suggests that they present myoepithelial differentiation, a characteristic feature based on which sclerosing adenopathy is diagnosed. In addition, some scholars have confirmed the existence of microfilaments in the cytoplasm of basal cells of sclerosing adenopathy using electron microscopy, which is consistent with the presence of actin filaments[25]. In rare cases, S100 protein immunoreactivity can be seen in prostate basal cell hyperplasias, such as adenoid basal cell carcinoma, adenoid cystic carcinoma, and atypical basal cell hyperplasia[26]. In addition, we have recently found that D2-40 is a sensitive marker of prostate basal cells[27]; therefore, D2-40 and the combination of P63 and cytokeratin can be used to differentiate sclerosing adenopathy from prostate cancer. However, in practice, due to the severe extrusion of basal cells and myoepithelial cells, the immunohistochemical expression results are not ideal, and some sclerosing adenopathy cases will express P504S to varying degrees, overlapping with the immunohistochemistry results of patients with prostate cancer, posing great challenges during pathological diagnosis.
Sclerosing adenopathy of the prostate is considered a rare variant of adenopathy whose biological behavior remains uncertain, and the research on the risk of prostate cancer following a diagnosis of sclerosing adenopathy is very limited. Sclerosing adenopathy might be one of the precursors of prostate adenocarcinoma; however, this is unlikely because of its rarity and lack of other evidence linking its clinic features or pathology to cancer. When patients with sclerosing adenopathy present with cellular atypia, it is considered atypical sclerosing adenopathy; however, we should emphasize the use of the term "atypical sclerosing adenopathy" does not imply that these lesions are precancerous. Some scholars have found that DNA aneuploidy occurs in some atypical sclerosing adenopathy cases, but all typical sclerosing adenopathy cases are DNA diploid[28]. Whether sclerosing adenopathy can be used as an independent feature to predict the risk of prostate cancer in men is not yet clear, and a large multi-institutional prospective study is needed for confirmation.
Sclerosing adenopathy has a similar histological pattern to that of the following lesions and is easily misdiagnosed as one of them. In this group of 12 patients with sclerosing adenopathy, two were misdiagnosed as having prostate adenocarcinoma and one was misdiagnosed as having nephrogenic adenoma by the primary pathologist. Therefore, prostate adenocarcinoma sclerosing adenopathy should be distinguished from the following lesions[1]. Prostate adenocarcinoma, which is the most likely misdiagnose. The identification points include: (1) Sclerosing adenopathy is mainly located in the peripheral area, with small and concentrated lesions, unlike cancer, that spreads and whose growth is invasive; (2) Sclerosing adenopathy is glandular hyperplasia with interstitial hyperplasia that is mucoid or fibrous; (3) An eosinophilic basement membrane material is seen around the ducts of patients with sclerosing adenopathy; (4) Mild-to-moderate dysplasia of the glandular epithelium may lead to subnucleation without significantly enlarged nucleoli; (5) There is no amyloid but a myxoid in the glandular follicular lumen. Furthermore, prostate cancer acini are lined with a layer of cuboidal or columnar cells without a distinctly flattened basal cell layer, as evidenced by the negative immunoreactivity for keratin 903[29,30]; and (6) Nephrogenic adenomas differentiate from sclerosing adenomas when they are tubular or solid. Nephrogenic adenomas are usually confined to the lamina propria, covering a single layer of short columnar or subcubical epithelial cells, among which some are shoe-stud-like cells, with round or oval nuclei, visible nucleoli, and most express markers such as CK7, CKH, CKL, and CEA, whereas they do not express the markers P63, PSA, PSAP, GATA3, and CK5/6. Atypical adenomatous hyperplasia is also nodular hyperplasia with clear borders; however, the lesions are often interspersed with a small number of large acinar with a normal structure in the proliferating small glands[31].
In addition, there are no studies that show that the PSA value is related to the occurrence and development of prostate sclerosing adenopathy. In this study, the PSA value of the vast majority of patients with sclerosing adenopathy was within the reference range. Therefore, patients with sclerosing adenopathy should be informed that long-term follow-up and observation are required. If the PSA value increases significantly compared to the original value, and clinical symptoms such as frequent urination, urgency, dysuria, hematuria, and dysuria appear, patients should promptly consult their doctor, since they may have prostate cancer.
