Published online Jun 16, 2022. doi: 10.12998/wjcc.v10.i17.5748
Peer-review started: October 30, 2021
First decision: March 3, 2022
Revised: March 14, 2022
Accepted: April 4, 2022
Article in press: April 4, 2022
Published online: June 16, 2022
Processing time: 221 Days and 17.3 Hours
Chondromyxoid fibroma (CMF) is an unusual benign tumour of cartilaginous tissues that may be confused with other malignant tumours. It is rarely seen in the cervical spine.
A 24-year-old young woman was admitted to the hospital because of neck and shoulder pain. Computed tomography, magnetic resonance imaging, X-ray and other imaging examinations of the cervical spine and laboratory-related indicators combined with intraoperative pathology revealed that the patient had cervical CMF. We performed total resection of the vertebral body and intervertebral disc, and internal fixation was performed to simultaneously maintain the stability of the entire spine. The clinical results from extensive resection were satisfactory. At the 2-year follow-up, the patient's symptoms had not recurred.
CMF is a benign primary bone tumour that is rarely located in the vertebral bone. Accurate initial diagnosis of these tumours is important for appropriate treatment. En bloc surgical resection of the tumour is the cornerstone of treatment.
Core Tip: The case was diagnosed and operated independently by our hospital, and the patient had a good prognosis. Because of the rarity of the case itself and the rare location of tumor growth and infiltration in the case, we not only introduced the case itself in detail, but also made a retrospective study of a series of related literatures.
- Citation: Li C, Li S, Hu W. Chondromyxoid fibroma of the cervical spine: A case report. World J Clin Cases 2022; 10(17): 5748-5755
- URL: https://www.wjgnet.com/2307-8960/full/v10/i17/5748.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i17.5748
Chondromyxoid fibroma (CMF) is a rare benign tumour of cartilaginous origin. It accounts for less than 0.5% of bone tumours and less than 2% of benign bone tumours[1]. CMF was first reported in 1948 by Jaffe and Lichtenstien[2], and it was defined by the World Health Organization as "a benign tumour characterized by lobules of spindle-shaped or stellate cells with abundant myxoid or chondroid intercellular material separated by zones of more cellular tissue rich in spindle-shaped or round cells with varying number of multinucleated giant cells of different sizes”. The morbidity rate is higher in male patients than female patients, and the male-to-female ratio is approximately 1.28:1[3]. Karyotype analysis of CMF tumour cells has demonstrated that all the cells contain clonal rearrangements of chromosome 6 and 6q13, which are not related to other types of bone and soft tissue tumours. Inv (6) (p25q13) was seen only in CMF. The persistent occurrence of 6q13 rearrangements suggests a specific oncogenic mechanism in CMFs that most likely involves the activation of oncogenes. CMF of the cervical spine is rare, but cases have been reported in various parts of the human body[4]. One case of CMF of the C7 vertebral body has been treated surgically. The operative procedure was successful, and the postoperative follow-up was satisfactory.
A 24-year-old young woman was admitted to the hospital because of "neck and shoulder pain for more than one month, aggravated with activity limitation for one day".
One month earlier, neck and shoulder pain began without obvious inducement, alternating on both sides, which was aggravated after fatigue and slightly relieved after rest. There was no sign of radiation pain, numbness or weakness of the upper limbs. One day before admission, the distending pain in the neck and shoulder was aggravated again, accompanied by limitation of neck movement.
The patient had been healthy in the past.
The patient, who had a daughter, with no family history of heredity.
Physical examination showed tenderness and percussion pain of the cervical spinous process. No radiating nerve pain was detected, and other related pathological signs were negative.
After admission, no obvious abnormalities were found in relevant laboratory examinations, female breast colour Doppler ultrasound, genitourinary system colour Doppler ultrasound or lung computed tomography (CT). A Tuberculosis(TB) test was also negative.
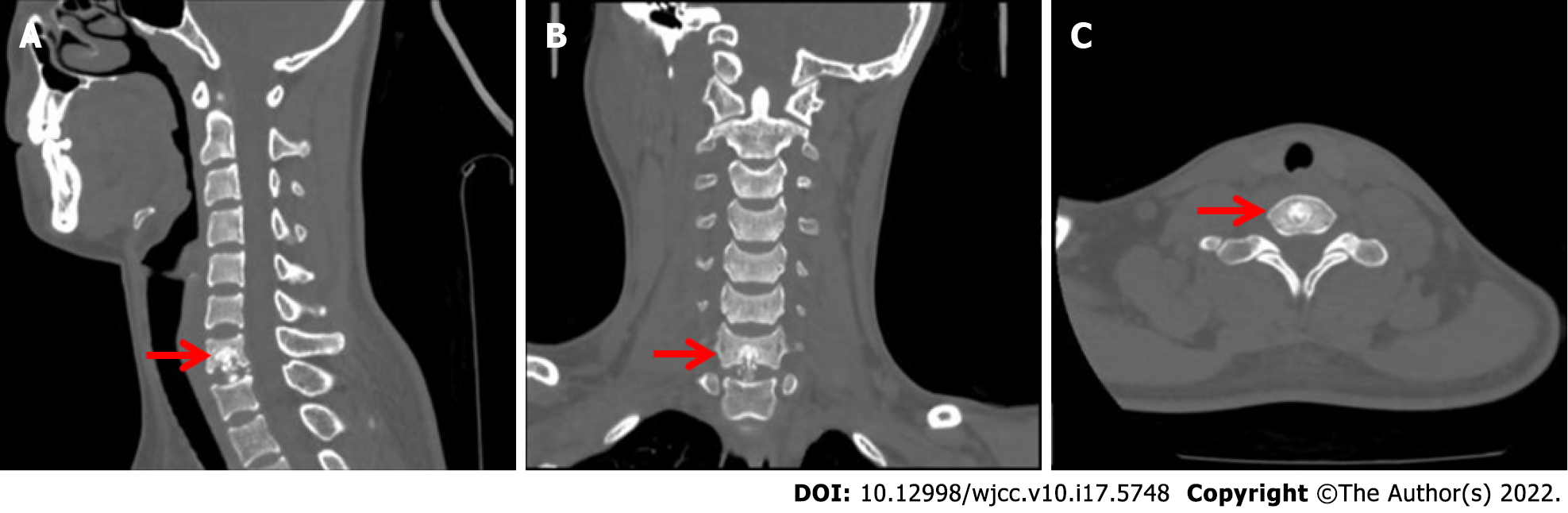
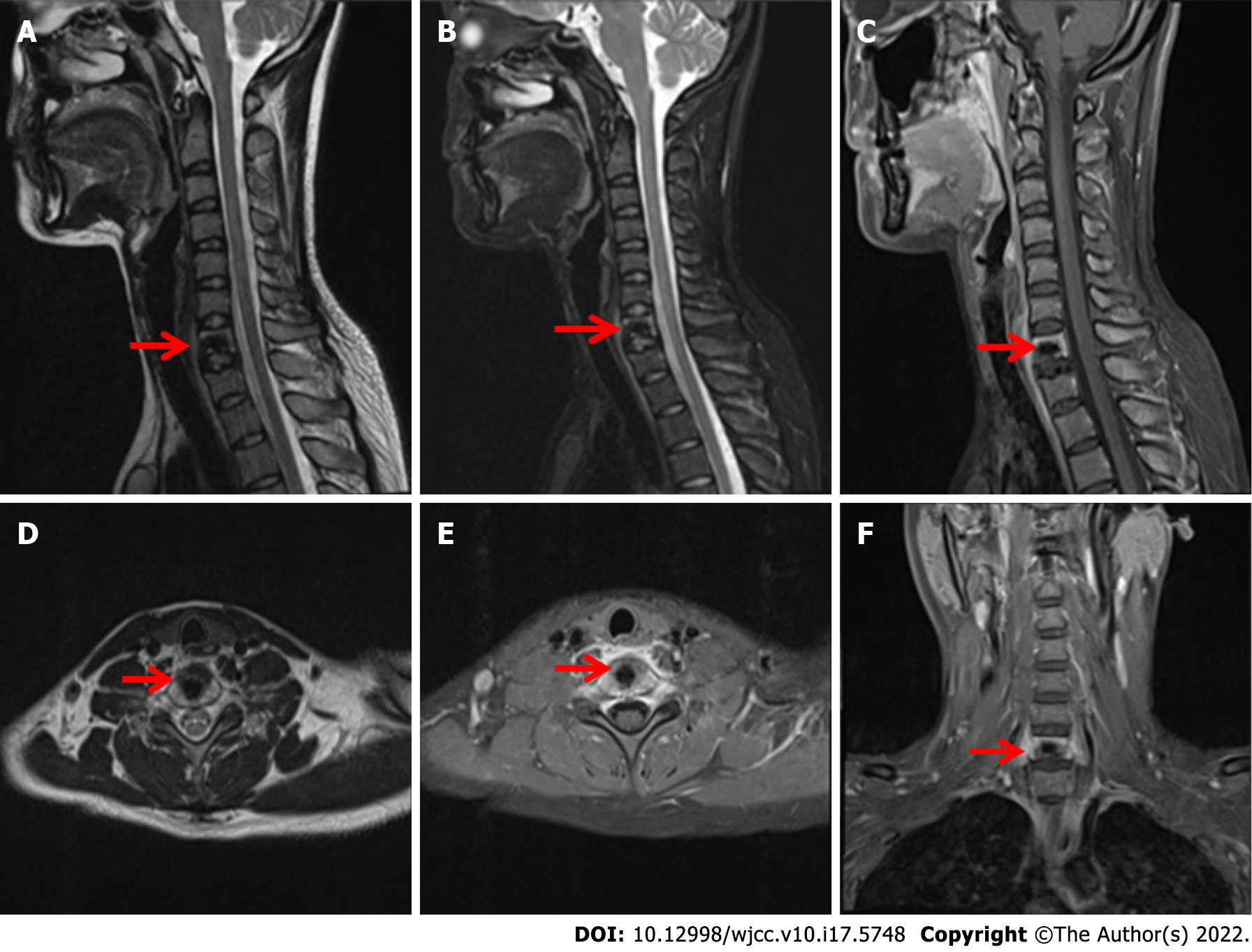
Cervical CT (Figure 1) showed that the bone structure of each cervical vertebral body was complete. The C7 vertebral body and C7/T1 intervertebral disc showed irregular high-density shadows with clear boundaries, and other bone density shadows showed no obvious abnormalities. Magnetic resonance imaging (MRI) (Figure 2) of the cervical vertebrae showed patchy abnormal signals at C7, low signals on T1-weighted images (T1WI), high and low mixed signals in T2WI and fat suppression images, swelling of adjacent soft tissue and inhomogeneous enhancement after enhancement. No abnormal signal was found in the C3/4-C6/7 intervertebral discs. The signal of the cervical spinal cord was normal.
The patient was diagnosed with cervical CMF.
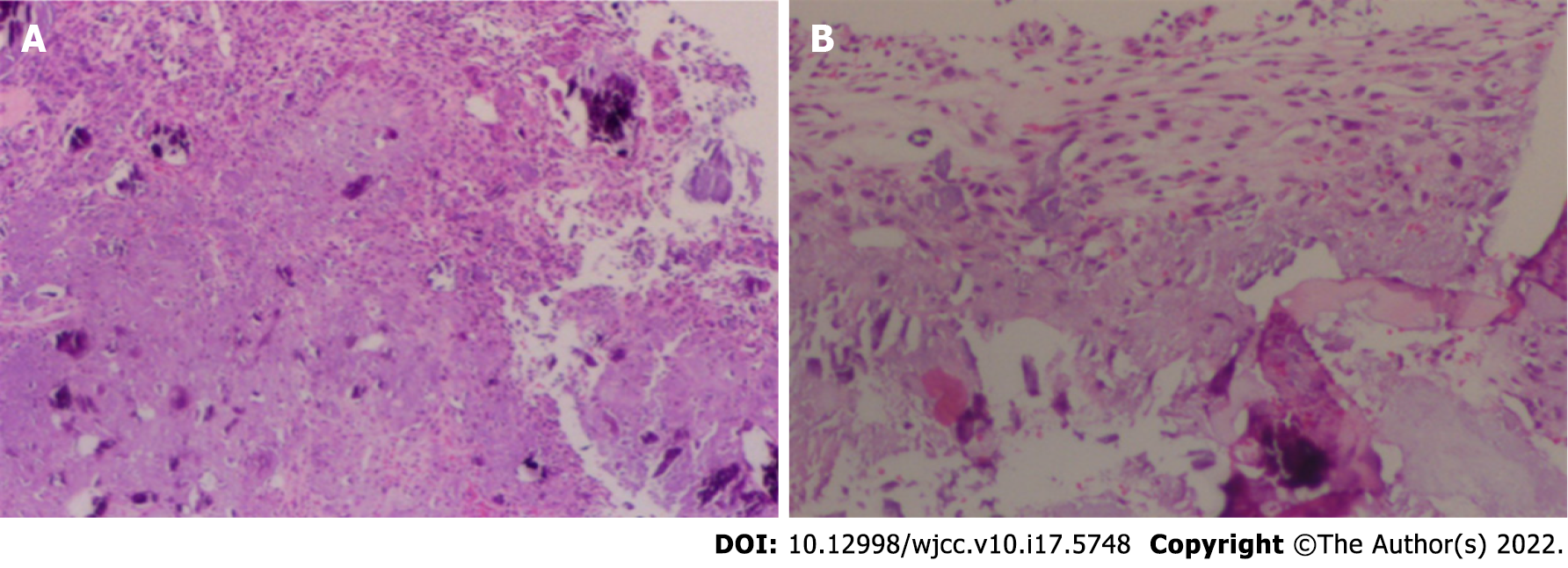
Under general anaesthesia with the patient in the supine position, We performed an anterior cervical surgery on the patient. C-arm fluoroscopy was used to locate the intervertebral space with a locating needle, and a distractor was placed in the centre of the front of the C6/7 and C7/T1 vertebral bodies to expand them. And subtotal resection of the C7 vertebral body was performed with a bone rongeur. A large amount of white chyle focus tissue in the vertebral body had eroded most of the bone in the vertebral body, and specimens were obtained for pathological examination (Figure 3). The cervical canal and nerve root canal were decompressed, and the adhesion of the spinal cord and bilateral nerve roots was released. The length from the lower edge of the C6 vertebral body to the upper edge of the T1 vertebral body was measured. A titanium cage of appropriate length was selected, and autogenous iliac bone was implanted into the titanium cage between C6 and T1. A hole was drilled in front of the C6 and T1 vertebral bodies, and titanium plate screws were installed. Before the end of the operation, C-arm fluoroscopy showed that the positions of the titanium plate and screw remained acceptable.
After the operation, the patient's symptoms were significantly alleviated, and the patient could walk independently. The neck was fixed with a common neck brace for 6 wk. At the 2-year follow-up, the patient's symptoms had not recurred.
Retrospective study of this case revealed no obvious abnormalities in the body from the preoperative screening of patients, with the exception of the cervical spine. Laboratory tests showed that relevant tumour markers and inflammatory markers were not significantly increased. The diagnosis was confirmed according to the comprehensive judgement of imaging examinations and intraoperative pathology. The diagnosis of CMF is difficult because of its overlapping features with other bony lesions. Pathology reveals that CMF has mucinous and fibrous components[5]. The tumour cells were primarily arranged in lobules characterised by sparse cells in the centre, dense cells in the periphery, and more cells (primarily spindle cells, multinucleated giant cells and chondroblasts) between the lobules. Tumour cells were arranged in a fusiform or stellate pattern in a myxoid stroma. Calcification occurred in more than one-third of these cases but rarely became prominent. Hyaline cartilage and chondroblastoma-like areas were not uncommon. Approximately 18% of the tumours showed odd nuclei, and trabecular perforations were rare. Immunohistochemically (IHC), the tumour was positive for S100, which suggests that it was a chondroid tumour. Diseases that may be differentiated from CMF include low grade chondrosarcoma, enchondroma, chondromyxoid fifibroma-like osteosarcoma, chondroblastoma, and giant cell tumor of bone[6].
The differential diagnosis between CMF and chondrosarcoma is crucial. CMF also presents as a low-grade malignancy with histological nuclear atypia similar to chondrosarcoma[7]. The treatment and prognosis of CMF and chondrosarcoma vary greatly. Approximately 22% of CMFs are misdiagnosed as chondrosarcoma, which is a common malignant tumour in many bone tumour diseases. Chon
Another disease that can be confused with CMF is osteosarcoma. There are rare low-grade osteosarcomas. Chondromyxoid fibromatoid osteosarcoma (CMF-OS) was first reported in 1989, which presented with the histological and biological behaviours of low malignancy. Microscopically, it had a CMF-like appearance that consisted of loosely packed cellular gates in a highly mucoid stroma background and lobulated by fibrovessels. The most salient feature in differentiating CMF from CMF-OS should be careful study of whether the bone is neoplastic, especially in lesions with malignant radiological features. IHC staining of vimentin-positive and S100-negative cells might be helpful in distinguishing CMF from CMF-OS. Zhong et al[8] summarised the differences between CMF-OS and CMF using age of morbidity, tumour location, imaging examination, medical history, IHC and genetic characteristics from a series of case reviews. Finally, based on the above differences, combined with the imaging and pathological features of our case, we concluded that the nature of the patient's tumor was cervical CMF.
Controversy about the imaging characteristics of CMF also exist. However, the literature suggests a multidisciplinary approach for distinguishing CMF from chondrosarcoma and concludes that CMF has some characteristic radiological characteristics in typical sites[9]. Tumours showing oval osteolytic lesions generally occur in the long or flat bones of young people. CMFs are isointense on T1WI and have moderate-to-high signal intensity or contrast peripheral enhancement on T2STIR/T2FS. Traditional "bite" features, low signal intensity margins on all MRI sequences, and the absence of or minimal bone marrow or soft tissue oedema might also be useful features.
CMF is generally a slow-growing asymptomatic lesion. Most CMFs are located in the metaphyseal region of the long bones, approximately one-third of which formed in the tibia, foot tubular bones, distal femur, and pelvis. Although CMF has a predilection site, most of the reported cases have been atypical. Therefore, we cannot avoid CMF in the differential diagnosis of bone tumours in various parts of the human body. The clinical manifestations vary according to the size, location and extent of the lesion. The common symptoms of CMF in cervical spine are neck and shoulder pain, limitation of movement, numbness and radiating pain of upper limbs after nerve root damage. Although CMF is a relatively rare benign tumour, vertebral CMF only accounts for 8% of all CMFs, and cervical CMF is even rarer, with fewer than 12 cases reported[10]. The features of vertebral CMF include the erosion of cortical bone, even beyond the extent of the periosteum into the surrounding soft tissue or spinal canal, and this feature indicates more serious destruction, which may indicate the malignant transformation of tumours. All cervical CMFs reported in the English literature over a 30-year period from 1991 to 2021 (Table 1) were retrieved and are summarized below.
| Ref. | Age and sex | Involved site | Clinical symptoms | Mode of treatment | Results and follow-up |
| Rivierez et al[11], 1991 | 41/F | Complex of part C5 vertebral body and posterior longitudinal ligament | Torticollis, upper limb pain | Stage 2 operation | No recurrence was found in 10 months of follow-up |
| Lopez et al[12], 2002 | 20/M | C2 vertebral body and transverse foramen | Intermittent pain in the neck after a fall; tenderness in the back muscles of the neck; limited neck rotation and lateral bending | Transoropharyngeal approach, C2 vertebra resection, fusion of the occipital to C4 vertebrae | Relieved pain and instability and had no recurrence within two years |
| Bala et al[13], 2006 | 36/M | C2 vertebral body with right posterior longitudinal ligament complex | Occasional, chronic neck pain | Under CT guidance, the tumour of the C2 vertebra was resected through a transoropharyngeal approach, and the right iliac bone was harvested and implanted | At 18 months of follow-up, the patient was pain-free. Imaging revealed a residual tumour volume surrounding the graft and the right vertebral artery |
| Jonatha et al[14], 2008 | 35/M | C7 vertebral body and left pedicle | Neck pain with limited movement; numbness and pain in the ulnar side of the left upper limb | C7 vertebra resection and autogenous iliac bone implantation | At eight years of follow-up, the patient had no neurological symptoms. Plain films and CT scans showed no progression of the tumour |
| Subach et al[15], 2010 | 27/F | C6 lamina and right pedicle, extending to the foraminal location | There was paraesthesia, pain, numbness in the right neck and radiation to the right upper limb. It has worsened over the past six months | The C6 lamina and the right pedicle were completely resected, and posterolateral C5-C7 fusion and posterior intersegmental fixation were performed | The numbness and tenderness of the right upper extremity had subsided by 9 months postoperatively; a solid bony fusion showed no evidence of tumour recurrence |
| Taghipour et al[10], 2015 | 36/F | Encapsulated mass involving the soft tissue of the posterior margin of C3 and C4 spinous processes and partially invading the bone of the C5 spinous process | Neck pain with radiating pain in the right upper extremity for 1 yr | Surgical treatment (details unknown) | Follow-up for 2 yr, no recurrence |
| Our case | 24/F | Involvement of the C7 vertebral body and C7/T1 intervertebral disc | Swelling and pain in the neck and shoulder with limitation of movement | Total C7 and C7/T1 discectomy with autogenous iliac bone graft | Follow-up for 14 months showed no recurrence. |
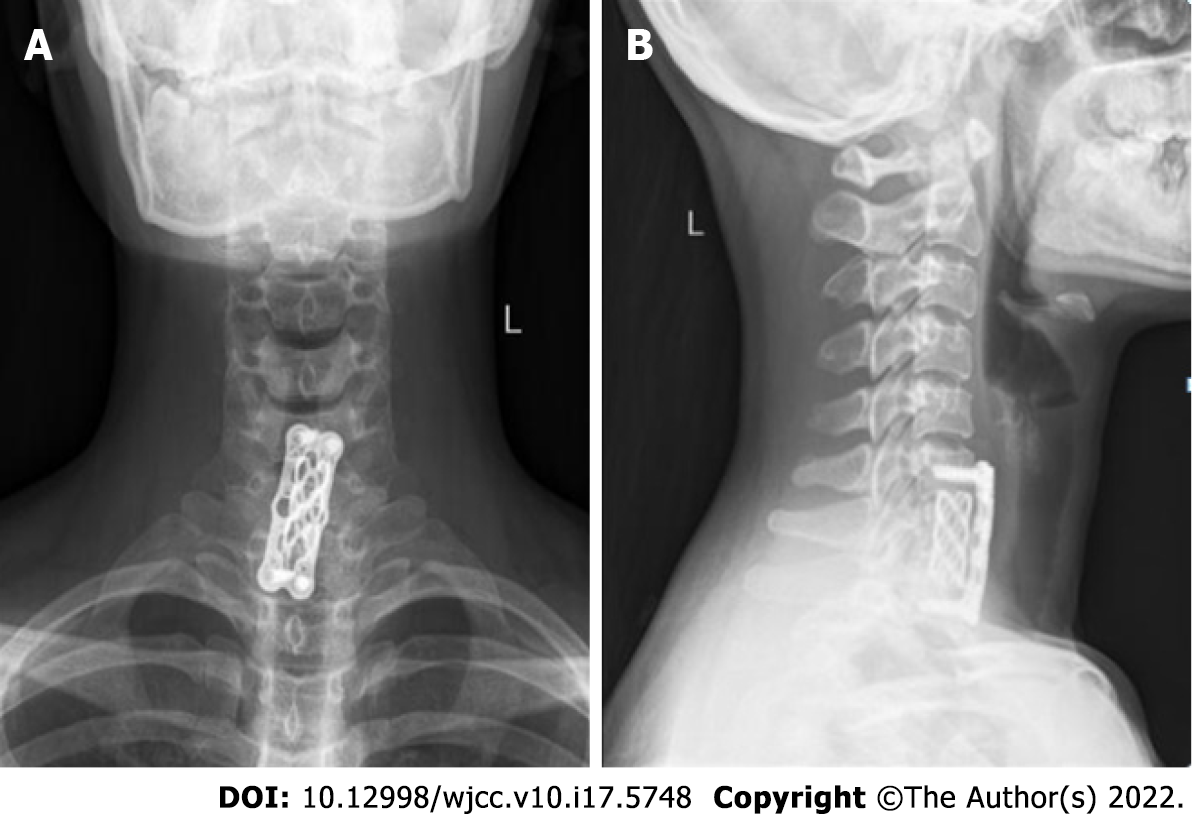
CMF was reported in upper and lower cervical vertebrae, and the tumour invades all parts of the vertebral body, transverse foramen and posterior soft tissue. The tumour had only caused obvious disc erosion in our case. We resected all of the tumours of the vertebral body and intervertebral disc via surgery and performed iliac bone implantation and anterior fusion. The occipital bone and atlas maintain 50% of the flexion and extension range of motion of the cervical spine, and the atlas and axis maintain 50% of the rotation range of motion of the vertebral body. The age of the patient was fully considered in the preoperative strategy. Anterior fusion of the two segments of the lower cervical spine was performed. Therefore, it would not have a significant impact on the patient's quality of life in the future. The internal fixation position of the patient was good, and no discomfort, such as neck movement or dysphagia, was shown (Figure 4).
An epidemiological survey of primary spinal tumours in Chinese patients showed that the proportion of benign tumours is larger than that of malignant tumours, and the proportion of male patients is larger than that of female patients. Benign tumours are more likely to occur at the age of 21 to 40 years old, and malignant tumours are more likely to occur at the age of 41 to 60 years old. The distribution of primary spinal tumours in the cervical, thoracic, lumbar and sacral segments is roughly the same, with most benign tumours occurring in cervical vertebrae and most malignant tumours appearing in sacral vertebrae. The age of morbidity and the location of the tumour in our case were consistent with the characteristics of primary spinal tumours in Chinese patients. Simple curettage, without filling the defect, leads to approximately 20% to 80% tumour recurrence[16]. This recurrence might result from incomplete tumour excision and leave pseudopod processes extending from the main tumour to the cavernous body (sutures) after simple curettage. Curettage plus bone grafting or cement fixation has a much lower recurrence rate of approximately 7%[17]. To prevent tumour recurrence, we performed total resection of the vertebral body and intervertebral disc, and internal fixation was performed to simultaneously maintain the stability of the entire spine.
CMF is a benign tumor that may be associated with active growth of cartilage at the predilection site. At present, active surgical treatment, such as resection or curettage, is recommended for such tumors. Regardless of which treatment is adopted, the tumor should be totally removed as far as possible, because the residual tumor will lead to recurrence of the lesion. To prevent spinal instability after tumor resection, internal fixation should be performed at the same time to maintain spinal stability. It has been reported in the literature that there remains a certain proportion of recurrence after total resection of the lesion, and the longest recurrence time is 30 years; therefore, long-term follow-up is recommended. In this case, the lesion was located in the cervical vertebra, the lesion was completely resected, and autogenous bone was transplanted at the same time. There was no tumor recurrence after more than two years of follow-up, but close follow-up should be continued.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Muthu S, India S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Soni R, Kapoor C, Shah M, Turakhiya J, Golwala P. Chondromyxoid Fibroma: A Rare Case Report and Review of Literature. Cureus. 2016;8:e803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | JAFFE HL, LICHTENSTEIN L. Chondromyxoid fibroma of bone; a distinctive benign tumor likely to be mistaken especially for chondrosarcoma. Arch Pathol (Chic). 1948;45:541-551. [PubMed] |
| 3. | Douis H, Saifuddin A. The imaging of cartilaginous bone tumours. II. Chondrosarcoma. Skeletal Radiol. 2013;42:611-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Yasuda T, Nishio J, Sumegi J, Kapels KM, Althof PA, Sawyer JR, Reith JD, Bridge JA. Aberrations of 6q13 mapped to the COL12A1 locus in chondromyxoid fibroma. Mod Pathol. 2009;22:1499-1506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | HemanthaKumar G, Sathish M. Diagnosis and Literature Review of Chondromyxoid Fibroma - A Pathological Puzzle. J Orthop Case Rep. 2019;9:101-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Wangsiricharoen S, Wakely PE Jr, Siddiqui MT, Ali SZ. Cytopathology of chondromyxoid fibroma: a case series and review of the literature. J Am Soc Cytopathol. 2021;10:366-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Vasudeva N, Shyam Kumar C, Ayyappa Naidu CR. Chondromyxoid Fibroma of Distal Phalanx of the Great Toe: A Rare Clinical Entity. Cureus. 2020;12:e7133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Zhong J, Si L, Geng J, Xing Y, Hu Y, Jiao Q, Zhang H, Yao W. Chondromyxoid fibroma-like osteosarcoma: a case series and literature review. BMC Musculoskelet Disord. 2020;21:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Cappelle S, Pans S, Sciot R. Imaging features of chondromyxoid fibroma: report of 15 cases and literature review. Br J Radiol. 2016;89:20160088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Taghipour Zahir S, Sefidrokh Sharahjin N, Sadlu Parizi F, Rahmani K. Chondromyxoid Fibroma of Two Cervical Vertebrae with Involvement of Surrounding Soft Tissue: Radiologic Diagnostic Dilemma. Iran J Radiol. 2015;12:e19273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Rivierez M, Richard S, Pradat P, Devred C. [Chondromyxoid fibroma of the cervical spine. Apropos of a case treated by partial vertebrectomy]. Neurochirurgie. 1991;37:264-268. [PubMed] |
| 12. | Lopez-Ben R, Siegal GP, Hadley MN. Chondromyxoid fibroma of the cervical spine: case report. Neurosurgery. 2002;50:409-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Bala A, Robbins P, Knuckey N, Wong G, Lee G. Spinal chondromyxoid fibroma of C2. J Clin Neurosci. 2006;13:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 14. | Jonathan A, Rajshekhar V, Chacko G. Chondromyxoid fibroma of the seventh cervical vertebra. Neurol India. 2008;56:84-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Subach BR, Copay AG, Martin MM, Schuler TC, Romero-Gutierrez M. An unusual occurrence of chondromyxoid fibroma with secondary aneurysmal bone cyst in the cervical spine. Spine J. 2010;10:e5-e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Zillmer DA, Dorfman HD. Chondromyxoid fibroma of bone: thirty-six cases with clinicopathologic correlation. Hum Pathol. 1989;20:952-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 92] [Article Influence: 2.6] [Reference Citation Analysis (4)] |
| 17. | Basak B, Haragan A, Shackcloth M, Thekkudan J. Chondromyxoid Fibroma of the Rib: A Rare Benign Tumor With Potential for Local Recurrence. Cureus. 2021;13:e19172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (1)] |