Published online Jun 16, 2022. doi: 10.12998/wjcc.v10.i17.5846
Peer-review started: December 23, 2021
First decision: January 27, 2022
Revised: February 2, 2022
Accepted: April 9, 2022
Article in press: April 9, 2022
Published online: June 16, 2022
Processing time: 167 Days and 15.6 Hours
Gallbladder perforation and gastrointestinal fistula are rare but serious complications of severe acute pancreatitis (SAP). However, neither spontaneous gallbladder perforation nor cholecysto-colonic fistula has been reported in acalculous acute pancreatitis patients.
A 31-year-old male presenting with epigastric pain was diagnosed with hypertriglyceridemia-related SAP. He suffered from multiorgan failure and was able to leave the intensive care unit on day 20. Three percutaneous drainage tubes were placed for profound exudation in the peripancreatic region and left paracolic sulcus. He developed spontaneous gallbladder perforation with symptoms of fever and right upper quadrant pain 1 mo after SAP onset and was stabilized by percutaneous drainage. Peripancreatic infection appeared 1 mo later and was treated with antibiotics but without satisfactory results. Then multiple colon fistulas, including a cholecysto-colonic fistula and a descending colon fistula, emerged 3 mo after the onset of SAP. Nephroscopy-assisted peripancreatic debridement and ileostomy were carried out immediately. The fistulas achieved spontaneous closure 7 mo later, and the patient recovered after cholecystectomy and ileostomy reduction. We presume that the causes of gallbladder perforation are poor bile drainage due to external pressure, pancreatic enzyme erosion, and ischemia. The possible causes of colon fistulas are pancreatic enzymes or infected necrosis erosion, ischemia, and iatrogenic injury. According to our experience, localized gallbladder perforation can be stabilized by percutaneous drainage. Pancreatic debridement and proximal colostomy followed by cholecystectomy are feasible and valid treatment options for cholecysto-colonic fistulas.
Gallbladder perforation and cholecysto-colonic fistula should be considered in acalculous SAP patients.
Core Tip: To the best of our knowledge, this is the first time that spontaneous gallbladder perforation and cholecysto-colonic fistula have been reported in patients with acalculous severe acute pancreatitis. Biliary obstruction due to peripancreatic effusions, pancreatic enzymes or infected necrosis erosion, ischemia, and iatrogenic injury might be related. According to our experience, localized gallbladder perforation can be stabilized by percutaneous drainage. Pancreatic debridement and proximal colostomy followed by cholecystectomy are feasible and valid treatment options for cholecysto-colonic fistulas.
- Citation: Wang QP, Chen YJ, Sun MX, Dai JY, Cao J, Xu Q, Zhang GN, Zhang SY. Spontaneous gallbladder perforation and colon fistula in hypertriglyceridemia-related severe acute pancreatitis: A case report. World J Clin Cases 2022; 10(17): 5846-5853
- URL: https://www.wjgnet.com/2307-8960/full/v10/i17/5846.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i17.5846
Pancreatic pseudocysts, wall-off necrosis, and peripancreatic abscess are well-known local complications of severe acute pancreatitis (SAP). There are few reports on rare complications of gallbladder perforation[1] or gastrointestinal fistula. Gallbladder perforation occurs in 2%-10% of patients with acute cholecystitis[2], usually occurs in elderly males[3], and is mostly associated with calculous cholecystitis[4]. Delays in diagnosis lead to a poor prognosis. One study reported that the morbidity and mortality of gallbladder perforation are 37.5% and 12.5%, respectively[4]. Gastrointestinal fistula most commonly occurs in the colon, with an incidence of 3.3% in acute pancreatitis (AP) and 15% in SAP[5], which can also involve the duodenum, stomach, and small intestine[6]. Compared with patients without colon complications, SAP patients with colon involvement have significantly higher morbidity and mortality (54% for colonic necrosis)[7]. However, neither gallbladder perforation nor cholecysto-colonic fistula has been recorded in acalculous AP patients. Herein, we present the first case of spontaneous gallbladder perforation and cholecysto-colonic fistula in a patient with acalculous SAP.
A 31-year-old male presented in the emergency room with epigastric pain for 3 d and loss of consciousness for 1 d.
This patient with a body mass index of 29.39 kg/m2 was admitted to the local hospital because of epigastric pain for 1 d after a fatty meal. He described the pain as persistent, severe, and radiating to the back, accompanied by nausea and vomiting. Local laboratory examination revealed that the serum amylase level was over 500 U/L (the upper normal limit was 135 U/L), and the triglyceride concentration was 44 mmol/L. The abdominal computed tomography (CT) scan showed pancreatic edema without gallstones. Considering hypertriglyceridemia-related AP, he received lipid-lowering (fenofibrate) and supportive treatment. Two days later, he was transferred to our hospital for worsened situations with loss of consciousness, anuria, and high fever.
The patient reported no remarkable history of past illness.
The patient liked fatty food, smoked 40 cigarettes per day for 10 years, and drank 500 mL of liquor per day for 10 years. There was no family history of malignant tumors.
The patient’s body temperature was 40 °C, heart rate was 180 beats per min, and respiratory rate was 40 breaths per min. Blood pressure and oxygen saturation could not be measured. Neurological examination revealed loss of light reflection from both pupils, and the Glasgow Coma Scale was E1V1M1. The abdomen was distended, tension was high, and bowel sounds were weak.
The auxiliary examination results at admission are shown in Table 1.
| Test item | Test result | Reference range |
| White blood cell (× 109/L) | 8.7 | 3.5-9.5 |
| Hemoglobin (g/L) | 78 | 120–160 |
| Platelet (× 109/L) | 206 | 100-350 |
| Alanine aminotransferase (U/L) | 230 | 9-50 |
| Alkaline phosphatase (U/L) | 67 | 45-125 |
| Total bilirubin (μmol/L) | 17.1 | 5.1-22.2 |
| Conjugated bilirubin (μmol/L) | 9.9 | 0-6.8 |
| Potassium (mmol/L) | 4.4 | 3.5-5.5 |
| Serum urea (mmol/L) | 19 | 2.78-7.14 |
| Serum creatinine (μmol/L) | 404 | 59-104 |
| Creatine kinase (U/L) | 42853 | 24-195 |
| Myoglobin (μg/L) | 88925 | 10-92 |
| High-sensitivity C-reactive protein (mg/L) | > 250 | < 3.0 |
| Erythrocyte sedimentation rate (mm/h) | > 140 | 0-15 |
| Procalcitonin (ng/mL) | 16 | < 0.25 |
| Blood cultures | Negative | Negative |
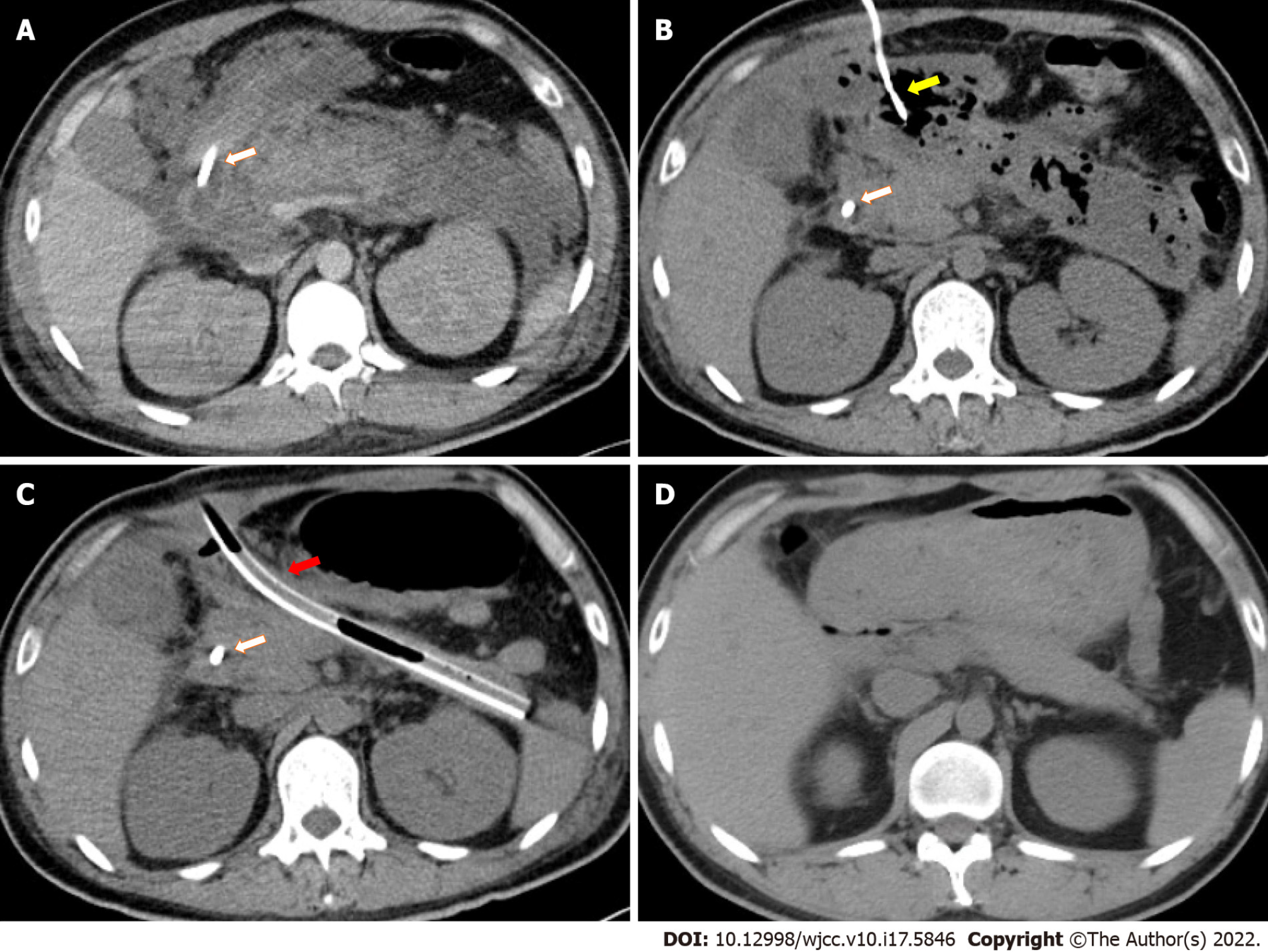
An enhanced CT scan showed swelling of the pancreas and profound effusions in the periphery of the pancreas, omental sac, and left paracolic sulcus (Figure 1A).
The patient was diagnosed with hypertriglyceridemia-related SAP with multiorgan failure, including shock, respiratory failure, acute renal failure, and rhabdomyolysis.
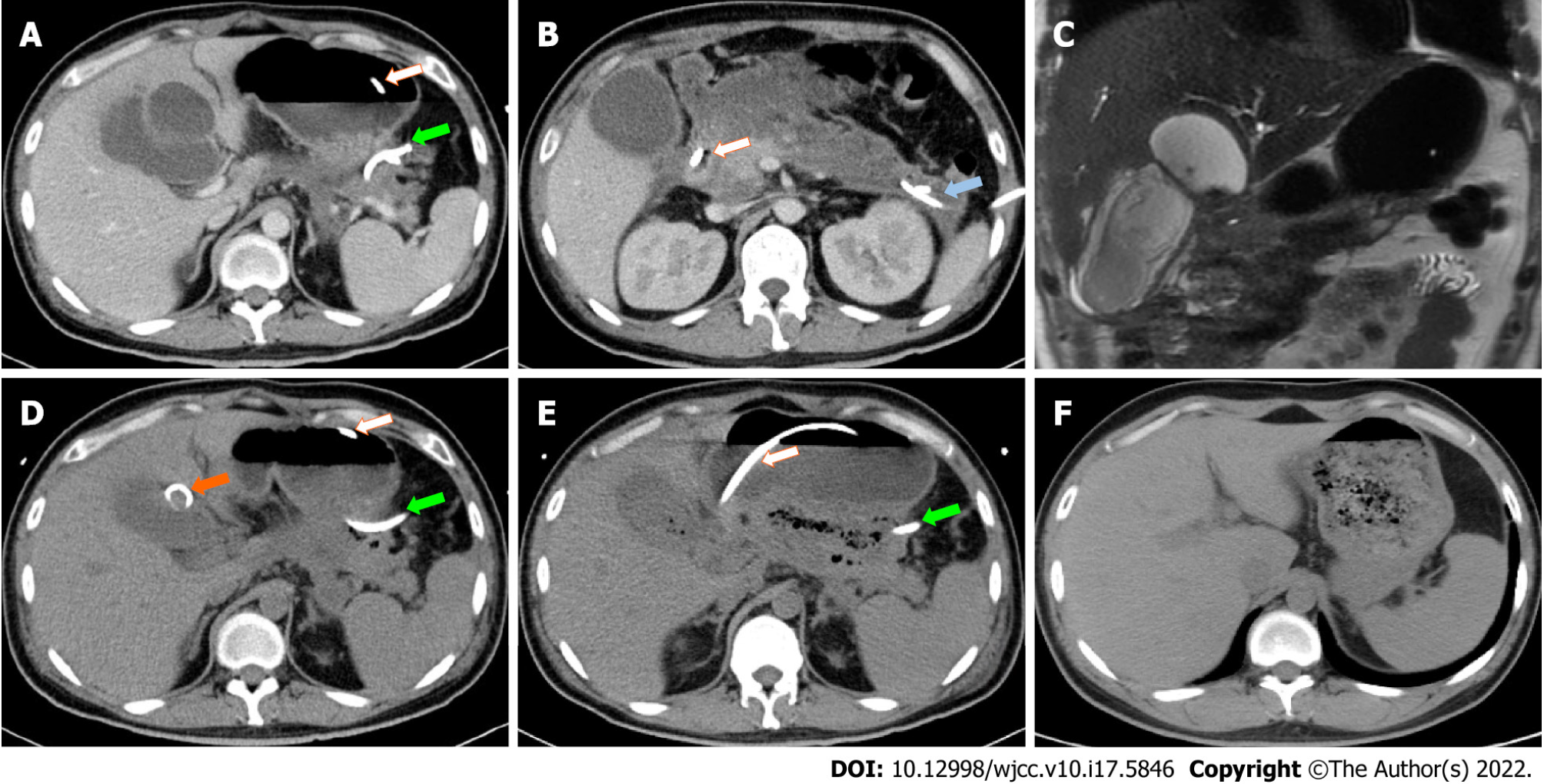
After supportive treatment including vasoactive agents, mechanical ventilation, kidney replacement therapy, and early enteral nutrition support the patient was stabilized and admitted to the general ward after spending 20 d in the intensive care units, during which time three percutaneous drainage tubes were placed for possible infection in necrosis collection and fluid effusions in the peripancreatic region and left paracolic sulcus (Figures 1B, 2A and 2B). The drainage fluid culture was negative.
One month after the onset of SAP, the patient developed fever and right upper quadrant pain with elevated conjugated bilirubin and alkaline phosphatase levels of 37.3 μmol/L and 340 U/L, respectively. Contrast-enhanced CT (Figure 2A) and magnetic resonance cholangiopancreatography (Figure 2C) revealed a cystic lesion adjacent to and communicating to a large gallbladder (Figure 2B), and gallbladder perforation was considered. Therefore, another drainage tube was placed into the cystic lesion percutaneously (Figure 2D). The drainage fluid was bile-like with an elevated total bilirubin level of 412 μmol/L.
Two months after the onset of SAP, fever came intermittently when abdominal CT showed gas in the necrotic tissue (Figure 1B), and the peripancreatic drainage fluid gradually turned into pus, which could be readily treated by antibiotics but recurred after antibiotics were ceased. One month later, when the radiologists replaced drainage tubes, feces were withdrawn from the gallbladder fossa and left paracolic sulcus. As indirect evidence of cholecysto-colonic fistula, CT showed gas in the gallbladder lumen (Figure 2E). Then, cholecysto-colonic fistula and descending colon fistula were confirmed via contrast examination. The patient immediately received nephroscopy-assisted debridement of peripancreatic necrosis and ileostomy. Peripancreatic and paracolic drains were changed to larger tubes (Figure 1C), and the abdominal cavity was flushed with normal saline every day. One month after surgery, the patient's body temperature returned to normal, and he was discharged from the hospital.
Six months after the onset of SAP, the patient gradually resumed oral intake with good tolerance, and all drains were removed 1 mo later. Seven months after ileostomy, a colonoscopy revealed spontaneous closure of colon fistulas, and abdominal CT showed the absorption of peripancreatic infectious necrosis (Figure 1D). The patient subsequently underwent cholecystectomy (Figure 2F) and ileostomy reversal. Pathology of the gallbladder suggested chronic inflammation of fibrous connective tissue. Since then, the patient has been symptom free for 5 mo. To illustrate the patient's medical history succinctly and clearly, we briefly summarize it in Table 2.
| Time since SAP onset | Clinical events |
| 11 d | Started jejunal nutrition |
| 1 mo | Gallbladder perforation |
| Percutaneous drainage | |
| 2 mo | Peripancreatic infection |
| Antibiotics and percutaneous drainage | |
| 3 mo | Cholecysto-colonic fistula and descending colon fistula |
| Peripancreatic debridement and ileostomy | |
| 4 mo | Normal body temperature |
| Discharged from hospital | |
| 6 mo | Started oral intake |
| 7 mo | All drains removed |
| 10 mo | Cholecystectomy and ileostomy reversal |
| 15 mo | Free from the symptoms after surgery |
Gallbladder perforation usually occurs days to weeks after acute cholecystitis[3]. It can cause diffuse peritonitis, or it can be surrounded by connective tissue, causing only localized peritonitis[2]. Gallbladder perforation is commonly seen in calculous cholecystitis and sometimes in cancer or trauma[8,9]. On the other hand, acalculous gallbladder perforation in AP is extremely rare. In this case, gallbladder perforation was diagnosed 1 mo after the onset of SAP. Fortunately, the bile was confined to the cystic lesion adjacent to gallbladder without causing generalized biliary peritonitis.
One of the possible mechanisms of gallbladder perforation is that poor bile drainage leads to increased pressure in the gallbladder, causing gallbladder ischemia and necrosis[10]. In this case, pancreatic edema and peripancreatic exudation might have caused biliary obstruction. Second, pancreatic enzymes can erode the adjacent gallbladder[3]. Our patient had profound effusions in the omental sac, which might contribute to damaging the integrity of the gallbladder walls. Third, hypotension will lead to insufficient blood supply to the gallbladder[10]. Our patient developed distributive shock shortly after SAP onset. Moreover, fasting after SAP onset and jejunal nutrition further increased the intraluminal pressure of the gallbladder according to animal models[11,12].
Colon complications of acute pancreatitis are uncommon and mainly include necrosis, perforation, fistula, and stricture[7,13,14]. Necrosis and perforation appear early in the course of necrotizing pancreatitis, usually within 1 mo, while fistulas and stricture usually occur several months later. In the current case, a cholecysto-colonic fistula, which has not been reported before, and a descending colon fistula were found during drain replacement 3 mo after the onset of SAP and 1 mo after peripancreatic infection.
Similar to gallbladder perforation, erosion of pancreatic enzymes and infectious necrosis, as well as hypotension from shock, can also cause disruption of the colon wall[15,16]. Iatrogenic injury could easily injure the intestine. However, a retrospective study ruled out percutaneous drainage as a risk factor for colon complications[13]. In the present case, it was difficult to fully rule out the possibility of puncture injury.
Local inflammation is prominent when colon fistula forms. Therefore, it is best not to repair the intestinal wall in the early stage. Pancreatic debridement and proximal colostomy are feasible options[17,18], after which some fistulas can thus be cured[7]. When local inflammation subsided, ostomy reduction was performed a mean of 248.1 d after the initial surgery[13]. Currently, there is no treatment recommendation for patients with gallbladder perforation or fistula in acalculous AP. According to our experience, localized gallbladder perforation can be stabilized by percutaneous drainage instead of urgent surgery. Pancreatic debridement and proximal colostomy followed by cholecystectomy after a period of 7 mo are feasible and valid treatment options for cholecysto-colonic fistulas.
Although gallbladder perforation and gastrointestinal fistula are rare complications in acalculous SAP patients, they should be considered for their poor prognosis. Pancreatic debridement and proximal colostomy followed by cholecystectomy after the infection is relieved are feasible and valid treatment options for cholecysto-colonic fistulas.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Hosoya S, Japan; Priyadarshi RN, India S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Perera M, Pham T, Toshniwal S, Lennie Y, Chan S, Houli N. A case of concomitant perforated acute cholecystitis and pancreatitis. Case Rep Surg. 2013;2013:263046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Quiroga-Garza A, Alvarez-Villalobos NA, Angeles-Mar HJ, Garcia-Campa M, Muñoz-Leija MA, Salinas-Alvarez Y, Elizondo-Omaña RE, Guzmán-López S. Localized gallbladder perforation: a systematic review of treatment and prognosis. HPB (Oxford). 2021;23:1639-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Başara I, Seçil M. Spontaneous asymptomatic gallbladder perforation. Quant Imaging Med Surg. 2014;4:212-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 4. | Derici H, Kara C, Bozdag AD, Nazli O, Tansug T, Akca E. Diagnosis and treatment of gallbladder perforation. World J Gastroenterol. 2006;12:7832-7836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 116] [Cited by in RCA: 139] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 5. | Dhadlie S, Ratnayake S. A rare case report of ascending colon perforation secondary to acute pancreatitis. Int J Surg Case Rep. 2019;55:62-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Andrew D, Shyam K, Johny J, Beaty S. Elderly male patient with gastrocolic fistula following severe acute necrotising pancreatitis. BMJ Case Rep. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Mohamed SR, Siriwardena AK. Understanding the colonic complications of pancreatitis. Pancreatology. 2008;8:153-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Zhang J, Shen G, Shi Y, Zhang C, Hong D, Jin L, Yang H, Sun W, Cai H, Hu Z, Wu W. Spontaneous acalculous gallbladder perforation in a man secondary to chemotherapy and radiation: A rare case report. Medicine (Baltimore). 2018;97:e0674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 9. | Katagiri T, Irisawa A, Wakabayashi H, Tsunoda T, Tomoda H, Saito R, Kinuta S. Idiopathic perforation of acalculous gallbladder after insertion of a transpapillary pancreatic stent. Endosc Int Open. 2016;4:E838-E840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Gunasekaran G, Naik D, Gupta A, Bhandari V, Kuppusamy M, Kumar G, Chishi NS. Gallbladder perforation: a single center experience of 32 cases. Korean J Hepatobiliary Pancreat Surg. 2015;19:6-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Psáder R, Sterczer A, Pápa K, Harnos A, Szilvási V, Pap A. Effect of enteral feeding on gallbladder function in dogs. Acta Vet Hung. 2012;60:211-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Ledeboer M, Masclee AA, Biemond I, Lamers CB. Gallbladder motility and cholecystokinin secretion during continuous enteral nutrition. Am J Gastroenterol. 1997;92:2274-2279. [PubMed] |
| 13. | Maatman TK, Nicolas ME, Roch AM, Lewellen KA, Al-Azzawi HH, Ceppa EP, House MG, Nakeeb A, Schmidt CM, Zyromski NJ. Colon Involvement in Necrotizing Pancreatitis: Incidence, Risk Factors, and Outcomes. Ann Surg. 2022;275:568-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Gondal B, Ogunsua AA, McIntosh L, Shaikh Y, Cave D. Perforation of the descending colon in severe acute pancreatitis: a case report and literature review. Pancreas. 2014;43:488-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Patterson M, Bernstein CN. A Rare Complication of Pancreatitis. Gastroenterology. 2021;160:1479-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Van Minnen LP, Besselink MG, Bosscha K, Van Leeuwen MS, Schipper ME, Gooszen HG. Colonic involvement in acute pancreatitis. A retrospective study of 16 patients. Dig Surg. 2004;21:33-38; discussion 39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Yoshikawa K, Lefor AK, Kubota T. Acute pancreatitis followed by retroperitoneal perforation of the descending colon and a duodenal fistula: Report of a case. Int J Surg Case Rep. 2020;72:599-602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Hozaka Y, Kurahara H, Mataki Y, Kawasaki Y, Iino S, Sakoda M, Mori S, Maemura K, Shinchi H, Natsugoe S. Successful treatment for severe pancreatitis with colonic perforation using video-assisted retroperitoneal debridement: A case report. Int J Surg Case Rep. 2018;52:23-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |