Published online Jun 16, 2022. doi: 10.12998/wjcc.v10.i17.5833
Peer-review started: December 13, 2021
First decision: March 7, 2022
Revised: March 18, 2022
Accepted: April 3, 2022
Article in press: April 3, 2022
Published online: June 16, 2022
Processing time: 177 Days and 15.1 Hours
Pulp revascularization has become a new method for the treatment of periapical diseases in young permanent teeth in recent years. Through root canal flushing and disinfection, avoiding mechanical preparation, guiding apical stem cells into the root canal and promoting the continuous development of tooth roots, it has achieved good clinical curative effects. But in adult patients with chronic periapical periodontitis with immature roots and open apices, apical barrier technology is often used to treat these teeth.
Pulp revascularization of a 26-year-old patient's tooth was performed using cefaclor instead of minocycline and iRoot BP instead of mineral trioxide aggregate as intracanal medication. The case was followed up for 36 mo. Observations showed evidence of regression of clinical signs and symptoms, resolution of apical periodontitis and no discolouration of affected teeth.
For adult patients with chronic periapical periodontitis with immature roots and open apices, pulp revascularisation showed favourable results in treating these teeth.
Core Tip: Pulp revascularization is especially suitable for young permanent teeth with incomplete apical development. However, few scholars have reported on adult teeth with apical periodontitis caused by an abnormal central tip being treated with dental pulp revascularization technology. The purpose of this case report was to describe the potential of using pulp revascularization to treat a permanent adult tooth.
- Citation: Yang YQ, Wu BL, Zeng JK, Jiang C, Chen M. Pulp revascularization on an adult mandibular right second premolar: A case report. World J Clin Cases 2022; 10(17): 5833-5840
- URL: https://www.wjgnet.com/2307-8960/full/v10/i17/5833.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i17.5833
Pulp revascularization has become a new method for the treatment of periapical diseases in young permanent teeth in recent years. Through root canal flushing and disinfection, avoiding mechanical preparation, guiding apical stem cells into the root canal and promoting the continuous development of tooth roots, it has achieved good clinical curative effects[1,2]. Initially, Holden et al[3] stimulated apical tissue bleeding to treat young permanent tooth periapical disease according to the mechanism of trauma healing caused by blood clots. They used the blood cells of necrotic teeth to regenerate tissue in the root canal but only formed approximately 2 mm of granulation tissue in the root tip. With the improvement of root canal disinfection and crown sealing technology, in 2001, Iwaya et al[4] placed antibiotics in the root canal for disinfection when treating a young permanent tooth patient with chronic periapical disease, stimulated apical bleeding and filled the root canal. Finally, the crown was tightly sealed with mineral trioxide aggregate (MTA). After 30 mo of follow-up, the root of the affected tooth continued to develop, the root canal wall thickened, the root tip closed, and an electrical activity test continued to show positive results[4]. An increasing number of clinicians have used this method and obtained similar results.
The biological mechanism of pulp revascularization is still unknown. The main process is thorough and effective root canal disinfection. Root canal disinfection and chemical irrigation are used to remove infectious materials in the root canal. Commonly used rinsing fluids include 1.5%-3% sodium hypochlorite solution and 17% EDTA solution[5]. After chemical preparation, the root canal is sealed with a triple antibacterial paste, which is generally composed of ciprofloxacin, metronidazole and minocycline[6,7]. During the treatment process, it is best to protect the residual dental pulp tissue, dental pulp stem cells and apical papillary stem cells. Studies have shown that stem cells that isolated from various problems of the oral cavity have emerged as important sources for bone and dental regulation, given stem cells plasticity, they can differentiate into specific cell lineages with a capacity of almost unlimited self-renewal and release of trophic/immunomodular factors[8-10]. These stem cells have different differentiation potentials induced by signalling molecules and the bioactive material MTA, which can form dental pulp and dentin and periodontal ligaments[11,12]. Subsequently, a regenerative scaffold based on blood clots is formed, and growth factors are provided. Some scholars have added platelet-rich plasma or platelet-rich fibrin[13]. The effect is good, but this approach involves blood product extraction and technical sensitivity. Its application prospects are still unknown. Finally, a tight crown seal is performed to provide a good environment for stem cell proliferation and differentiation, thereby promoting the continued development of tooth roots. Here we present a male adult patient with chronic periapical periodontitis with immature roots and open apices, and this tooth used pulp revascularisation to show a favourable result.
A 26-year-old man presented with an abscess for more than one month associated with a right mandibular posterior tooth.
A 26-year-old male presented for more than one month history of an abscess on the right mandibular posterior tooth. The patient did not receive any treatment for the affected tooth.
The patient had no history of any previous disease.
The patient had no personal and family history.
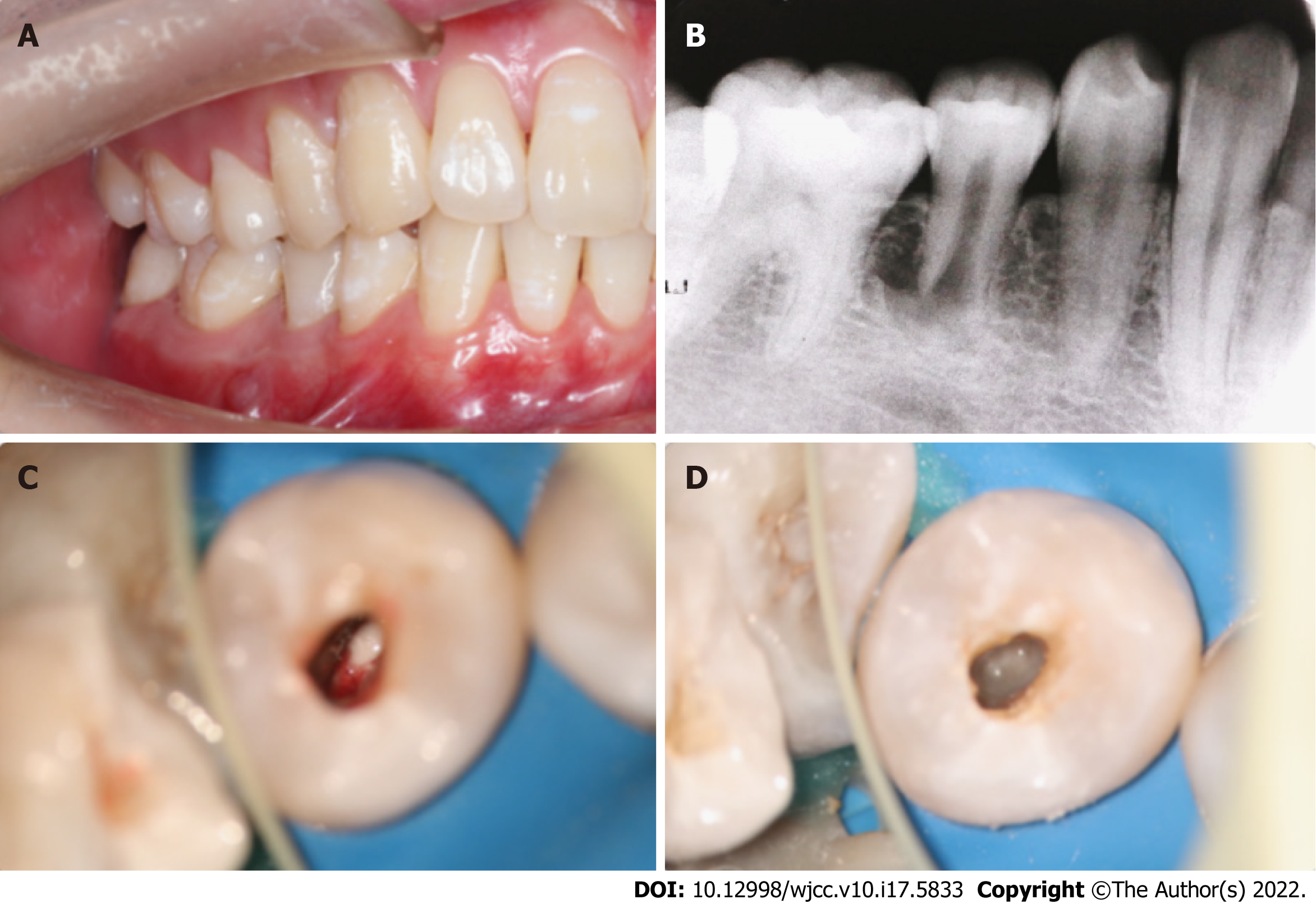
An extraoral examination revealed no abnormalities. There were no palpable lymph nodes in the head and neck. An intraoral examination showed that the abscess was localized buccally in the periapical region of the right mandibular second premolar (Figure 1A). Pressing the abscess showed yellowish white pus discharging. The occlusal surface of the affected tooth showed an abnormal central cusp worn away, a mild response to percussion/palpation, a periodontal pocket probing depth within normal limits (3-4 mm), and no pathologic tooth mobility. The tooth did not show any response to cold and hot pulp sensitivity tests.
A radiographic examination revealed the presence of a periapical lesion associated with an immature root with an apical (Figure 1B).
Based on the patient’s history and clinical and radiographic examinations, the right mandibular second premolar was diagnosed with chronic periapical periodontitis.
Treatment options, including pulp revascularization technology and apical barrier technology, were presented to the patient. The advantages and disadvantage of the two technologies were discussed with the patient. The patient was informed that the outcome of pulp revascularization for adult permanent teeth with persistent apical periodontitis was unknown. The patient opted for pulp revascularization. The timeline of this case is presented in Table 1.
| Timeline | Events | |
| September 30, 2018 | First treatment visit | Triple antibiotic paste was used to seal the root canal |
| October 15, 2018 | Second treatment visit | Penetrated the periapical tissue and provoked bleeding into the canal |
| October 16, 2018 | Third treatment visit | The access cavity was restored with light-curing composite resin |
| April 23, 2019 | 6-mo follow-up | The periapical lesion had slightly decreased in size |
| October 22, 2019 | 12-mo follow-up | The periapical lesion was smaller than before |
| October 14, 2020 | 24-mo follow-up | The periapical lesion showed further radiographic evidence of healing |
| October 23, 2021 | 36-mo follow-up | Indicating reparation of the periapical lesion |
Local anaesthesia with 2% lidocaine containing 1:100000 epinephrine was administered. The tooth was isolated with a rubber dam, and the pulp cavity was accessed with a carbide bur under a microscope. Pus could be seen in the root canal (Figure 1C). The root canal was rinsed with 3% sodium hypochlorite and 17% EDTA and dried with sterile paper points. Granulation tissue could be seen at the apical. The working length (WL) 0.5 mm short of the radiographic apex was determined with an electronic apex locator and periapical radiography (WL = 15 mm). Due to the large apical foramen, chemical preparation was emphasized, and mechanical preparation was assisted. Low-concentration (0.1-1.0 mg/mL) triple antibiotic paste was used to seal the root canal (Figure 1D), which was composed of ciprofloxacin, metronidazole and cefaclor (1:1:1). The access cavity was closed with a sterile cotton pellet and temporary restorative material.
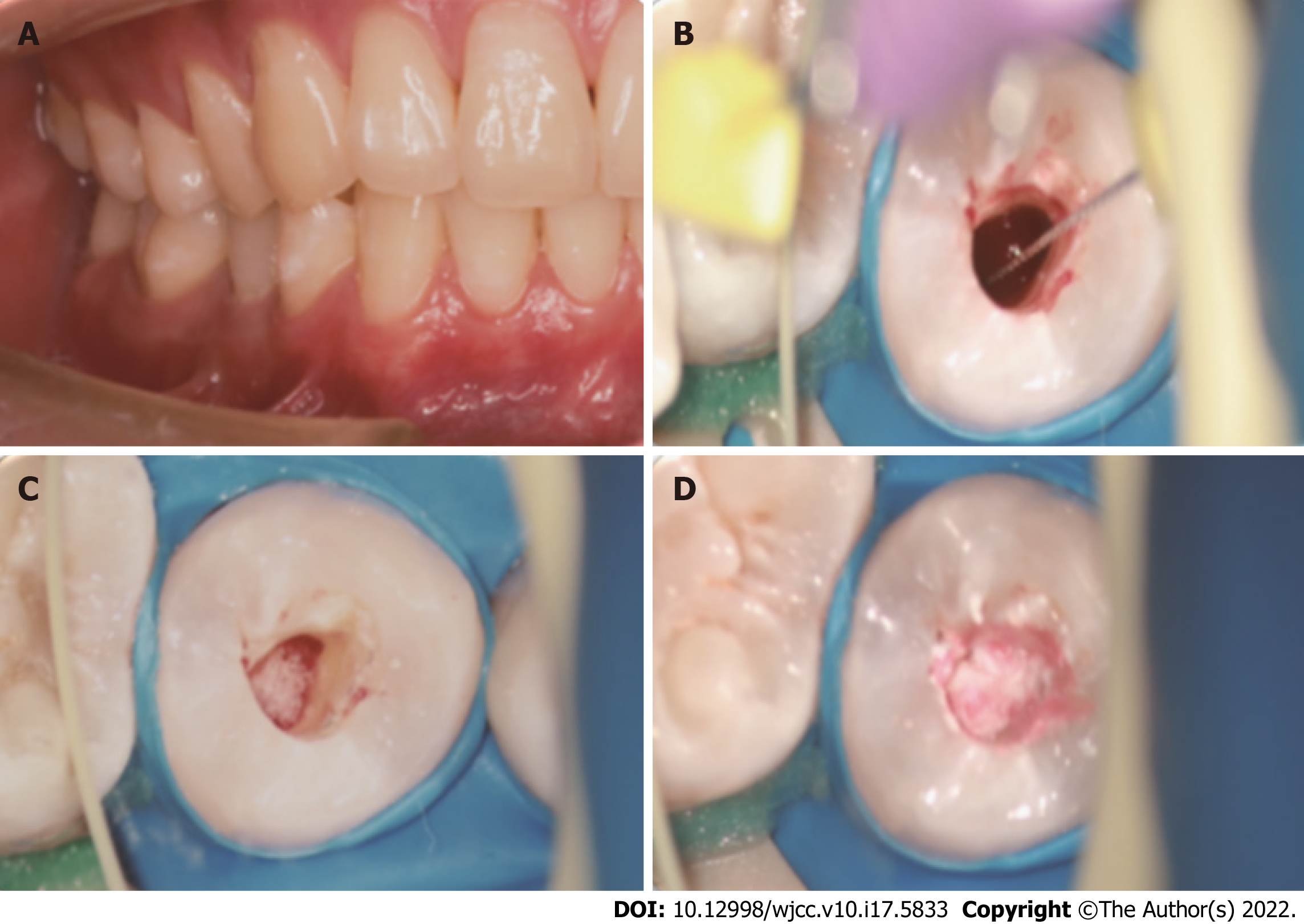
Two weeks after the first treatment visit, the localized sinus had subsided, and the tooth was asymptomatic (Figure 2A). Local anaesthesia with 2% mepivacaine without a vasoconstrictor was administered. The tooth was isolated with a rubber dam, and the seal was removed with a carbide bur under a microscope. Triple antibacterial paste in the canal was removed with copious amounts of 3% sodium hypochlorite and 17% EDTA irrigation and dried with paper points. A #10 K-file was used to penetrate the periapical tissue and provoke periapical bleeding into the canal, reaching the position of the enamel-cemental junction (Figure 2B). After the bleeding became semicoagulated, an absorbable gelatine sponge was placed as a stop point (Figure 2C), and then an iRoot BP of approximately 3-mm thickness was placed over the absorbable gelatine sponge (Figure 2D). A moist cotton pellet was placed over the iRoot BP, and the access cavity was closed with temporary restorative material.
One day after the second treatment visit, the temporary restorative material and cotton pellet were removed from the access cavity. It was determined that the iRoot BP had completely hardened. The access cavity was restored with light-curing composite resin.
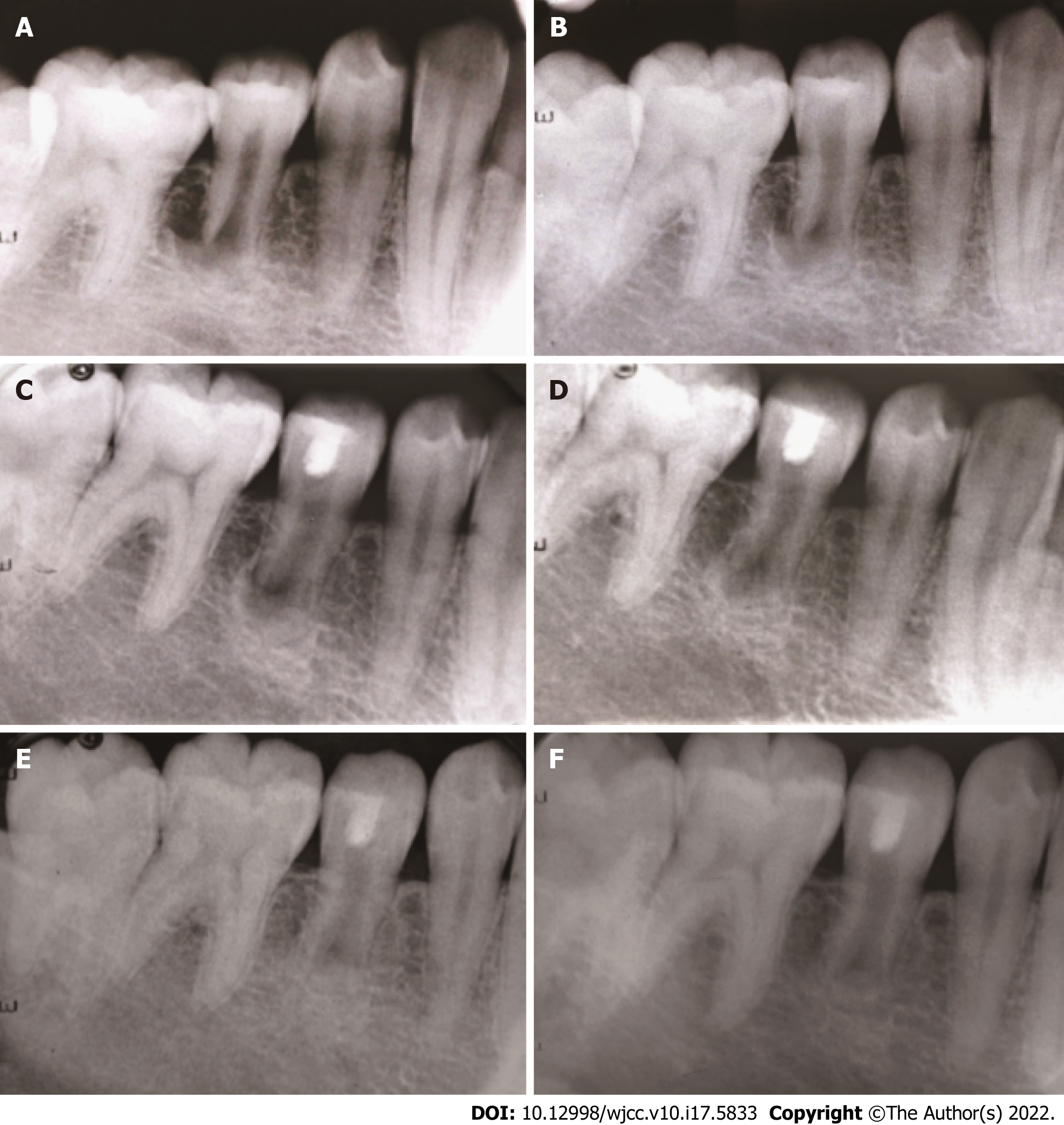
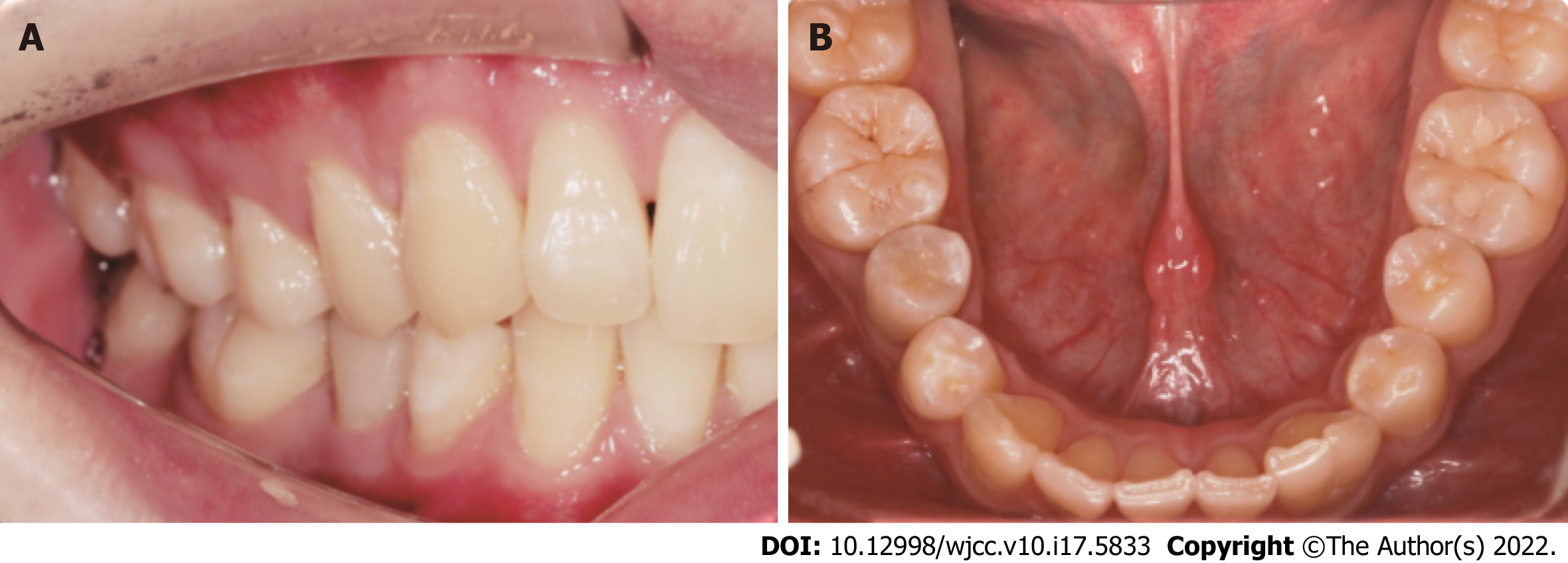
At the 6-mo follow-up, the periapical lesion had slightly decreased in size (Figure 3C). At the 12-mo follow-up, the periapical lesion was smaller than before (Figure 3D). At the 24-mo follow-up visit, the periapical lesion showed further radiographic evidence of healing, showing the improvement of pulp revascularization treatment (Figure 3E). The same was observed at the 36-mo follow-up visits, indicating reparation of the periapical lesion (Figure 3F). At the same time, the affected teeth did not discolouration (Figure 4A and B). However, the tooth of the canal walls did not thicken, and the apex also did not appear to have closed and did not respond to pulp tests with cold, heat, and electric pulp tests.
In the present case report, we have described the potential of using pulp revascularization to treat adult permanent teeth with apical periodontitis. In adult patients with chronic periapical periodontitis with immature roots and open apices, apical barrier technology is often used to treat these teeth. Apical barrier technology refers to the use of MTA to form an immediate artificial barrier in the apical area for sealing[14]. However, this technology has a high cost and difficult clinical manipulation. In addition, clinical treatment and follow-up visits found that apical barrier technology caused less root development and no thickening of the root wall or extension of the root length[15]. In this case, pulp revascularization with easy clinical manipulation was used, and the final effect on affected teeth was good. However, there is inadequate literature to support that pulp revascularization has a good effect on adult patients with immature roots and open apical teeth[15].
At present, there is no consistent standard for evaluating the efficacy of pulp revascularization. The curative effect has been mainly based on clinical manifestation, pulp vitality examination and radiographic examination. According to the American Association of Endodontists guidelines, the primary goals are healing apical periodontitis and eliminating clinical symptoms. Increased thickening of the canal walls and/or continued root development as well as a positive response to cold and hot pulp sensitivity tests are desirable but not essential to determine success[16]. In this case, the periapical lesions healed, but root development stopped and remained in a static state. The reason may be the lack and low activity of stem cells in adult permanent teeth, although stem cells cannot provide the best function. Therefore, the selection and implantation of endogenous or exogenous biological scaffolds may be a very important step in the treatment of adult permanent teeth. However, at present, there is no literature citing which kind of scaffold has a better curative effect on adult permanent teeth.
A common complication of revascularization is tooth discoloration. Previous studies have suggested that tooth discoloration is related to the triple antibiotic paste. Minocycline is considered to form a chelate with calcium ions in dentinal tubules, which changes the refractive index of teeth and causes tooth discoloration[17]. Cefaclor is an antibiotic alternative to minocycline. Thibodeau et al[18] and Dabbagh et al[19] proposed replacing minocycline with cefaclor and reported successful regenerative treatment using this technique. It has also been suggested that the possible mechanism of tooth discoloration may be related to the interaction between MTA and blood and the blockade of dentinal tubules[20]. In this case, there was no obvious discoloration of the affected teeth, which may be because cefaclor was used instead of minocycline and iRoot BP was used instead of MTA. Some studies have found that iRoot BP promotes increased alkaline phosphatase activity compared with MTA, and iRoot BP has better biocompatibility and repair performance and promotes the expression of factors related to odontogenic differentiation, so it has a higher biomineralization ability and induces dentin differentiation[21]. Until a large number of reliable clinical cases prove that cefaclor and iRoot BP improve the success rate, this approach can be used according to the principle of evidence-based medicine.
In this case, it was possible to observe that pulp revascularisation in immature roots with open apical teeth was clinically and radiographically relatively successful, as was evident in the 36 mo of follow-up, with repair of the periapical lesion. Such facts demonstrate that immature roots with open apical teeth, necrotic pulp and apical periodontitis can be treated using revascularization. It may be preferable to fill the root canals with the host's own vital tissue rather than with artificial material. However, randomized, prospective clinical trials are needed to demonstrate that the treatment outcome of pulp revascularisation is better.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Dentistry, oral surgery and medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Capparè P, Italy; Ghaffar KA, Egypt S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Saoud TM, Huang GT, Gibbs JL, Sigurdsson A, Lin LM. Management of Teeth with Persistent Apical Periodontitis after Root Canal Treatment Using Regenerative Endodontic Therapy. J Endod. 2015;41:1743-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Brogni JK, Vitali FC, Cardoso IV, Dos Santos JD, Prado M, Alves AMH, Duque TM. A second attempt at pulp revascularisation on an immature traumatised anterior tooth: a case report with two-year follow-up. Aust Endod J. 2021;47:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Holden T, Nygaard-Ostby B. [Treatment of pulp necrosis developing before the root is fully formed]. Nor Tannlaegeforen Tid. 1968;78:740-743. [PubMed] |
| 4. | Iwaya SI, Ikawa M, Kubota M. Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dent Traumatol. 2001;17:185-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 418] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 5. | Zeng Q, Nguyen S, Zhang H, Chebrolu HP, Alzebdeh D, Badi MA, Kim JR, Ling J, Yang M. Release of Growth Factors into Root Canal by Irrigations in Regenerative Endodontics. J Endod. 2016;42:1760-1766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | do Couto AM, Espaladori MC, Leite APP, Martins CC, de Aguiar MCF, Abreu LG. A Systematic Review of Pulp Revascularization Using a Triple Antibiotic Paste. Pediatr Dent. 2019;41:341-353. [PubMed] |
| 7. | Montero-Miralles P, Martín-González J, Alonso-Ezpeleta O, Jiménez-Sánchez MC, Velasco-Ortega E, Segura-Egea JJ. Effectiveness and clinical implications of the use of topical antibiotics in regenerative endodontic procedures: a review. Int Endod J. 2018;51:981-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Tetè G, Capparè P, Gherlone E. New Application of Osteogenic Differentiation from HiPS Stem Cells for Evaluating the Osteogenic Potential of Nanomaterials in Dentistry. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Capparè P, Tetè G, Sberna MT, Panina-Bordignon P. The Emerging Role of Stem Cells in Regenerative Dentistry. Curr Gene Ther. 2020;20:259-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 10. | Tetè G, D'Orto B, Nagni M, Agostinacchio M, Polizzi E, Agliardi E. Role of induced pluripotent stem cells (IPSCS) in bone tissue regeneration in dentistry: a narrative review. J Biol Regul Homeost Agents. 2020;34:1-10. [PubMed] |
| 11. | Kaukua N, Shahidi MK, Konstantinidou C, Dyachuk V, Kaucka M, Furlan A, An Z, Wang L, Hultman I, Ahrlund-Richter L, Blom H, Brismar H, Lopes NA, Pachnis V, Suter U, Clevers H, Thesleff I, Sharpe P, Ernfors P, Fried K, Adameyko I. Glial origin of mesenchymal stem cells in a tooth model system. Nature. 2014;513:551-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 322] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 12. | Chrepa V, Henry MA, Daniel BJ, Diogenes A. Delivery of Apical Mesenchymal Stem Cells into Root Canals of Mature Teeth. J Dent Res. 2015;94:1653-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 13. | Ulusoy AT, Turedi I, Cimen M, Cehreli ZC. Evaluation of Blood Clot, Platelet-rich Plasma, Platelet-rich Fibrin, and Platelet Pellet as Scaffolds in Regenerative Endodontic Treatment: A Prospective Randomized Trial. J Endod. 2019;45:560-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 14. | Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod. 1999;25:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 713] [Cited by in RCA: 660] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 15. | Kim SG, Malek M, Sigurdsson A, Lin LM, Kahler B. Regenerative endodontics: a comprehensive review. Int Endod J. 2018;51:1367-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 258] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 16. | AAE. Clinical Considerations for a Regenerative Procedure. 2016. |
| 17. | Akcay M, Arslan H, Yasa B, Kavrık F, Yasa E. Spectrophotometric analysis of crown discoloration induced by various antibiotic pastes used in revascularization. J Endod. 2014;40:845-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Thibodeau B, Trope M. Pulp revascularization of a necrotic infected immature permanent tooth: case report and review of the literature. Pediatr Dent. 2007;29:47-50. [PubMed] |
| 19. | Dabbagh B, Alvaro E, Vu DD, Rizkallah J, Schwartz S. Clinical complications in the revascularization of immature necrotic permanent teeth. Pediatr Dent. 2012;34:414-417. [PubMed] |
| 20. | Santos LGPD, Chisini LA, Springmann CG, Souza BDM, Pappen FG, Demarco FF, Felippe MCS, Felippe WT. Alternative to Avoid Tooth Discoloration after Regenerative Endodontic Procedure: A Systematic Review. Braz Dent J. 2018;29:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Lin J, Zeng Q, Wei X, Zhao W, Cui M, Gu J, Lu J, Yang M, Ling J. Regenerative Endodontics Versus Apexification in Immature Permanent Teeth with Apical Periodontitis: A Prospective Randomized Controlled Study. J Endod. 2017;43:1821-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 129] [Article Influence: 16.1] [Reference Citation Analysis (0)] |