In conclusion, sclerosing adenopathy of the prostate is a morphological abnormality with characteristic histological features and immunohistochemical profiles. All the available evidence indicates that it is a benign condition that does not require treatment and is not a precancerous condition of prostate cancer. It is important to note, however, that many of the cases encountered to date have had a short follow-up period, and that it may often co-exist with other unrelated cancers in older men. The pathogenesis of this lesion is unclear, but its most striking feature seems to be the ability of cells to differentiate and proliferate. Studying and better understanding the features of sclerosing adenopathy should lead to its appropriate conservative management.
Sclerosing adenopathy of the prostate is a morphological abnormality with characteristic histological features and immunohistochemical profiles. All the available evidence indicates that it is a benign condition that does not require treatment and is not a precancerous condition of prostate cancer. It is important to note, however, that many of the cases encountered to date have had a short follow-up period, and that it may often co-exist with other unrelated cancers in older men. The pathogenesis of this lesion is unclear, but its most striking feature seems to be the ability of cells to differentiate and proliferate. Studying and better understanding the features of sclerosing adenopathy should lead to its appropriate conservative management.
Sclerosing adenopathy of the prostate is a focal proliferative lesion, and the same name is also proposed for the prostate lesion due to its similarity in appearance to sclerosing adenopathy of the breast. Due to the presence of dense small acini in the proliferative stroma, the morphology is similar to that of prostate adenocarcinoma, which brings great challenges to both imaging diagnosis and pathological diagnosis. In addition, a small proportion of sclerosing adenopathy of the prostate co-exists with adenocarcinoma, which makes the diagnosis of this lesion more difficult. So far, there is still a lack of a large number of clinical data sample libraries for clinical pathologists to study in-depth, and further exploration is needed in the future. Since we are aware of the importance of this lesion morphology, the clinical features, pathological morphology, and immunohistochemical phenotype of prostate sclerosing lesions were retrospectively analyzed in this study to further increase the importance of this lesion.
The objective is to investigate the clinicopathological features, diagnosis, and immunohistochemical phenotypes that distinguish prostate sclerosing adenopathy from other conditions.
This study explores the clinicopathological features, diagnosis, and immunohistochemical phenotypes that distinguish prostate sclerosing adenopathy from other conditions. We believe that our study makes a significant contribution to the literature because we show that this condition is benign, does not require treatment, and is not a precancerous condition of prostate cancer. However, notably, many of the cases encountered to date have had a short follow-up period, and this condition may often co-exist with other unrelated cancers in older men.
The clinical data, laboratory tests, pathological morphology, and immunohistochemical phenotypes of 12 cases of prostatic sclerosing adenopathy were retrospectively analyzed, and the relevant literature was reviewed.
The authors summarized the age, clinical symptoms, medical history, serum total prostate-specific antigen (PSA) value, surgical findings, surgical methods, follow-up time, and follow-up results of 12 patients with prostate sclerosing adenopathy in detail (Table 1). The results of the study showed that the patients were all elderly men, with an average age of 71.7 years. The patients had symptoms of hematuria, frequent urination, urgency, and dysuria to varying degrees. Different degrees of prostate hyperplasia was seen during digital rectal examination and surgery, and the bladder was the most common. Lateral lobe hyperplasia is predominant. The mean postoperative follow-up time was 27.6 mo, and only 1 patient died of bladder cancer. In addition, 11 of the 12 patients had PSA values within the normal reference range. From the above description, it is not difficult to find that the clinical features of sclerosing adenopathy of the prostate are similar to those of benign prostatic hyperplasia and prostate cancer, and most of the patients' PSA values are Within the normal range, suggesting that sclerosing adenopathy may be a benign lesion. Pathologically, the lesions are very complex, with single or mixed glandular tubular, cord-like and linear structures, and focal distribution in benign prostate glands. Unlike prostate adenocarcinoma, immunohistochemical Expression of the basal cell (such as P63, CK5/6) and myoepithelial (Calponin, S100, SMA) markers, provides a very meaningful value for the identification of the two. In practice, when it is difficult to identify prostate sclerosing adenopathy and prostate cancer, we can use immunohistochemical markers to distinguish them. In addition, we can also use immunohistochemical PSA, and PSAP to identify sclerosing adenopathy and other neoplastic lesions such as nephrogenic adenoma. The above pathological characteristics can provide effective help for the follow-up in-depth study of the prostate. However, even though we performed a comprehensive systematic analysis of sclerosing adenopathy of the prostate, this study has some limitations. First, although we predicted a certain relationship between sclerosing adenopathy of the prostate and PSA values, there is still a lack of direct evidence for the two relevance of the person. Secondly, the sample size of this study is too small, and it is necessary to further supplement the sample size and explore it in depth. Finally, whether sclerosing adenopathy is a precancerous lesion of prostate cancer also lacks direct evidence to further prove. These issues need to be further explored in future work.
Sclerosing adenopathy of the prostate is a morphological abnormality with characteristic histological features and immunohistochemical profiles. All the available evidence indicates that it is a benign condition that does not require treatment and is not a precancerous condition of prostate cancer. It is important to note, however, that many of the cases encountered to date have had a short follow-up period, and that it may often co-exist with other unrelated cancers in older men. The pathogenesis of this lesion is unclear, but its most striking feature seems to be the ability of cells to differentiate and proliferate. Studying and better understanding the features of sclerosing adenopathy should lead to its appropriate conservative management.
Patients should be informed that long-term follow-up and observation are required. If the PSA value increases significantly compared to the original value, and clinical symptoms such as frequent urination, urgency, dysuria, hematuria, and dysuria appear, patients should promptly see their doctor, since they may have prostate cancer.
Provenance and peer review: Unsolicited article; Externally peer-reviewed.
Peer-review model: Single-blind
Specialty type: Pathology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cassell III AK, Liberia; Marickar F, India S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Hansel DE, Herawi M, Montgomery E, Epstein JI. Spindle cell lesions of the adult prostate. Mod Pathol. 2007;20:148-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 2. | Jones EC, Clement PB, Young RH. Sclerosing adenosis of the prostate gland. A clinicopathological and immunohistochemical study of 11 cases. Am J Surg Pathol. 1991;15:1171-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Sakamoto N, Tsuneyoshi M, Enjoji M. Sclerosing adenosis of the prostate. Histopathologic and immunohistochemical analysis. Am J Surg Pathol. 1991;15:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Grignon DJ, Ro JY, Srigley JR, Troncoso P, Raymond AK, Ayala AG. Sclerosing adenosis of the prostate gland. A lesion showing myoepithelial differentiation. Am J Surg Pathol. 1992;16:383-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Chen KT, Schiff JJ. Adenomatoid prostatic tumor. Urology. 1983;21:88-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Gleason DF. Atypical hyperplasia, benign hyperplasia, and well differentiated adenocarcinoma of the prostate. Am Surg Pathol. 1985;9:53-67. |
| 7. | Young RH, Clement PB. Sclerosing adenosis of the prostate. Arch Pathol Lab Med. 1987;111:363-366. [PubMed] |
| 8. | Helpap B. The biological significance of atypical hyperplasia of the prostate. Virchows Arch A Pathol Anat Histol. 1980;387:307-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 75] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Sesterhenn IA, Mostofi FK, Davis CJ. Fibroepithelial nodules of the prostate simulating carcinoma. Lab Investig 1988; 58: 83A. |
| 10. | Chen KT. Adenomatoid prostatic tumor versus sclerosing adenosis of the prostate. Am J Surg Pathol. 1990;14:989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Young RH, Clement PB. 'Pseudoadenomatoid' tumour of prostate. Histopathology. 1990;16:420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Ronnett BM, Epstein JI. A case showing sclerosing adenosis and an unusual form of basal cell hyperplasia of the prostate. Am J Surg Pathol. 1989;13:866-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | O'Malley FP, Grignon DJ, Shum DT. Usefulness of immunoperoxidase staining with high-molecular-weight cytokeratin in the differential diagnosis of small-acinar lesions of the prostate gland. Virchows Arch A Pathol Anat Histopathol. 1990;417:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 66] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Srigley JR, Dardick I, Hartwick RW, Klotz L. Basal epithelial cells of human prostate gland are not myoepithelial cells. A comparative immunohistochemical and ultrastructural study with the human salivary gland. Am J Pathol. 1990;136:957-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 15. | Collina G, Botticelli AR, Martinelli AM, Fano RA, Trentini GP. Sclerosing adenosis of the prostate. Report of three cases with electronmicroscopy and immunohistochemical study. Histopathology. 1992;20:505-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Parker C, Muston D, Melia J, Moss S, Dearnaley D. A model of the natural history of screen-detected prostate cancer, and the effect of radical treatment on overall survival. Br J Cancer. 2006;94:1361-1368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Evans AJ, Henry PC, Van der Kwast TH, Tkachuk DC, Watson K, Lockwood GA, Fleshner NE, Cheung C, Belanger EC, Amin MB, Boccon-Gibod L, Bostwick DG, Egevad L, Epstein JI, Grignon DJ, Jones EC, Montironi R, Moussa M, Sweet JM, Trpkov K, Wheeler TM, Srigley JR. Interobserver variability between expert urologic pathologists for extraprostatic extension and surgical margin status in radical prostatectomy specimens. Am J Surg Pathol. 2008;32:1503-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 18. | Cheng L, Bostwick DG. Atypical sclerosing adenosis of the prostate: a rare mimic of adenocarcinoma. Histopathology. 2010;56:627-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Luque RJ, Lopez-Beltran A, Perez-Seoane C, Suzigan S. Sclerosing adenosis of the prostate. Histologic features in needle biopsy specimens. Arch Pathol Lab Med. 2003;127:e14-e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Bostwick DG, Chang L. Overdiagnosis of prostatic adenocarcinoma. Semin Urol Oncol. 1999;17:199-205. [PubMed] |
| 21. | Berney DM, Fisher G, Kattan MW, Oliver RT, Møller H, Fearn P, Eastham J, Scardino P, Cuzick J, Reuter VE, Foster CS; Trans-Atlantic prostate group. Pitfalls in the diagnosis of prostatic cancer: retrospective review of 1791 cases with clinical outcome. Histopathology. 2007;51:452-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Bussolati G, Alfani V, Weber K, Osborn M. Immunocytochemical detection of actin on fixed and embedded tissues: its potential use in routine pathology. J Histochem Cytochem. 1980;28:169-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Hara K, Ito M, Takeuchi J, Iijima S, Endo T, Hidaka H. Distribution of S-100b protein in normal salivary glands and salivary gland tumors. Virchows Arch A Pathol Anat Histopathol. 1983;401:237-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 96] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Haimoto H, Hosoda S, Kato K. Differential distribution of immunoreactive S100-alpha and S100-beta proteins in normal nonnervous human tissues. Lab Invest. 1987;57:489-498. [PubMed] |
| 25. | Ghadially FN. Intracytoplasmatic filaments. In: Ghadially FN, editor. Ultrastructural Pathology of the Cell and Matrix, 3rd ed. London: Butterworths, 1988: 839-936. |
| 26. | Kuroda N, Katto K, Ohtsuki Y, Hes O, Michal M, Inoue K, Ohara M, Mizuno K, Lee GH. Hybrid sclerosing adenosis and basal cell hyperplasia of the prostate. Med Mol Morphol. 2010;43:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Kuroda N, Katto K, Tamura M, Shiotsu T, Nakamura S, Ohtsuki Y, Hes O, Michal M, Inoue K, Ohara M, Mizuno K, Lee GH. Immunohistochemical application of D2-40 as basal cell marker in evaluating atypical small acinar proliferation of initial routine prostatic needle biopsy materials. Med Mol Morphol. 2010;43:165-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Gill HK, Ioffe OB, Berg WA. When is a diagnosis of sclerosing adenosis acceptable at core biopsy? Radiology. 2003;228:50-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Shao S, Yao M, Li X, Li C, Chen J, Li G, Jia C, Wu R. Conventional and contrast-enhanced ultrasound features in sclerosing adenosis and correlation with pathology. Clin Hemorheol Microcirc. 2021;77:173-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Hedrick L, Epstein JI. Use of keratin 903 as an adjunct in the diagnosis of prostate carcinoma. Am J Surg Pathol. 1989;13:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 119] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Devaraj LT, Bostwick DG. Atypical basal cell hyperplasia of the prostate. Immunophenotypic profile and proposed classification of basal cell proliferations. Am J Surg Pathol. 1993;17:645-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 64] [Article Influence: 2.0] [Reference Citation Analysis (0)] |