Published online Jun 26, 2022. doi: 10.12998/wjcc.v10.i18.5957
Peer-review started: December 27, 2021
First decision: January 23, 2022
Revised: February 2, 2022
Accepted: April 22, 2022
Article in press: April 22, 2022
Published online: June 26, 2022
Processing time: 171 Days and 4 Hours
An emerging area of interest in understanding disease phenotypes is systems genomics. Complex diseases such as diabetes have played an important role towards understanding the susceptible genes and mutations. A wide number of methods have been employed and strategies such as polygenic risk score and allele frequencies have been useful, but understanding the candidate genes harboring those mutations is an unmet goal. In this perspective, using systems genomic approaches, we highlight the application of phenome-interactome networks in diabetes and provide deep insights. LINC01128, which we previously described as candidate for diabetes, is shown as an example to discuss the approach.
Core Tip: Comprehensive genome-wide phenome-interactome networks are essential to identify candidate biomarkers such as LINC01128.
- Citation: Rout M, Kour B, Vuree S, Lulu SS, Medicherla KM, Suravajhala P. Diabetes mellitus susceptibility with varied diseased phenotypes and its comparison with phenome interactome networks. World J Clin Cases 2022; 10(18): 5957-5964
- URL: https://www.wjgnet.com/2307-8960/full/v10/i18/5957.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i18.5957
Diabetes mellitus occurs as a result of insufficient insulin production or impaired insulin sensitivity, and it has become a serious threat to people's health[1,2]. It is a heterogeneous problem with numerous aetiologies comprising three main types, viz., type 1 diabetes mellitus (T1DM), type 2 diabetes mellitus (T2DM), and gestational diabetes mellitus (GDM). Understanding the biological mechanisms associated would allow us to identify candidate proteins and genes[3]. The emergence of genome-wide association studies (GWASs) has substantially enhanced our understanding of the genetic basis of disease risk in the past few years. Prior to the introduction of GWASs in 2006, very little information was available about the genes that influence common complicated or multifactorial diseases and quantitative traits. These research findings imply that susceptibility to prevalent diseases is influenced by a variety of genetic topologies, including common genetic variants with minimal effects and uncommon variants with substantial impact sizes[4-6]. Nevertheless, the combination of candidate T2DM genes discovered using GWASs does not fully confirm established features of disease pathogenesis. Several system-level approaches have been used to bridge the gap between genome and phenome correlation[7]. Computational analyses of disease linked genes using interactome and toxicogenomic data help us to connect T2DM candidate genes found in GWAS with disease pathophysiology, including abnormal pancreatic cell formation and function, and insulin sensitivity. On the other hand, computational predictions of potential proteins/genes are less expensive and time-saving than experimental methods[8,9]. In order to unravel the genetic roots of common disorders, it is necessary to understand the complexity of the gene–phenotype connection. Recent research employing the human interactome and phenome has uncovered not just common phenotypic and genetic overlap between diseases but also a modular architecture of the genetic landscape of human diseases, opening up new avenues for reducing the complexity of human diseases[10,11]. Because diseases are rarely caused by the malfunction of a single protein, a more comprehensive and robust interactome is essential for identifying groups of interconnected proteins associated with disease aetiology[12].
The phenome interaction networks are used to study a wide range of phenotypic traits based on the analysis of the complete genome; it follows a genotypic to phenotypic approach in order to analyse the phenotypic traits[13]. The diseases with overlapping clinical signs can be predicted because of the mutation in different genes which are playing a role in similar functions. More recently, the studies on humans as well as model organisms have revealed that the primary or secondary association between proteins can also be one of the reasons of the same phenotype that means the mutation in particular protein along with its direct or indirect association with a single or multiple proteins can be responsible for overlapping of the clinical manifestations[14]. The opposite scenario can also be analysed using a phenome-interactome network, in case of pleiotropy, the cases in which a single gene is responsible for different phenotypic traits[15]. The protein-protein interaction (PPI) network models are used to analyse the phenomic traits, which in turn is helpful in understanding cell signalling and drug development in the diseased as well as normal cell physiology; basically, it is important to understand almost every process of the cell. PPI networks are the mathematical representation of physical interaction between similar or different proteins for the analysis of phenomes. The mathematical representation of interaction among different proteins in PPIs is based upon graph theory where the proteins are represented as nodes and edges to depict the type of interaction between two different interacting proteins[16]. PPI networks help to find the genes for a particular disease with a huge accuracy and when PPIs are implemented on the large datasets, it could lead to prediction of novel gene candidates[11]. The phenome interaction networks are quite important to understand and mine the genes associated with a particular disease. The genes that are responsible for similar functions have a higher chance of having the same phenotypes; therefore, understanding phenotypic as well as genotypic data is a must in order to understand the origination and development of a disease at the systems biology level for the better treatment[17]. The origin and cause of several complex diseases including cancer, diabetes, and obesity can be understood by PPI network analysis[18].
GDM is categorised as insulin resistance leading to hyperglycemia during pregnancy, which mostly retracts after parturition. According to the World Health Organization, the prevalence rate is 15.8% accounting to about 20.4 million live births, with the majority of cases in pregnant women above the age of 35 years. The International Diabetes Federation in 2019 estimated a prevalence of 28.5% in India with incidence varying in each state due to challenges in screening strategies and paucity of consensus among physicians and healthcare providers in prepartum and postpartum management of GDM[19]. The diagnostic criteria may differ worldwide, and understanding the pathophysiology is crucial as it affects both the mother and the fetus during gestation, delivery, and later stages of life making them susceptible to diabetes, obesity, and cardiovascular complications in the long term[20]. Major challenges that have governed this disease are the guidelines for screening and diagnosis. The testing criteria are different with varying forms of oral glucose tolerance test being followed worldwide[21]. Management of GDM is another challenge as both the mother and fetus are at risk in their current milieu. Studies have highlighted the importance of treating GDM, reducing the risk of perinatal morbidity and improving post-delivery outcomes[22]. Glucose intolerance leads to the manifestation of the disease, hence the benchmark of GDM treatment should be glycaemic control which is achieved through lifestyle intervention such as diet and exercise, pharmacological intervention such as insulin, oral drugs, and herbal medicines, and finally postnatal management[23].
Pregnant women with GDM have an inherent risk of developing T2DM post-delivery or later on in life. The offspring is also susceptible to any form of diabetes postnatally or in the long term. The genetic factors responsible for GDM and future risk of developing T2DM through epidemiological and physiological studies reveal commonality in susceptibility loci, which implies that most of the diabetes genes are involved in causing GDM. The few key genes that share common variants are KCNJ11, GCK, HNF1A, TCF7L2, CDKAL1, KCNQ1, CDKN2A, MTNR1B, SRR, HHEX, TCF2, SLC30A8, and IGF2BP2[24,25]. Genetic similarities between T1DM and GDM is less studied, and a study among Asian Indian women with GDM showed the presence of pancreatic autoantibodies like GAD which is a biomarker for T1DM[26]. Maturity onset diabetes of young (MODY) has different types and each type is characterised by a single gene, and few studies have shown that mutations in HNF1A and HNF4A are MODY genes which predispose to GDM[27].
Integrating phenotypic data with genotypic data through a computationally created high-confidence interaction network to analyse human diseases concurrently defines a phenome-interactome network[14]. An organized study on genes expressed in thigh subcutaneous adipose tissue of Asian Indian Type 2 Diabetes Mellitus revealed evidence of “sick thigh fat” as a causative disease. The phenome-interactome network had a significant correlation of differentially expressed genes (DEGs) and hub proteins with its phenotypic traits obtained at the clinical, biochemical, and radiological, cellular, and molecular levels, thus enumerating their role in T2DM, T1DM, and obesity[28]. RNA-seq analysis enables identification of differentially expressed genes and their role in a disease. The depth of the literature available on RNA-seq analysis performed on pregnant ladies with GDM is negligible. The GDM is a condition in which the intrauterine milieu, especially the placenta, plays a central role in altering the course of the fetus. Hence, having an understanding of the key genes regulated in the placenta is paramount for the disease diagnosis. Most of the literature available on RNA-seq analysis is centred on identifying DEGs in the placenta, umbilical cord, and amniocytes[29-32]. Studies have identified that non-coding RNAs such as long non-coding (lnc)RNAs, microRNAs, and circular RNAs play a central role in GDM pathogenesis. MicroRNAs have been identified as non-invasive early diagnostic biomarkers for GDM[33]. LncRNA-associated feed-forward loops network had a strong correlation between dysregulated glucose metabolism and hormone regulation in GDM cases[34]. The mechanism governing the pathophysiology of the disease is still not clear and the studies available are limited. Hence, the current problem is to understand the genetic background that affects both the mother and fetus with changes in the intrauterine environment and thus identify early diagnostic biomarkers. GDM is associated with a number of comorbidities due to the multifactorial nature of the disease. A study to identify key genes involved in GDM maternal and placental milieu revealed associations with T2DM, T1DM, obesity, hyperglycaemia, preeclampsia, neonatal diabetes, MODY, neurological disorders, cardiovascular disease, preeclampsia, hepatitis C, rheumatoid arthritis, and neoplasms[35]. Hence, the need to identify genes governing this disease and the variations that might affect the phenotype needs to be understood.
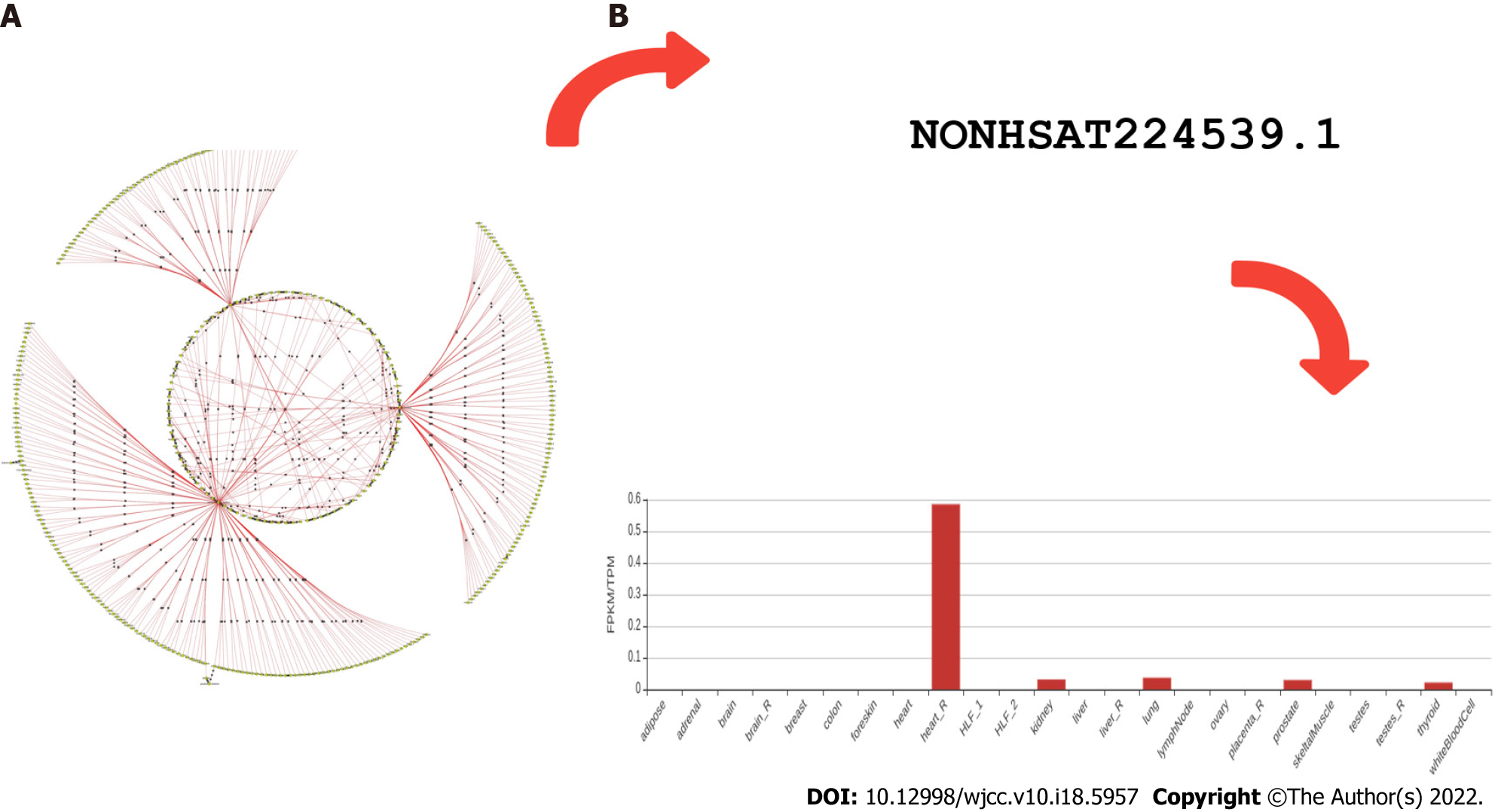
As glucose level in the body is regulated by insulin, a hormone (peptide) which increases the glucose uptake and its assimilation. However, insulin resistance is stated when it becomes unable to perform this function in a diabetic patient. On the other hand, the beta cell continuously secretes insulin to make up and maintain balance but it results in hyperinsulinemia[36]. This increased level will trigger the production of IGF-1 from liver cells. IGF-1 will then bind to its tyrosine kinase receptor IGF-1R and stimulate various metabolic and mitogenic signalling pathways to control processes like cancer cell proliferation, differentiation, and apoptosis. Later, some downstream targets like PI3KB and rat sarcoma-mitogen-activated protein kinase/extracellular signal regulated kinase signaling pathways get stimulated. PI3KB signaling has a role in cancer cell survival and migration, while the rat sarcoma mitogen-activated protein kinase/extracellular signal regulated kinase signaling pathway controls cancer cell proliferation and metabolism[37]. Hence, patients who have diabetes show increased levels of IGF-1, bringing in them more susceptibility towards a higher risk of developing different cancers like breast, prostate, and colorectal cancer[38]. However, the growth factor IGF-II which shares locus with lncH19 (IGF-II/H19) forms an imprinted gene. This silencing is found disrupted in different cancers including prostate cancer. The association of adipose tissue and obesity is a known risk factor for both T2DM and prostate cancer by disturbing cellular environments. As a result, hyperglycaemia or inflammatory metabolic situations are hypothesized to be the cause of this loss of imprinting (LOI)[39]. Differentially expressed lncRNA (LINC01128) is already known to increase the rate of cervical cancer progression and is also predicted as a biomarker of gestational hypertension[40,41]. Similarly, Pradeep Tiwari et al[28] in 2019 suggested that LINC01128 could serve as a biomarker for diabetes diagnosis and prognosis (Figure 1). Metformin, an antidiabetic drug from several studies, has been proved to not only effect on glucose metabolism but also show interactions with androgen receptors. It plays a role in stabilizing prostate specific antigen (PSA) levels[42]. In certain therapy, another commonly used method for T2DM, it is reported that glucagon-like peptide-1 receptor expression plays an anti-prostate cancer effect. It is helping in attenuating cell cycle progression. So, its forceful activation to express can be a potential therapeutic approach[43]. Therefore, both metformin and certain therapies help in blocking cell cycle progression by reducing mTOR activity[44]. Hypogonadism (decrease in level of testosterone) is also found associated with both diabetes and prostate cancer (PCa). A fall in its serum level is capable of causing high graded PCa. Hence, T2DM is suggested to be a crucial predictor of high graded PCa especially with benign prostatic hyperplasia[45]. For early possible detection, PSA levels are broadly used, but its concentration shows variation due to several other comorbidities, age, and lifestyle, which makes it to demand more precise analysis of test results. Based on a linear aggression analysis, there is a fall in PSA in patients who are taking antidiabetics and obese people on hemodilution. This establishes an inverse relationship between diabetes obesity and PSA level. Such study suggests to deliberately check the PSA level, especially in diabetic and obese patients[46]. Both PCa and DM incidence is rising parallel with age. Despite the fact diabetes mellitus reduces the risk of PCa, DM can also increase its mortality[47]. The understanding of association between DM and PCa is still insufficient. Moreover, obesity makes its pathophysiology a more complex situation[48].
In a study, GEO datasets of osteosarcoma (OS) were analysed for LINC01128 expression to clear its oncogenic role. It revealed that increased expression of LINC01128 in OS patients is accompanied with their shorter survival. However, its knockdown turned down the proliferation, migration, and invasion. In OS, LINC01128 is identified to work as a sponge in triggering Wnt/β-Catenin signaling by promoting MMP2 expression through miR-299-3p[49]. In promoting cervical cancer development again, it functions as a sponge for miR-383-5p[50]. In cervical cancer tissues, the expression of LINC01128 is found significantly high and its fall suggests that it might lower the SFN (stratifin) at both the mRNA and protein levels. SFN, a known potential biomarker in cervical cancer, is also majorly expressed in the early stage of lung adenocarcinomas. It clearly explains how LINC01128 could accelerate cell processes like cell proliferation, migration, and invasion and even can inhibit the apoptosis through SFN upregulation and release by binding miR-383-5p and also working as its antagonist[51,52]. miR-383 is under regulation of LINC01128. However, overexpression of miR-383 in T2DM serum reverses the cell apoptosis under high glucose in mouse β cells by TLR4 and APOC3 suppression[53]. Also, high LINC01128 was seen in stage III-IV CRC and mediated PRMT5 function, which is a mediator of methylation of proteins[54]. In pancreatic cancer, it was found as an EMT-LPS (epithelial mesenchymal transition related lncRNA prognostic signature) molecule[55].
The phenome-interactome networks have been a powerful approach to understand and characterize networks. There is a greater scope of relevance underlying the pathophysiology mentioned above. To fully comprehend the importance of phenome-interactome networks and diabetes associated metabolism, it is vital to ensure that there is a healthy diet regimen followed which also addresses the clinical implications of its absorption, bioavailability, and human health benefits. Integrated systems approaches can be used to discover the novel genes and pathways with an emphasis on the molecular physiological insights gained through systems/nutrigenomic modules and thereby candidate DEGs could be detected. Furthermore, standard operating procedures, recommendations, and guidelines in consideration of the aforementioned diabetes phenotypes for better dissemination of phenome-interactome predictions will help avoid the risk of over/under treatment. In addition, post next generation sequencing, a large focus nowadays should be on the development of NGS/genotyping panels which can set a precedent for a global consortium effort bridging the gap between the nutritional deficiency diseases and diabetes.
The authors gratefully acknowledge Arvinpreet Kaur, Mehak Chopra, Berenice, Kiran Telukunta, Anshu Bharadwaj, Harpreet Singh, and Purnima Sharma for subtle scientific deliberations.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Lei Y, China; Wang W, China; Zeng Y, China S-Editor: Liu JH L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365:1333-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1582] [Cited by in RCA: 1609] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 2. | Brunetti A, Chiefari E, Foti D. Recent advances in the molecular genetics of type 2 diabetes mellitus. World J Diabetes. 2014;5:128-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 80] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 3. | Tang X, Hu X, Yang X, Fan Y, Li Y, Hu W, Liao Y, Zheng MC, Peng W, Gao L. Predicting diabetes mellitus genes via protein-protein interaction and protein subcellular localization information. BMC Genomics. 2016;17 Suppl 4:433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Gibson G. Rare and common variants: twenty arguments. Nat Rev Genet. 2012;13:135-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 882] [Cited by in RCA: 838] [Article Influence: 64.5] [Reference Citation Analysis (1)] |
| 5. | Visscher PM, Brown MA, McCarthy MI, Yang J. Five years of GWAS discovery. Am J Hum Genet. 2012;90:7-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1644] [Cited by in RCA: 1587] [Article Influence: 122.1] [Reference Citation Analysis (0)] |
| 6. | Hirschhorn JN, Gajdos ZK. Genome-wide association studies: results from the first few years and potential implications for clinical medicine. Annu Rev Med. 2011;62:11-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Jain P, Vig S, Datta M, Jindel D, Mathur AK, Mathur SK, Sharma A. Systems biology approach reveals genome to phenome correlation in type 2 diabetes. PLoS One. 2013;8:e53522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Prokopenko I, McCarthy MI, Lindgren CM. Type 2 diabetes: new genes, new understanding. Trends Genet. 2008;24:613-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 177] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 9. | Florez JC. Newly identified loci highlight beta cell dysfunction as a key cause of type 2 diabetes: where are the insulin resistance genes? Diabetologia. 2008;51:1100-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 208] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 10. | Wu X, Liu Q, Jiang R. Align human interactome with phenome to identify causative genes and networks underlying disease families. Bioinformatics. 2009;25:98-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Oti M, Snel B, Huynen MA, Brunner HG. Predicting disease genes using protein-protein interactions. J Med Genet. 2006;43:691-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 372] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 12. | Alanis-Lobato G. Mining protein interactomes to improve their reliability and support the advancement of network medicine. Front Genet. 2015;6:296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Hebbring SJ. The challenges, advantages and future of phenome-wide association studies. Immunology. 2014;141:157-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 14. | Lage K, Karlberg EO, Størling ZM, Olason PI, Pedersen AG, Rigina O, Hinsby AM, Tümer Z, Pociot F, Tommerup N, Moreau Y, Brunak S. A human phenome-interactome network of protein complexes implicated in genetic disorders. Nat Biotechnol. 2007;25:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 660] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 15. | Cronin RM, Field JR, Bradford Y, Shaffer CM, Carroll RJ, Mosley JD, Bastarache L, Edwards TL, Hebbring SJ, Lin S, Hindorff LA, Crane PK, Pendergrass SA, Ritchie MD, Crawford DC, Pathak J, Bielinski SJ, Carrell DS, Crosslin DR, Ledbetter DH, Carey DJ, Tromp G, Williams MS, Larson EB, Jarvik GP, Peissig PL, Brilliant MH, McCarty CA, Chute CG, Kullo IJ, Bottinger E, Chisholm R, Smith ME, Roden DM, Denny JC. Phenome-wide association studies demonstrating pleiotropy of genetic variants within FTO with and without adjustment for body mass index. Front Genet. 2014;5:250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Agapito G, Guzzi PH, Cannataro M. Visualization of protein interaction networks: problems and solutions. BMC Bioinformatics. 2013;14 Suppl 1:S1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Li Y, Patra JC. Genome-wide inferring gene-phenotype relationship by walking on the heterogeneous network. Bioinformatics. 2010;26:1219-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 261] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 18. | Sun J, Zhao Z. A comparative study of cancer proteins in the human protein-protein interaction network. BMC Genomics. 2010;11 Suppl 3:S5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Morampudi S, Balasubramanian G, Gowda A, Zomorodi B, Patil AS. The Challenges and Recommendations for Gestational Diabetes Mellitus Care in India: A Review. Front Endocrinol (Lausanne). 2017;8:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers. 2019;5:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 991] [Article Influence: 165.2] [Reference Citation Analysis (1)] |
| 21. | Mpondo BC, Ernest A, Dee HE. Gestational diabetes mellitus: challenges in diagnosis and management. J Diabetes Metab Disord. 2015;14:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS; Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352:2477-2486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2150] [Cited by in RCA: 2125] [Article Influence: 106.3] [Reference Citation Analysis (0)] |
| 23. | Langer O. A spectrum of glucose thresholds may effectively prevent complications in the pregnant diabetic patient. Semin Perinatol. 2002;26:196-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Huerta-Chagoya A, Vázquez-Cárdenas P, Moreno-Macías H, Tapia-Maruri L, Rodríguez-Guillén R, López-Vite E, García-Escalante G, Escobedo-Aguirre F, Parra-Covarrubias A, Cordero-Brieño R, Manzo-Carrillo L, Zacarías-Castillo R, Vargas-García C, Aguilar-Salinas C, Tusié-Luna T. Genetic determinants for gestational diabetes mellitus and related metabolic traits in Mexican women. PLoS One. 2015;10:e0126408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 25. | Watanabe RM. Inherited destiny? Genome Med. 2011;3:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Unnikrishnan R, Shanthi Rani CS, Anjana RM, Uthra SC, Vidya J, Sankari GU, Venkatesan U, Rani SJ, Mohan V. Postpartum development of type 1 diabetes in Asian Indian women with gestational diabetes. Indian J Endocrinol Metab. 2016;20:702-706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Weng J, Ekelund M, Lehto M, Li H, Ekberg G, Frid A, Aberg A, Groop LC, Berntorp K. Screening for MODY mutations, GAD antibodies, and type 1 diabetes--associated HLA genotypes in women with gestational diabetes mellitus. Diabetes Care. 2002;25:68-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Tiwari P, Saxena A, Gupta N, Medicherla KM, Suravajhala P, Mathur SK. Systems Genomics of Thigh Adipose Tissue From Asian Indian Type-2 Diabetics Revealed Distinct Protein Interaction Hubs. Front Genet. 2018;9:679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Cao M, Zhang L, Lin Y, Li Z, Xu J, Shi Z, Chen Z, Ma J, Wen J. Circular RNA expression profiles in umbilical cord blood exosomes from normal and gestational diabetes mellitus patients. Biosci Rep. 2020;40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Magee TR, Ross MG, Wedekind L, Desai M, Kjos S, Belkacemi L. Gestational diabetes mellitus alters apoptotic and inflammatory gene expression of trophobasts from human term placenta. J Diabetes Complications. 2014;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 31. | Pinney SE, Joshi A, Yin V, Min SW, Rashid C, Condon DE, Wang PZ. Exposure to Gestational Diabetes Enriches Immune-Related Pathways in the Transcriptome and Methylome of Human Amniocytes. J Clin Endocrinol Metab. 2020;105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Wang H, She G, Zhou W, Liu K, Miao J, Yu B. Expression profile of circular RNAs in placentas of women with gestational diabetes mellitus. Endocr J. 2019;66:431-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 33. | Zhu Y, Tian F, Li H, Zhou Y, Lu J, Ge Q. Profiling maternal plasma microRNA expression in early pregnancy to predict gestational diabetes mellitus. Int J Gynaecol Obstet. 2015;130:49-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 34. | Fu X, Cong H, Zhao S, Li Y, Liu T, Sun Y, Lv N. Construction of Glycometabolism- and Hormone-Related lncRNA-Mediated Feedforward Loop Networks Reveals Global Patterns of lncRNAs and Drug Repurposing in Gestational Diabetes. Front Endocrinol (Lausanne). 2020;11:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Rout M, Lulu S S. Molecular and disease association of gestational diabetes mellitus affected mother and placental datasets reveal a strong link between insulin growth factor (IGF) genes in amino acid transport pathway: A network biology approach. J Cell Biochem. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Godsland IF. Insulin resistance and hyperinsulinaemia in the development and progression of cancer. Clin Sci (Lond). 2009;118:315-332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 37. | Poloz Y, Stambolic V. Obesity and cancer, a case for insulin signaling. Cell Death Dis. 2015;6:e2037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 38. | Adekola K, Rosen ST, Shanmugam M. Glucose transporters in cancer metabolism. Curr Opin Oncol. 2012;24:650-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 278] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 39. | Kingshott G, Biernacka K, Sewell A, Gwiti P, Barker R, Zielinska H, Gilkes A, McCarthy K, Martin RM, Lane JA, McGeagh L, Koupparis A, Rowe E, Oxley J, Holly JMP, Perks CM. Alteration of Metabolic Conditions Impacts the Regulation of IGF-II/H19 Imprinting Status in Prostate Cancer. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 40. | Xue F, Song X, Zhang S, Niu M, Cui Y, Wang Y, Zhao T. Long non-coding RNA TMPO-AS1 serves as a tumor promoter in pancreatic carcinoma by regulating miR-383-5p/SOX11. Oncol Lett. 2021;21:255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 41. | Xu J, Fan L, Qi F, Xiu X. Screening of Biomarkers for Hypertension Susceptibility in Pregnancy Proc Anticancer Res. 2020; 4. |
| 42. | Taussky D, Delouya G. Impact of diabetes and metformin use on prostate cancer. Scand J Urol. 2020;54:508-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 43. | Shigeoka T, Nomiyama T, Kawanami T, Hamaguchi Y, Horikawa T, Tanaka T, Irie S, Motonaga R, Hamanoue N, Tanabe M, Nabeshima K, Tanaka M, Yanase T, Kawanami D. Activation of overexpressed glucagon-like peptide-1 receptor attenuates prostate cancer growth by inhibiting cell cycle progression. J Diabetes Investig. 2020;11:1137-1149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 44. | Lin E, Garmo H, Van Hemelrijck M, Adolfsson J, Stattin P, Zethelius B, Crawley D. Association of type 2 diabetes mellitus and antidiabetic medication with risk of prostate cancer: a population-based case-control study. BMC Cancer. 2020;20:551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | Ohwaki K, Endo F, Shimbo M, Fujisaki A, Hattori K. Comorbidities as predictors of incidental prostate cancer after Holmium laser enucleation of the prostate: diabetes and high-risk cancer. Aging Male. 2017;20:257-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 46. | Kobayashi M, Mizuno T, Yuki H, Kambara T, Betsunoh H, Nukui A, Abe H, Fukabori Y, Yashi M, Kamai T. Association between serum prostate-specific antigen level and diabetes, obesity, hypertension, and the laboratory parameters related to glucose tolerance, hepatic function, and lipid profile: implications for modification of prostate-specific antigen threshold. Int J Clin Oncol. 2020;25:472-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Knura M, Garczorz W, Borek A, Drzymała F, Rachwał K, George K, Francuz T. The Influence of Anti-Diabetic Drugs on Prostate Cancer. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 48. | Kelkar S, Oyekunle T, Eisenberg A, Howard L, Aronson WJ, Kane CJ, Amling CL, Cooperberg MR, Klaassen Z, Terris MK, Freedland SJ, Csizmadi I. Diabetes and Prostate Cancer Outcomes in Obese and Nonobese Men After Radical Prostatectomy. JNCI Cancer Spectr. 2021;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 49. | Yao Q, Chen T. LINC01128 regulates the development of osteosarcoma by sponging miR‐299‐3p to mediate MMP2 expression and activating Wnt/β‐catenin signalling pathway. Journal of cellular and molecular medicine. 2020;24:14293-14305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 50. | He J, Ling L, Liu Z, Ren X, Wan L, Tu C, Li Z. Functional interplay between long non-coding RNAs and the Wnt signaling cascade in osteosarcoma. Cancer Cell International. 2021;21:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Hu Y, Ma Y, Liu J, Cai Y, Zhang M, Fang X. LINC01128 expedites cervical cancer progression by regulating miR-383-5p/SFN axis. BMC Cancer. 2019;19:1157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 52. | Li X, Yuan J, Cao Q, Xie A, Chen J. MicroRNA-383-5p inhibits the proliferation and promotes the apoptosis of gastric cancer cells by targeting cancerous inhibitor of PP2A. Int J Mol Med. 2020;46:397-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 53. | Cheng X, Huang Y, Yang P, Bu L. miR-383 ameliorates high glucose-induced β-cells apoptosis and hyperglycemia in high-fat induced diabetic mice. Life Sci. 2020;263:118571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 54. | Zhao Z, Yang YB, Li XY, Li XG, Chu XD, Lin ZB, Zhang YR, Guo YG, Ding H, Pan YL, Wang L, Pan JH. Comprehensive Analysis of N6-Methyladenosine-Related lncRNA Signature for Predicting Prognosis and Immune Cell Infiltration in Patients with Colorectal Cancer. Dis Markers. 2021;2021:8686307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 55. | Deng Y, Hu H, Xiao L, Cai T, Gao W, Zhu H, Wang S, Liu J. Identification of EMT-Related lncRNAs as a Potential Prognostic Biomarker and Therapeutic Targets for Pancreatic Adenocarcinoma. 2021. |