Published online Jun 16, 2022. doi: 10.12998/wjcc.v10.i17.5764
Peer-review started: November 8, 2021
First decision: January 11, 2022
Revised: January 13, 2022
Accepted: April 21, 2022
Article in press: April 21, 2022
Published online: June 16, 2022
Processing time: 213 Days and 5.2 Hours
Multiple primary malignancies (MPMs) refer to more than one primary malignancy in the same or separate organs of the same patient, and MPMs are considered when different histological characteristics are detected in epidemiological studies. Herein, we report a case presumed to be primary pancreatic cancer with multiple liver metastases by positron-emission tomography/com
A 50-year-old man was referred to our hospital due to abdominal discomfort for 2 mo. Abdominal CT at a local hospital revealed a pancreatic mass with multiple liver nodules. After being transferred to our hospital, PET/CT confirmed all these lesions to have elevated metabolic activity, and therefore primary pancreatic cancer with multiple liver metastases was considered. EUS-guided liver aspiration unexpectedly found signet-ring cells with a high Ki-67 positive rate (20%), while EUS-guided pancreatic aspiration detected pancreatic neuroendocrine cells with a relatively low Ki-67 positive rate (1%). The final diagnosis from the multidisciplinary team was simultaneous liver and pancreatic MPMs. The patient returned to his local hospital for neoadjuvant chemotherapy and surgery, and he is still alive during the 6-mo postoperative follow-up.
Although rare, MPMs should be considered when treating pancreatic mass with suspected metastatic lesions, and EUS-FNA has proved minimally invasive and accurate.
Core Tip: We report a rare case of synchronous multiple primary liver and pancreatic malignancies confirmed by endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA), although this patient was first diagnosed as having primary pancreatic cancer with multiple liver metastases by computed tomography and positron-emission tomography/computed tomography. Although rare, multiple primary malignancies should be considered in patients with pancreatic mass and suspected metastatic lesions, and EUS-FNA has proven to be a minimally invasive and accurate preoperative diagnosis method.
- Citation: Yang J, Zeng Y, Zhang JW. Simultaneous multiple primary malignancies diagnosed by endoscopic ultrasound-guided fine-needle aspiration: A case report. World J Clin Cases 2022; 10(17): 5764-5769
- URL: https://www.wjgnet.com/2307-8960/full/v10/i17/5764.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i17.5764
Multiple primary malignancies (MPMs) refer to more than one primary malignancy in the same or separate organs of the same patient, and MPMs are considered when different histological characteristics are detected[1]. Simultaneous malignancies are defined as malignancies that are diagnosed at the same time or during the staging of the first malignancy, while synchronous and metachronous malignancies were usually distinguished by a 2-mo or 6-mo time point in different databases[2,3]. In patients with digestive system MPMs, it is infrequent for liver or pancreatic cancer patients to have both primary malignancies detected simultaneously[4]. Treatment strategies and associated prognoses of patients with digestive system MPMs are significantly different from those patients with primary digestive cancer and distant metastasis.
Preoperative endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) is an essential breakthrough in the endoscopic field and a substantial procedure to evaluate benign and malignant gastrointestinal tract lesions and nearby organs[5]. Taking pancreatic cancer as an example, the overall survival of the preoperative EUS-FNA group was significantly higher than that of the non-FNA group, and there was no remarkable difference in the tumor recurrence rate or peritoneal implantation rate between the two groups[6].
Herein, we report a case presumed to be primary pancreatic cancer with multiple liver metastases by positron-emission tomography/computed tomography (PET/CT) and confirmed to be synchronous liver and pancreatic MPMs by EUS-FNA.
A 50-year-old Chinese man was referred to our hospital due to abdominal discomfort for approximately 2 mo.
Approximately 1 mo previously, this patient was admitted to a local hospital due to elevated blood amylase. He denied jaundice, vomiting, and gastrointestinal bleeding. He was diagnosed with acute pancreatitis, but CT revealed an enlarged pancreatic head and suspected liver metastases. Thus, he was referred to our hospital for further management.
The patient had no remarkable medical history.
This patient had a 30-year smoking history (half a pack per day) and has not quit smoking. He denied any family history of cancer.
After admission, the patient’s physical examination revealed no abnormality.
Blood analysis revealed elevated CA19-9 [106 U/mL (0-27 U/mL)], amylase [450 U/L (0-110 U/L)], and lipase [3795 U/L (0-300 U/L)]. Alpha-fetoprotein was within normal limits.
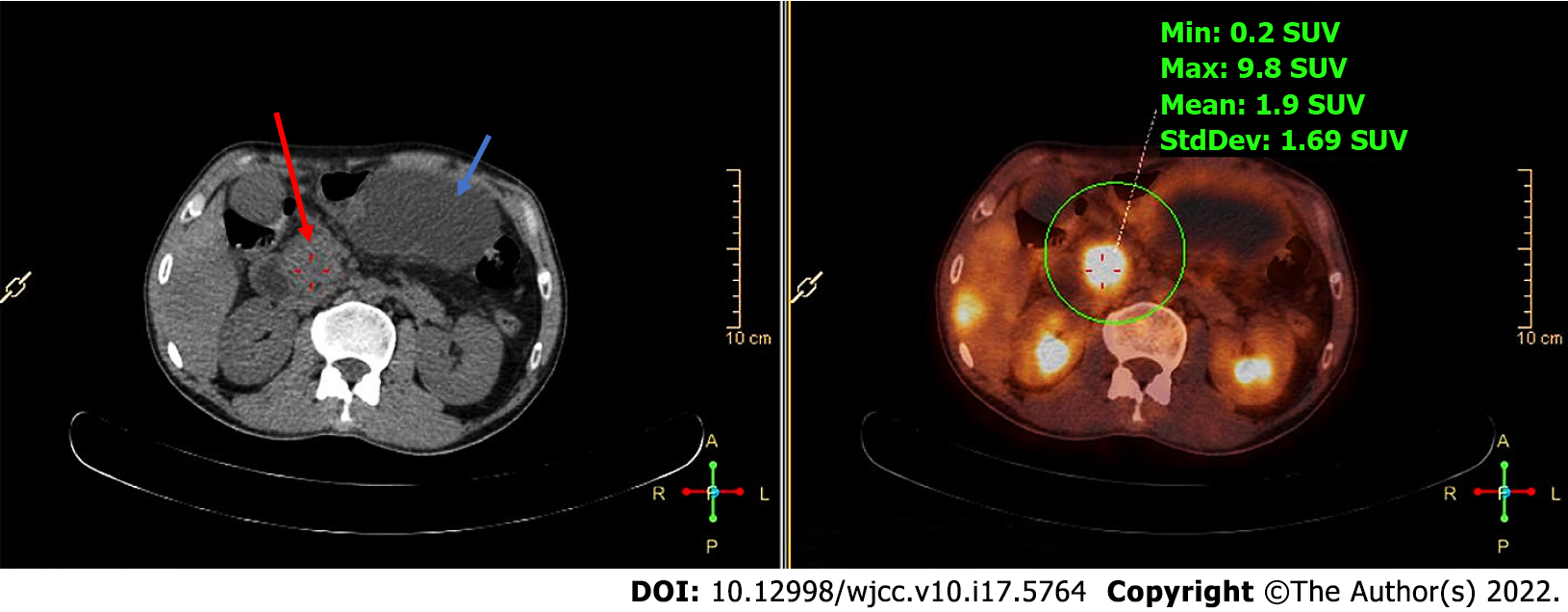
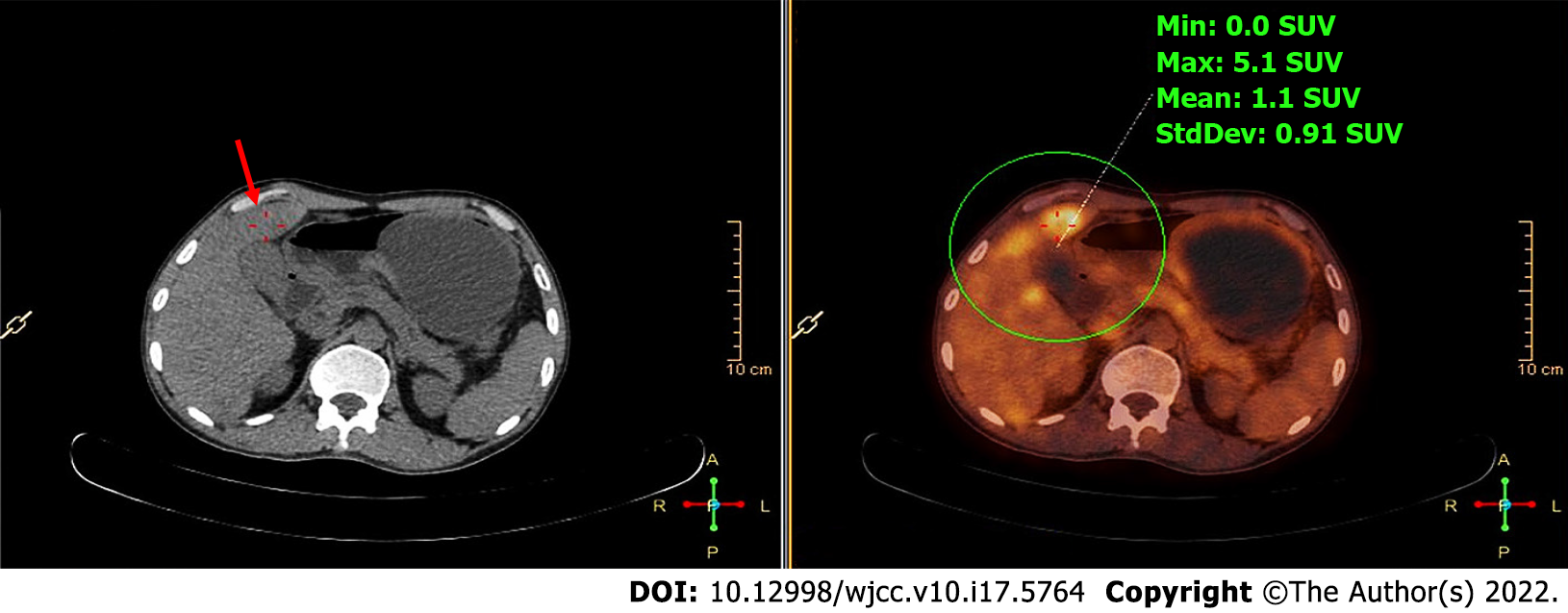
After admission, PET/CT detected increased soft tissues with elevated metabolic activity in the pancreatic head (Figure 1) and multiple liver nodules with increased metabolic activity (Figure 2), and therefore the initial diagnosis was primary pancreatic cancer with multiple liver metastases.
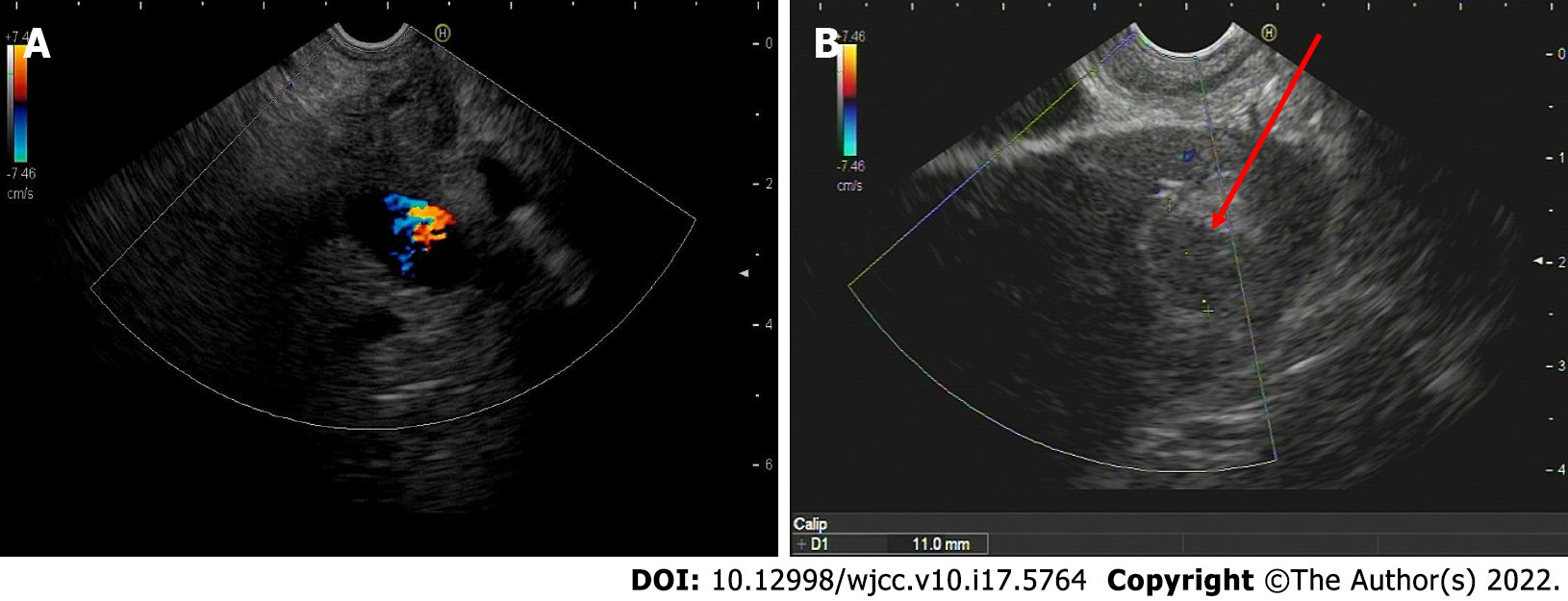
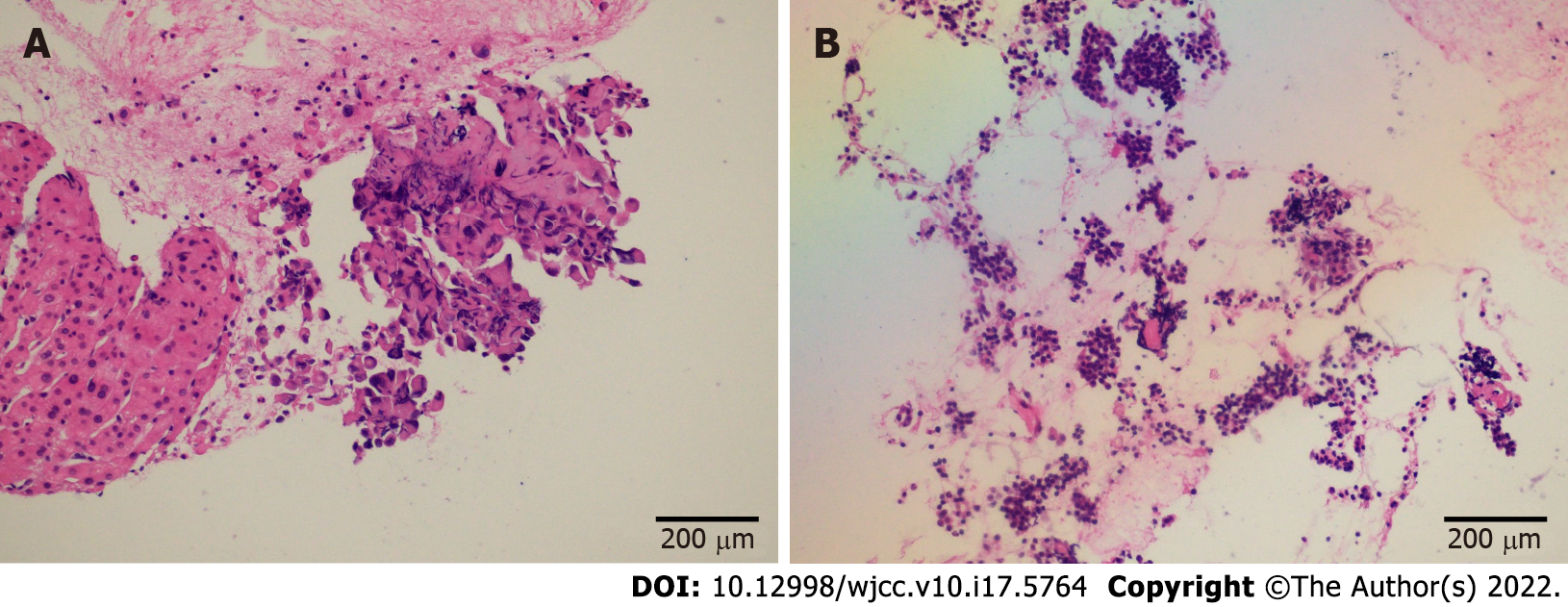
EUS confirmed an enlarged hypoechoic pancreatic head (Figure 3A) and multiple hypoechoic liver masses (Figure 3B). A linear Pentax echoendoscope (Hoya Co., Tokyo, Japan) and color Doppler flow imaging were employed to determine the puncture site. No malignant cells were detected in the fluid inside the peripancreatic cystic lesion extracted by EUS-FNA. EUS-FNA biopsy was performed with two 19-gauge needles (Boston Scientific Co., Natick, United States). EUS-guided liver aspiration unexpectedly revealed signet-ring cells (Figure 4A) with a high Ki-67 positive rate (20%), while EUS-guided pancreatic aspiration with another aspiration needle detected pancreatic neuroendocrine cells (Figure 4B) with a relatively low Ki-67 positive rate (1%). Three senior pathologists at our medical university confirmed that the considerable differences in immunohistochemical results indicated that the pancreatic mass and multiple liver nodules were not metastatic lesions from the other.
The gastric mucosa punctured by the EUS-guided liver aspiration needle was further inspected by magnifying endoscopy. No abnormal microsurface or microvessel was identified, and no malignant cells were found in deep excavation biopsies. The possibility of gastric signet-ring cell carcinoma was excluded. The patient’s colonoscopy was negative.
A multi-disciplinary team of pathologists, radiologists, and clinicians was convened. The final diagnoses were listed as follows: (1) Simultaneous liver and pancreatic MPMs (hepatic signet ring cell adenocarcinoma and pancreatic neuroendocrine tumor); and (2) Pancreatic pseudocyst.
The patient returned to his local hospital for neoadjuvant chemotherapy (apatinib, 500 mg, once per day) and left liver resection, and postoperative pathological results confirmed the diagnoses of hepatic signet ring cell adenocarcinoma, pancreatic neuroendocrine tumor, and post-necrotic pancreatic pseudocyst.
The patient is still alive at the 6-mo postoperative follow-up.
To the best of our knowledge, this is the first report of simultaneous liver and pancreatic MPMs preoperatively diagnosed by EUS-FNA in Asian patients. This case will help prompt clinicians to consider other possibilities besides primary pancreatic cancer with liver metastasis when dealing with similar issues and raise their attention to routinely perform preoperative EUS-FNA in patients with presumed malignancies.
With the continuous progress of medical techniques and the extension of life expectancy, the incidence of MPMs has increased gradually. Most patients with MPMs were male and elderly patients (> 50 years old), and the leading location in all MPMTs was the digestive system[7]. Compared with all other MPMs, liver malignancies revealed the fewest MPMs occurrences[8], and it is rarer to confirm both liver and pancreatic malignancies by EUS-FNA simultaneously. Lai et al[9] reported a 56-year-old Caucasian woman with a pancreatic mass and a single liver nodule. Her EUS-FNA cytology revealed pancreatic ductal adenocarcinoma, while she underwent a liver core biopsy and was confirmed to have hepatocellular carcinoma. The absence of EUS-FNA for liver biopsy may be due to the location of her liver lesion. In addition, Zhang et al[10] reported a 70-year-old man with pathologically confirmed pancreatic metastasis of hepatocellular carcinoma. Therefore, in patients with pancreatic masses and multiple liver nodules, clinicians should consider the following three possibilities: Primary pancreatic cancer with liver metastasis, primary liver cancer with pancreatic metastasis, and MPMs.
Correct diagnosis is the first and essential step in treating patients with malignancies. PET/CT, one of the sophisticated imaging methods that have been increasingly used in recent years, is playing a considerable role in the diagnosis of MPMs[11]. Compared with the inability to obtain specimens from PET/CT, the accuracy and safety advantages have been proven in the process of securing cell and tissue specimens via EUS-FNA[12-14]. EUS-FNA and associated procedures are expected to play an increasingly important role in the preoperative diagnosis of MPMs and other suspected malignancies.
This male patient in the present case had a smoking history of up to 30 years. Males and patients with a smoking history were considered to have a higher risk of developing multiple MPMs[1,15]. Therefore, these patients need to be consciously inspected for the possibility of MPMs. Liver lesions are more often considered metastases than primary tumors[16], and thus the presumed diagnosis of this patient was primary pancreatic cancer with multiple liver metastases. However, the pathological results of EUS-guided liver and pancreatic aspiration unexpectedly confirmed a rare case of MPMs. This case also reminds us that a pathological biopsy should always be the final and definite diagnosis in patients with suspected malignancies[17].
A rare case of simultaneous liver and pancreatic MPMs has been confirmed by pathological biopsies of EUS-guided liver and pancreatic aspiration. MPMs should be considered in patients with pancreatic mass and suspected metastatic lesions, and EUS-FNA is a minimally invasive and accurate diagnostic method.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Chinese Society of Gastroenterology.
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Corvino A, Kalayarasan R S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Gao CC
| 1. | Vogt A, Schmid S, Heinimann K, Frick H, Herrmann C, Cerny T, Omlin A. Multiple primary tumours: challenges and approaches, a review. ESMO Open. 2017;2:e000172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 367] [Article Influence: 45.9] [Reference Citation Analysis (1)] |
| 2. | Friedrich RE. Primary and second primary cancer in 649 patients with malignancies of the maxillofacial region. Anticancer Res. 2007;27:1805-1818. [PubMed] |
| 3. | Xiong J, Su Y, Bing Z, Zhao B. Survival between synchronous and non-synchronous multiple primary cutaneous melanomas-a SEER database analysis. PeerJ. 2020;8:e8316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Ikubo A, Matsufuji S, Morifuji Y, Koga H, Kobarai T, Kouya N, Sakai M, Samejima R, Kojima K, Tabuchi M, Yunotani S. Clinical Features, Prognosis, Diagnostic Approaches and Treatment of Multiple Primary Malignancies in the Digestive System. Anticancer Res. 2019;39:6863-6870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Cazacu IM, Luzuriaga Chavez AA, Saftoiu A, Vilmann P, Bhutani MS. A quarter century of EUS-FNA: Progress, milestones, and future directions. Endosc Ultrasound. 2018;7:141-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 6. | Alghamdi A, Palmieri V, Alotaibi N, Martel M, Barkun AN, Zogopoulos G, Chaudhury P, Chen YI. Sa1468 Preoperative EUS-guided FNA is associated with better overall survival in resectable pancreatic cancer when compared to upfront surgery without preoperative tissue acquisition: a systematic review and meta-analysis. Gastrointestinal Endoscopy. 2020;91:AB204. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Lv M, Zhang X, Shen Y, Wang F, Yang J, Wang B, Chen Z, Li P, Li S. Clinical analysis and prognosis of synchronous and metachronous multiple primary malignant tumors. Medicine (Baltimore). 2017;96:e6799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 8. | Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist. 2007;12:20-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 680] [Cited by in RCA: 728] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 9. | Lai JZ, Zhou Y, Cao D. Synchronous Pancreatic Ductal Adenocarcinoma and Hepatocellular Carcinoma: Report of a Case and Review of the Literature. Anticancer Res. 2018;38:3009-3012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Zhang Y, Han T, Wang D, Li G, Zhang Y, Yang X, Chen T, Zheng Z. Hepatocellular carcinoma with pancreatic mass as the first symptom: a case report and literature review. Ann Palliat Med. 2019;8:740-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Pang L, Liu G, Shi H, Hu P, Li B, Cheng D. Nineteen cases with synchronous multiple primary cancers studied by 18F-FDG PET/CT. Hell J Nucl Med. 2017;20:36-40. [PubMed] |
| 12. | Yoshinaga S, Itoi T, Yamao K, Yasuda I, Irisawa A, Imaoka H, Tsuchiya T, Doi S, Yamabe A, Murakami Y, Ishikawa H, Saito Y. Safety and efficacy of endoscopic ultrasound-guided fine needle aspiration for pancreatic masses: A prospective multicenter study. Dig Endosc. 2020;32:114-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Ichim VA, Chira RI, Mircea PA, Nagy GA, Crisan D, Socaciu MA. Accuracy of endoscopic ultrasound-guided biopsy of focal liver lesions. Med Ultrason. 2020;22:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Matsumoto K, Takeda Y, Onoyama T, Kawata S, Kurumi H, Koda H, Yamashita T, Isomoto H. Endoscopic ultrasound-guided fine-needle aspiration biopsy - Recent topics and technical tips. World J Clin Cases. 2019;7:1775-1783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Amer MH. Multiple neoplasms, single primaries, and patient survival. Cancer Manag Res. 2014;6:119-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 16. | Corvino A, Corvino F, Radice L, Catalano O. Synchronous mucinous colonic adenocarcinoma and multiple small intestinal adenocarcinomas: report of a case and review of literature. Clin Imaging. 2015;39:538-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Matsubayashi H, Sasaki K, Ono S, Abe M, Ishiwatari H, Fukutomi A, Uesaka K, Ono H. Pathological and Molecular Aspects to Improve Endoscopic Ultrasonography-Guided Fine-Needle Aspiration From Solid Pancreatic Lesions. Pancreas. 2018;47:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |