Published online Jun 16, 2022. doi: 10.12998/wjcc.v10.i17.5702
Peer-review started: November 16, 2021
First decision: February 14, 2022
Revised: February 24, 2022
Accepted: April 2, 2022
Article in press: April 2, 2022
Published online: June 16, 2022
Processing time: 204 Days and 17.4 Hours
Patients with paroxysmal nocturnal hemoglobinuria (PNH) have a clonal population of blood cells deficient in glycosylphosphatidylinositol-anchored (GPI-anchored) proteins, most of the time resulting from a mutation in the X-linked gene PIGA. We report a patient with PNH resulting from a rare biallelic PIGT mutation on chromosome 20.
A 47-year-old man was referred to our hospital for febrile pancytopenia. The patient reported a history of recurrent urticaria and arthralgia and he presented during 3 mo recurrent acute dermo-hypodermitis and aseptic meningitidis. Based on clinical cases published with PIGT-PNH, with clinically typical PNH and autoinflammatory symptoms, we treated our patients with repeated infusions of eculizumab to decrease autoinflammatory symptoms and then we performed an allogeneic stem cell transplantation (allo-SCT) with a mismatched unrelated donor. Our patient experienced no acute Graft vs Host disease (GvHD) and a moderate chronic GvHD and is now considered cured at 24 mo after allo-SCT.
This case report suggests that allo-SCT should be considered to cure PIGT-PNH patients.
Core Tip: Paroxysmal nocturnal hemoglobinuria with autoinflammatory symptoms has been described in 4 cases with PIG-T mutations (PIGT-PNH entity). We report the fifth case in the world. For the first time we treated him with an allogeneic hematopoietic stem cell transplantation (allo-SCT) after repeated infusions of eculizumab to decrease autoinflammatory symptoms. Allo-SCT was performed with a mismatched unrelated donor and no excess of alloreactivity or toxicity was observed. We think that this new case report with a review of literature will help physicians to have a focus on PIGT-PNH. It suggests that allogeneic SCT should be considered as a curative treatment option for this disease.
- Citation: Schenone L, Notarantonio AB, Latger-Cannard V, Fremeaux-Bacchi V, De Carvalho-Bittencourt M, Rubio MT, Muller M, D'Aveni M. Allogeneic stem cell transplantation-A curative treatment for paroxysmal nocturnal hemoglobinuria with PIGT mutation: A case report. World J Clin Cases 2022; 10(17): 5702-5707
- URL: https://www.wjgnet.com/2307-8960/full/v10/i17/5702.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i17.5702
Paroxysmal nocturnal hemoglobinuria (PNH) is an acquired hematopoietic stem cell (HSC) disorder. Deficient HSCs give rise to a clonal population of blood cells deficient in proteins anchored with glycosylphosphatidylinositol (GPI-anchored), a glycolipid moiety that secures 100 different proteins to the cell surface[1]. In 2019, 4 patients with typical PNH and autoinflammatory symptoms, including recurrent aseptic meningitis, were found to have a germline point mutation in one PIGT allele, with the other PIGT allele being removed by somatic deletion of a 20q region implicated in hematological malignancies. Analyses of patient leukocytes revealed free GPI expressed on the cell surface, triggering autoinflammation through increased IL-1β secretion, activation of the lectin pathway of complement and production of C5b-9 complexes[2]. Therefore, eculizumab treatment abrogates not only intravascular hemolysis but also autoinflammation. We report the fifth case of PIGT-PNH and the first time that allogeneic hematopoietic stem cell transplantation has been applied as treatment. This procedure was readily feasible with no excess alloreactivity or toxicity.
A 47-year-old man was referred to our hospital for pancytopenia with PNH cloning and meningitidis and urticaria.
Upon admission to our hospital, he presented with fever, sudden brownish urine and altered consciousness with mild pancytopenia (hemoglobin 93 g/L, platelets 137.109/L and leukocytes 2.109/L).
The patient reported a history of recurrent urticaria and arthralgia since he was 30-years-old. Three months and one month prior, he was hospitalized for acute dermohypodermitis with pancytopenia and no documented microbiologic agent. He was successfully treated with piperacillin and tazobactam for 14 d.
No special family history was reported.
Examination revealed urticaria and symptoms of meningitis including headache and stiff neck. His meningitis symptoms were resolved at 3 d after initiation of meropenem. During hospitalization, he experienced 4 episodes of aseptic meningitis and general fatigue, arthralgia and urticaria preceded each episode. He recovered quickly within 3 d from the last episode of meningitis with corticosteroids and without antibiotics. The patient developed severe chronic hemolysis after the first meningitidis episode.
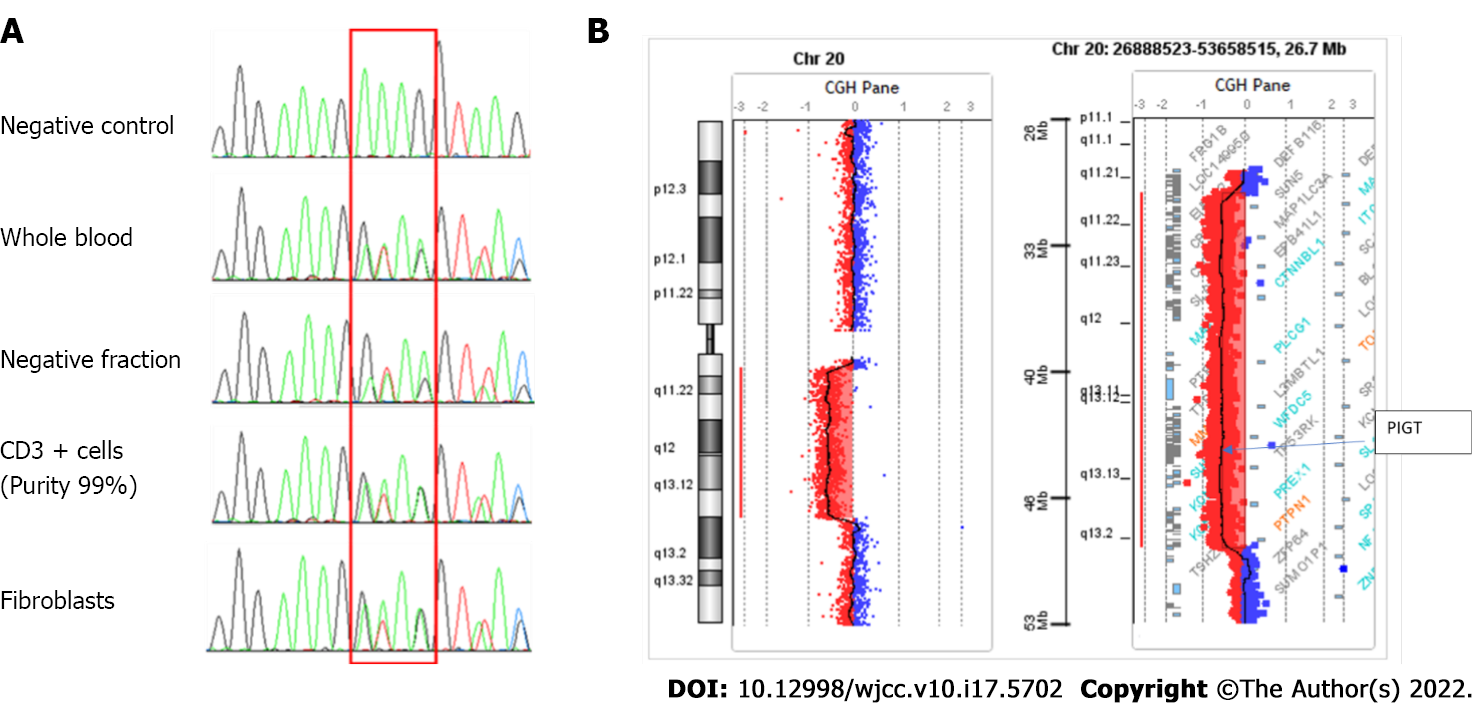
C reactive protein levels were mildly elevated at 20 mg/L. Examination of lumbar cerebrospinal fluid showed 307 polymorphonuclear leukocytes/mm3. No bacteria, fungi, viruses or mycobacteria were identified, nor were autoantibodies. A biopsy from one urticarial lesion revealed mixed inflammatory (neutrophils and monocytes) infiltrate. Flow cytometric analysis of both erythrocytes and granulocytes indicated deficiency of GPI-anchored proteins (Figure 1); complement system dosing showed a normal CH50 Level. Factor H and Factor I plasma concentrations and anti-Factor H antibodies were also normal. Examination of cellular morphology based on bone marrow aspiration revealed multilineage dysplasia with no excess blasts (< 2%). Medullar cytogenetic analysis detected a 20q deletion in the karyotype, and Sanger sequencing highlighted a deletion of 4 nucleotides (NM_015937.6:c.766_769del) in exon 6 (p. Lys256ThrfsTer38) leading to a frameshift and a premature stop codon. This mutation was found in the heterozygous state in both T lymphocytes and in the negative cellular fraction, suggesting a constitutional anomaly. These results were confirmed using another sample consisting of DNA extracted from fibroblast culture cells collected after skin biopsy. This finding is reported only once in the ClinVar database (RCV000735856.1). According to the CGH array, we detected a large somatic deletion of 18 Mb from 20q11.21 to 20q13.13, an area including the entire PIGT gene. This 20q deletion associated with heterozygous constitutional mutation of PIGT leads to biallelic inactivation of the gene (Figure 2).
Cerebral magnetic resonance imaging results were normal.
PIGT-PNH.
All the patient's symptoms, including urticaria, arthralgia, headache/meningitidis and hemolysis, completely disappeared after eculizumab was administered regularly. Finally, after 8 mo on eculizumab treatment, the pancytopenia worsened (hemoglobin 90 g/L, platelets 67.109/L and leukocytes 1.109/L), and the patient presented a sepsis secondary to a catheter-related bacteriemia of staphylococcus epidermidis resistant to methicillin. Bone marrow tests revealed 8% blast. We decided to transplant the patient because of the episode of severe infection and bone marrow smear results. The decision of transplantation was difficult, because in common PNH caused by mutation of PIGA, there is a high risk of developing GVHD, especially in patients older than 40-years-old with no sibling donors. No data were available about transplantation in PNH caused by mutation of PIGT, and our patient had no sibling or matched unrelated donors. However, recent retrospective studies demonstrated promising results with HLA-mismatched/haploidentical hematopoietic stem cell transplantation after reduced intensity conditioning and GVHD prophylaxis with post-Transplant cyclophosphamide in refractory severe aplastic anemia patients. Moreover, inflammatory symptoms in our patient were totally controlled by eculizumab. We hypothesized that it could be a good time for transplantation. Therefore, allogeneic hematopoietic stem cell transplantation with peripheral blood stem cells from an HLA-mismatched unrelated donor was carried out after a reduced-intensity conditioning regimen consisting of thiotepa (5 mg/m2 at day -7), a total fludarabine dose of 150 mg/m2 (30 mg/m2 from day -5 to day -1), and total intravenous (i.v.) busulfan 6.4 mg/kg (3.2 mg/kg/d on days -4 and -3). Graft vs host disease (GvHD) prophylaxis consisted of posttransplant cyclophosphamide (50 mg/kg/j on days +3 and +4), cyclosporine A (starting on day +5 at 3 mg/kg/day) as a continuous i.v. infusion, and i.v. MMF (starting on day +5 at 15 mg/kg every 12 h). A dose of 6×106 CD34+/kg body weight was infused.
We observed rapid myeloid engraftment, with a time for neutrophils > 0.5 × 109/L and platelet recovery (> 20.109/L) of 15 d and 16 d, respectively. Chimerism was complete donor at 1, 3, 12 and 18 mo posttransplant. No acute GvHD was observed. Six months after transplantation, he developed moderate chronic hepatic and skin GvHD that improved by enhancing the calcineurin inhibitor and starting 1 mg/kg/d corticosteroid therapy. At the time of writing, at 24 mo after transplantation, chronic GvHD is in complete remission with no immunosuppressant.
PNH is a clonal disorder involving blood cells deficient in glycosylphosphatidylinositol-anchored (GPI-anchored) proteins[1,3], which is often caused by a deficient initial step in GPI anchor synthesis, as catalyzed by the GPI-GlcNAc transferase encoded by the X-chromosomal gene PIGA[4-6]. However, 22 PIG genes participate in the biosynthesis and protein attachment of GPI[7,8]. The PIGT gene on chromosome 20, at position 20q13.12 with 12 exons, encodes phosphatidylinositol-glycan biosynthesis class T protein (PIG-T), a subunit of the heteropentameric GPI transamidase complex that facilitates attachment of GPI anchors to proteins[9]. Four cases of PHN with recurrent inflammatory symptoms have been reported[2] with PIGT defects and successfully treated with eculizumab. In 2013, PNH due to 2 mutation events was reported: a germline splice site mutation and a somatic deletion in PIGT (c.1401-2A>G), as identified by next-generation sequencing[10]. In 2018, a second patient with long-term severe urticaria and joint pain before developing PNH harbored similar mutations in PIGT (c.250G>T) and exhibited recurrent aseptic meningitis in addition to inflammatory symptoms[11]. Both cases clearly improved with eculizumab treatment. In 2019, 2 additional patients with PHN and inflammatory symptoms were reported: one had chronic lymphocytic leukemia, and the other carried the JAK2-V617F mutation. Both patients harbored germline mutations in one PIGT allele (one patient with c.761_764delGAAA and the other with c.197delA) associated with somatic deletions, including the entire PIGT gene in the second allele without PIGA gene abnormalities[2]. In known cases of PHN with PIGT disruption, one of the PIGT alleles is removed due to a somatic deletion of varying size, including the common deleted region (CDR) of 1.9 Mb, which is close to the centromeric region, often described in myeloid malignancies with 20q deletion. Based on a family segregation study, PIGT haploinsufficiency is not sufficient for the development of autoinflammatory symptoms. In our case, the development of MDS with 20q deletion was an indispensable additional abnormality resulting in biallelic inactivation of PIGT, explaining the PNH. If mutations in both PIGA and PIGT can induce PNH, recurrent inflammatory symptoms, including meningitis, are in particular found with PIGT mutations. Therefore, some authors have proposed creating a new entity named PIGT-PHN[2]. In PNH-PIGT syndrome, cytokine dosing suggests that increased free GPI might over activate NLRP3 inflammasomes in mononuclear cells with strong IL-1β and IL-18 responses. IL-18 is produced by activated inflammasomes[12,13] and is also produced during clinical GvHD. NLRP3 is known to play a role in enhancing GvHD[14]. Our case report is the fifth published case of PIGT-PNH. Among 4 patients previously described, 3 patients were partially controlled with corticosteroids, colchicine, diphenhydramine, cromoglycin, azathioprine, mycophenolate mofetil, dapsone, anakinra and canakinumab. Only eculizumab treatment abrogates autoinflammation for one patient. We confirm that eculizumab is the best treatment to abrogate intravascular hemolysis and autoinflammation. Because we know that complement activation and inflammatory dysregulation before allo-SCT might be associated to a higher incidence of severe acute GvHD in patients, our main concern was about the toxicity of this procedure. We report fort the first time that allogeneic hematopoietic stem cell transplantation is a readily feasible procedure with no excess alloreactivity or toxicity.
Allogeneic stem cell transplantation has not been reported for treating PIGT-PNH, yet this therapy addresses the concern regarding a high risk of alloreactivity and toxicity in patients with activated NLRP3 inflammasomes in mononuclear cells. Our case is the first to be successfully treated with allo-SCT, and no toxicity (especially GvHD) was observed.
We thank the patient for his full consent.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Hematology
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Saito M, Japan S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Wu YXJ
| 1. | Brodsky RA. Advances in the diagnosis and therapy of paroxysmal nocturnal hemoglobinuria. Blood Rev. 2008;22:65-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Höchsmann B, Murakami Y, Osato M, Knaus A, Kawamoto M, Inoue N, Hirata T, Murata S, Anliker M, Eggermann T, Jäger M, Floettmann R, Höllein A, Murase S, Ueda Y, Nishimura JI, Kanakura Y, Kohara N, Schrezenmeier H, Krawitz PM, Kinoshita T. Complement and inflammasome overactivation mediates paroxysmal nocturnal hemoglobinuria with autoinflammation. J Clin Invest. 2019;129:5123-5136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | Bessler M, Mason P, Hillmen P, Luzzatto L. Somatic mutations and cellular selection in paroxysmal nocturnal haemoglobinuria. Lancet. 1994;343:951-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 115] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Takahashi M, Takeda J, Hirose S, Hyman R, Inoue N, Miyata T, Ueda E, Kitani T, Medof ME, Kinoshita T. Deficient biosynthesis of N-acetylglucosaminyl-phosphatidylinositol, the first intermediate of glycosyl phosphatidylinositol anchor biosynthesis, in cell lines established from patients with paroxysmal nocturnal hemoglobinuria. J Exp Med. 1993;177:517-521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 114] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Takeda J, Miyata T, Kawagoe K, Iida Y, Endo Y, Fujita T, Takahashi M, Kitani T, Kinoshita T. Deficiency of the GPI anchor caused by a somatic mutation of the PIG-A gene in paroxysmal nocturnal hemoglobinuria. Cell. 1993;73:703-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 745] [Cited by in RCA: 726] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 6. | Miyata T, Takeda J, Iida Y, Yamada N, Inoue N, Takahashi M, Maeda K, Kitani T, Kinoshita T. The cloning of PIG-A, a component in the early step of GPI-anchor biosynthesis. Science. 1993;259:1318-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 374] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 7. | Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7976] [Cited by in RCA: 10459] [Article Influence: 697.3] [Reference Citation Analysis (0)] |
| 8. | Kinoshita T, Fujita M, Maeda Y. Biosynthesis, remodelling and functions of mammalian GPI-anchored proteins: recent progress. J Biochem. 2008;144:287-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 211] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 9. | Ohishi K, Inoue N, Kinoshita T. PIG-S and PIG-T, essential for GPI anchor attachment to proteins, form a complex with GAA1 and GPI8. EMBO J. 2001;20:4088-4098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 139] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Krawitz PM, Höchsmann B, Murakami Y, Teubner B, Krüger U, Klopocki E, Neitzel H, Hoellein A, Schneider C, Parkhomchuk D, Hecht J, Robinson PN, Mundlos S, Kinoshita T, Schrezenmeier H. A case of paroxysmal nocturnal hemoglobinuria caused by a germline mutation and a somatic mutation in PIGT. Blood. 2013;122:1312-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Kawamoto M, Murakami Y, Kinoshita T, Kohara N. Recurrent aseptic meningitis with PIGT mutations: a novel pathogenesis of recurrent meningitis successfully treated by eculizumab. BMJ Case Rep. 2018;2018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Barker BR, Taxman DJ, Ting JP. Cross-regulation between the IL-1β/IL-18 processing inflammasome and other inflammatory cytokines. Curr Opin Immunol. 2011;23:591-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 13. | Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2289] [Cited by in RCA: 2624] [Article Influence: 164.0] [Reference Citation Analysis (0)] |
| 14. | Koehn BH, Apostolova P, Haverkamp JM, Miller JS, McCullar V, Tolar J, Munn DH, Murphy WJ, Brickey WJ, Serody JS, Gabrilovich DI, Bronte V, Murray PJ, Ting JP, Zeiser R, Blazar BR. GVHD-associated, inflammasome-mediated loss of function in adoptively transferred myeloid-derived suppressor cells. Blood. 2015;126:1621-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |