Published online Jun 16, 2022. doi: 10.12998/wjcc.v10.i17.5667
Peer-review started: December 21, 2021
First decision: March 10, 2022
Revised: March 18, 2022
Accepted: April 9, 2022
Article in press: April 9, 2022
Published online: June 16, 2022
Processing time: 169 Days and 9.7 Hours
Branch duct-intraductal papillary mucinous neoplasms (BD-IPMNs) are the most common pancreatic cystic tumours and have a low risk of malignant trans
To assess both diameter and volume growth rate of BD-IPMNs and evaluate their correlation with the development of malignant characteristics.
Computed tomography scans and magnetic resonance imaging exams were retrospectively reviewed. The diameter was measured on three planes, while the volume was calculated by segmentation: The volume of the entire cyst was determined by manually drawing a region of interest along the edge of the neoplasm on each consecutive slice covering the whole lesion; therefore, a three-dimensional volume of interest was finally obtained with the calculated value expressed in cm3. Changes in size over time were measured. The development of worrisome features was evaluated.
We evaluated exams of 98 patients across a 40.5-mo median follow-up time. Ten patients developed worrisome features. Cysts at baseline were significantly larger in patients who developed worrisome features (diameters P = 0.0035, P = 0.00652, P = 0.00424; volume P = 0.00222). Volume growth rate was significantly higher in patients who developed worrisome features (1.12 cm3/year vs 0 cm3/year, P = 0.0001); diameter growth rate was higher as well, but the difference did not always reach statistical significance. Volume but not diameter growth rate in the first year of follow-up was higher in patients who developed worrisome features (0.46 cm3/year vs 0 cm3/year, P = 0.00634).
The measurement of baseline volume and its variation over time is a reliable tool for the follow-up of BD-IPMNs. Volume measurement could be a better tool than diameter measurement to predict the development of worrisome features.
Core Tip: Branch duct-intraductal papillary mucinous neoplasms (BD-IPMNs) are the most common pancreatic cystic tumours. Size is the most important risk factor in patients with BD-IPMNs. A high diameter growth-rate is associated with malignancy as well. In our study, we demonstrated that volume is associated with the development of worrisome features and that a higher volume growth-rate can lead to a higher risk of worrisome features. Moreover, in our cohort, volume growth-rate in the first year of follow-up predicted the development of worrisome features. Based on these data, measuring volume could be a better tool than the diameter to predict early BD-IPMNs malignant transformation.
- Citation: Innocenti T, Danti G, Lynch EN, Dragoni G, Gottin M, Fedeli F, Palatresi D, Biagini MR, Milani S, Miele V, Galli A. Higher volume growth rate is associated with development of worrisome features in patients with branch duct-intraductal papillary mucinous neoplasms. World J Clin Cases 2022; 10(17): 5667-5679
- URL: https://www.wjgnet.com/2307-8960/full/v10/i17/5667.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i17.5667
Pancreatic intraductal papillary mucinous neoplasms (IPMN), in their branch-duct form (BD-IPMN) are the most frequent cystic tumours of the exocrine pancreas and harbour a low risk of malignant transformation[1,2]. For this reason, it is essential to stratify the risk of malignancy in order to plan the most appropriate follow-up for each patient.
The current guidelines suggest an individual risk-based screening with computed tomography (CT) scan, magnetic resonance imaging (MRI) or endoscopic ultrasound (EUS)[3-5]. Cyst diameter growth rate is one of the parameters that is associated with the development of malignant features in patients with BD-IPMN[6,7]. However, only the International Guidelines by Tanaka et al[4] consider the cyst diameter growth rate as a worrisome feature. Recently, a nomogram based on cyst size, pancreatic duct size, presence of mural nodule, serum carbohydrate antigen (CA) 19.9 and carcinoembryonic antigen (CEA) was constructed to predict malignancy in BD-IPMNs[8]. However, none of the current guidelines take into account the volume of the cysts and its evolution over time, although it has recently been shown that this is a highly reproducible parameter with low inter-observer variability[9]. To date, there are no studies investigating the correlation between cyst volume growth rate and the risk of malignant degeneration in patients with BD-IPMN.
In light of the lack of agreement between current guidelines, we believe it would be useful to implement the available evidence regarding the risk factors for malignancy of pancreatic BD-IPMN. Moreover, assessing cyst volume growth rate may enable the identification of patients who would benefit from a close follow-up, distinguishing them from those for whom it would be more useful to delay check-ups over time or even interrupt them for cost-effective reasons.
In this study, we assess the growth rate (both diameter and volume) of low-risk BD-IPMNs and evaluate its correlation with the development of malignant characteristics. Moreover, we aim to evaluate the possible superiority of measuring cyst volume instead of cyst diameters in predicting poor outcomes.
We designed a single-centre, retrospective study at our tertiary referral centre. In accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments, we evaluated data of 148 patients who were referred to our pancreatic disease outpatient clinic (Gastroenterology Unit, Careggi University Hospital, Florence, Italy) for regular IPMN follow-up. We searched in the digital clinical records using a free text entry (“IPMN” or “intraductal papillary mucinous neoplasms” or “pancreatic cyst” and “branch-duct” or “BD”) into a 10-years-period (between July 2011 and July 2021). The study was approved by the local Ethical Committee of Careggi University Hospital on July 13th 2021 (protocol number: 20256_oss).
We included all adult patients with a known BD-IPMN who had at least two contrast-enhanced MRI studies or CT scans at our centre and a 12-mo minimum follow-up time. For patients who had multifocal BD-IPMN, the largest cyst was considered. Exclusion criteria were paediatric age (< 18 years), prior history of pancreatic cancer or surgery, history of acute or chronic pancreatitis, patients with worrisome features or high-risk stigmata (mentioned later) at first imaging study and patients who had their imaging studies in other clinical centres or for whom radiological study images were unavailable.
IPMN were defined as a grape-like cluster of small cysts originating from a branch of the pancreatic duct, a multilocular cyst with finger-like projections or a single, lobulated cyst communicating with the main pancreatic duct (MPD)[6,10]. Mucinous fluid at fine-needle aspiration and cyst fluid cytology consistent with IPMN were additional criteria.
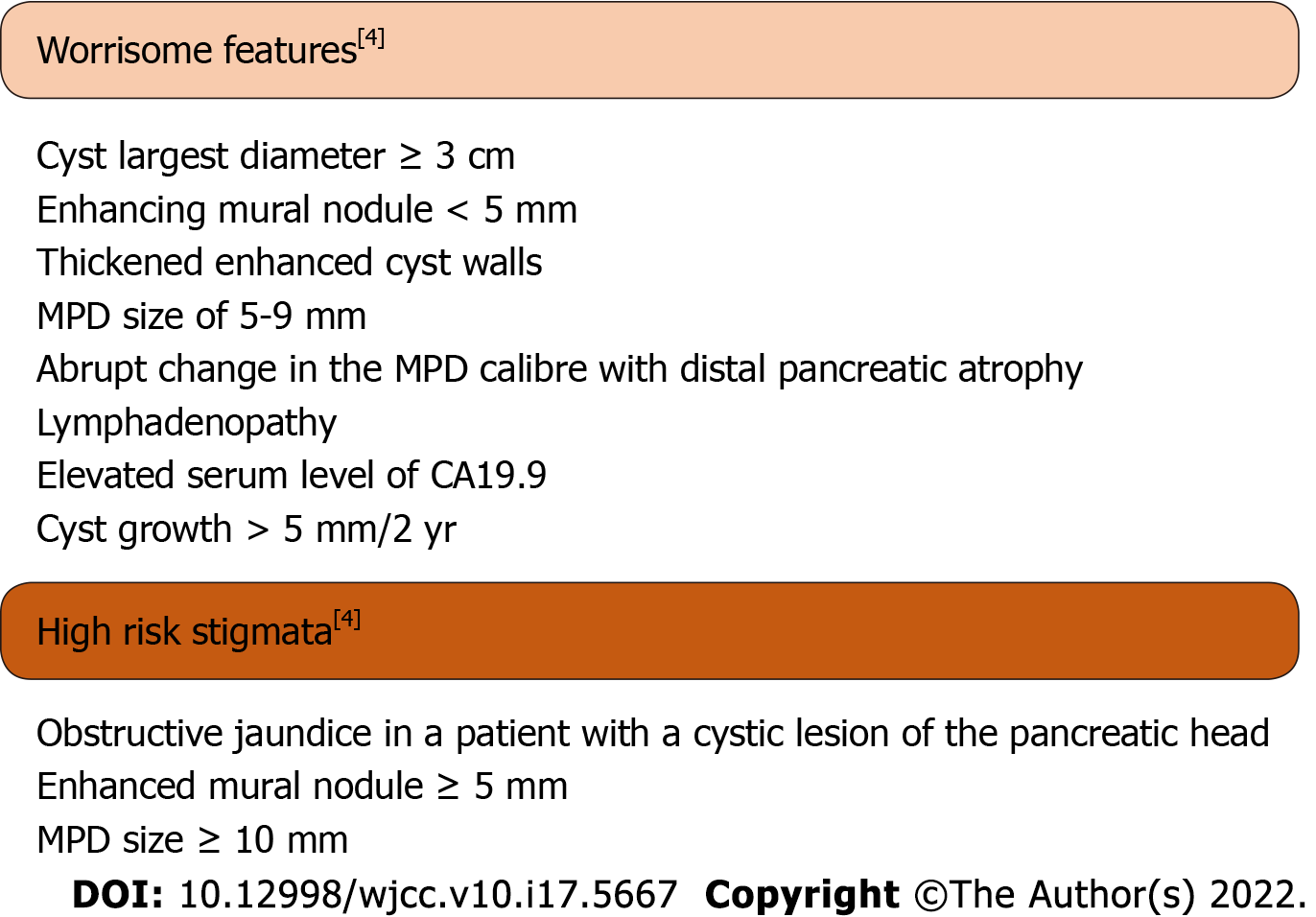
Worrisome features and high-risk stigmata were defined as in the 2017 Fukuoka guidelines (Figure 1). Worrisome features include cyst of ≥ 3 cm, enhancing mural nodule < 5 mm, thickened enhanced cyst walls, MPD size of 5-9 mm, abrupt change in the MPD calibre with distal pancreatic atrophy, lymphadenopathy, an elevated serum level of CA19.9 and a rapid rate of cyst growth > 5 mm/2 years. High-risk stigmata include obstructive jaundice in a patient with a cystic lesion of the pancreatic head, enhanced mural nodule ≥ 5 mm and MPD size ≥ 10 mm[4].
For each patient we collected demographic and clinical data including sex, age at start of follow-up, smoking habits, familial history, diabetes history and follow-up duration (in months). When possible, we collected biochemical data including serum CA19.9 and CEA. Serum CA19.9 was considered high if > 37 IU/L, as reported in a recent update by Ciprani et al[11]. When available, we also collected data regarding EUS examination including cyst dimension (two largest diameters) and (if FNA was performed) cyst fluid CEA, amylases and cytology characteristics.
All CT investigations were carried out on a 128-slice CT scanner (Brilliance iCT, Philips Medical Systems, Netherlands). Patients were scanned in the supine position with cranio-caudal breath-hold scans. All patients underwent non-contrast and contrast-enhanced CT scanning with a slice thickness of 2-3 mm. Iodinated contrast medium (iopromide 370 mg I/mL - Ultravist®, Bayer Schering, Berlin) was injected into the antecubital vein at a flow rate of 3-4 mL/s using an automatic injector, immediately followed by a saline flush. The dose of the contrast medium was administered according to the patient’s body weight [mL/kg body weight: 80-100 mL (< 80 kg) or 100-120 mL (> 80 kg)]. Contrast-enhanced CT images were acquired during the arterial (30-35 s), portal venous (80-90 s) and late phase (> 180 s).
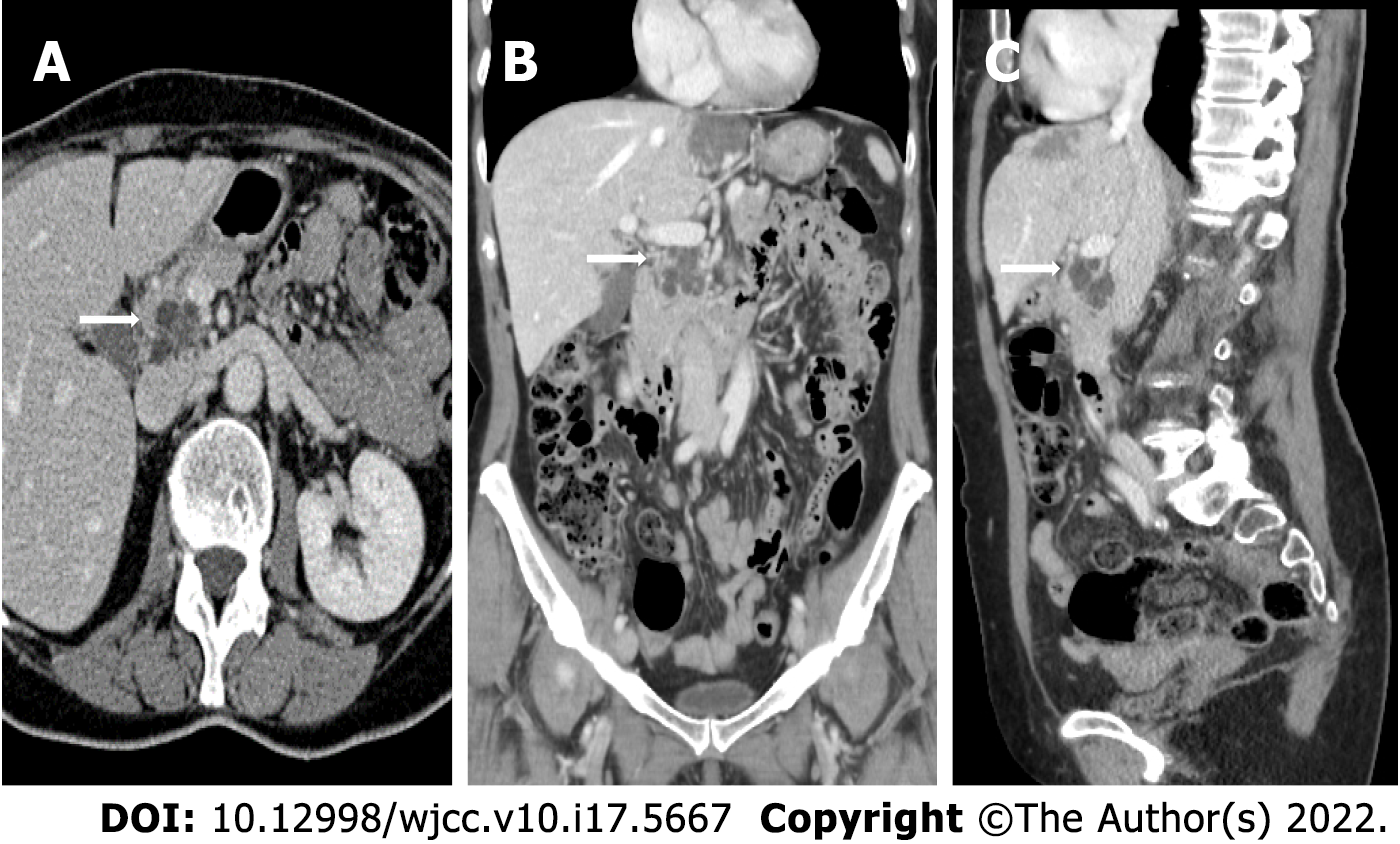
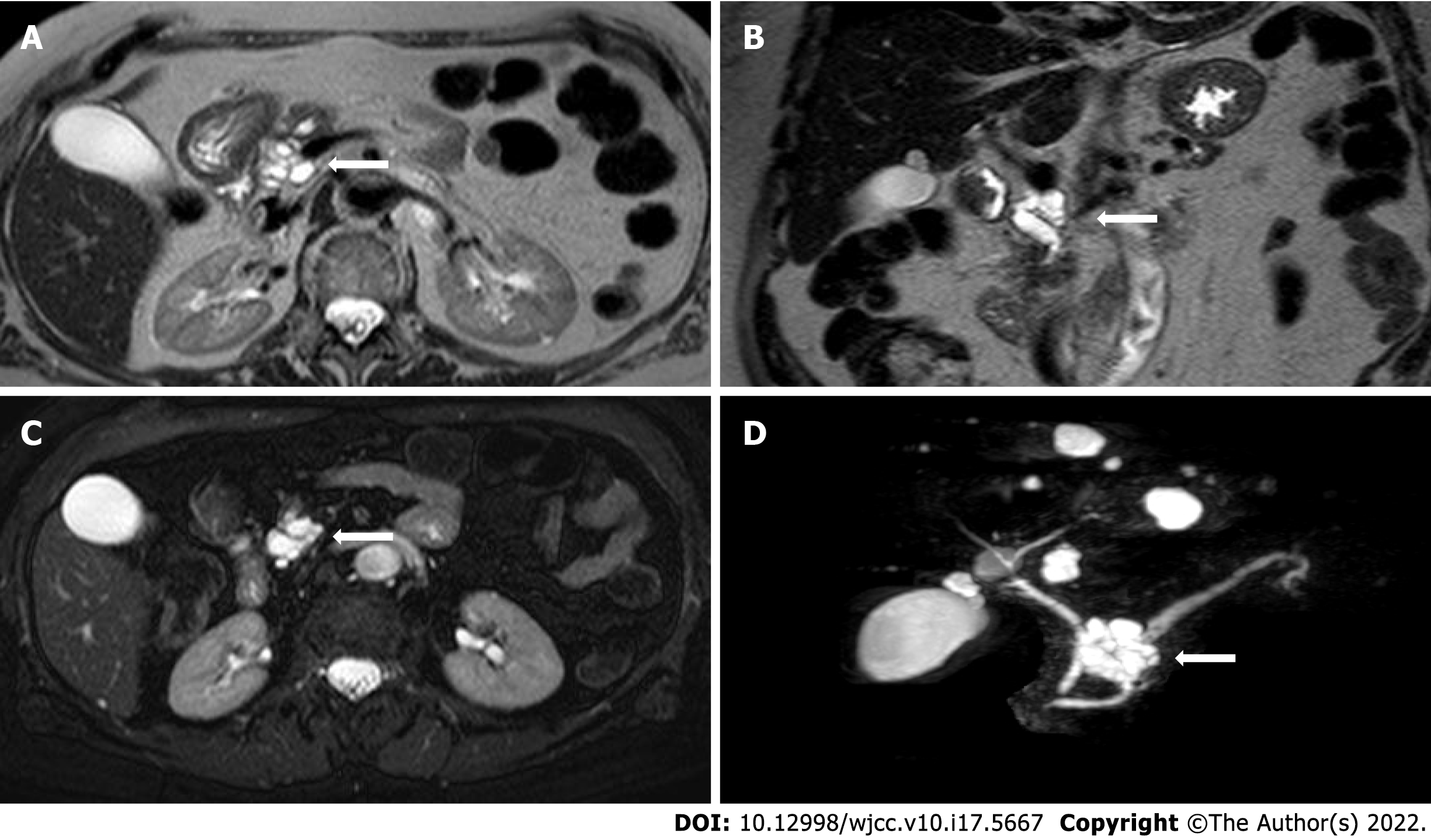
All MRI examinations were carried out on a 1.5 Tesla MRI scanner (MAGNETOM Aera, Siemens, Germany). MRI protocol for IPMN included: T2-weighted Turbo Spin Echo (TSE) or Single Shot TSE sequence acquired in axial and coronal planes; T1-weighted gradient echo (GRE) in-phase and out-of-phase sequence acquired in the axial plane; diffusion weighted imaging (DWI) (b value = 0; 100; 500; 800) acquired in the axial plane and ADC map; cholangiographic sequences, that is T2-weighted Single Shot TSE 2D (radial) fat-sat sequences and T2-weighted volume (3D) TSE fat-sat sequences (with maximum intensity projection reconstructions). The dynamic study was obtained during the intravenous administration of gadolinium chelates contrast agents (gadoteridol 279.3 mg/mL - Prohance®, Bracco Diagnostics Inc., Germany; 0.2 mL/kg of body weight) at a rate of 2-3 mL/s followed by saline injection with a triphasic technique: pancreatic (35-45 s), portal venous (80-90 s), and late phase (> 180 s). The dynamic study was performed with T1-weighted volume GRE sequences (3D), with selective fat saturation acquired in the axial plane. The slice thickness necessarily included the entire biliary tree and pancreatic ductal system. Figure 2 and Figure 3 report examples of our patients’ CT and MRI images.
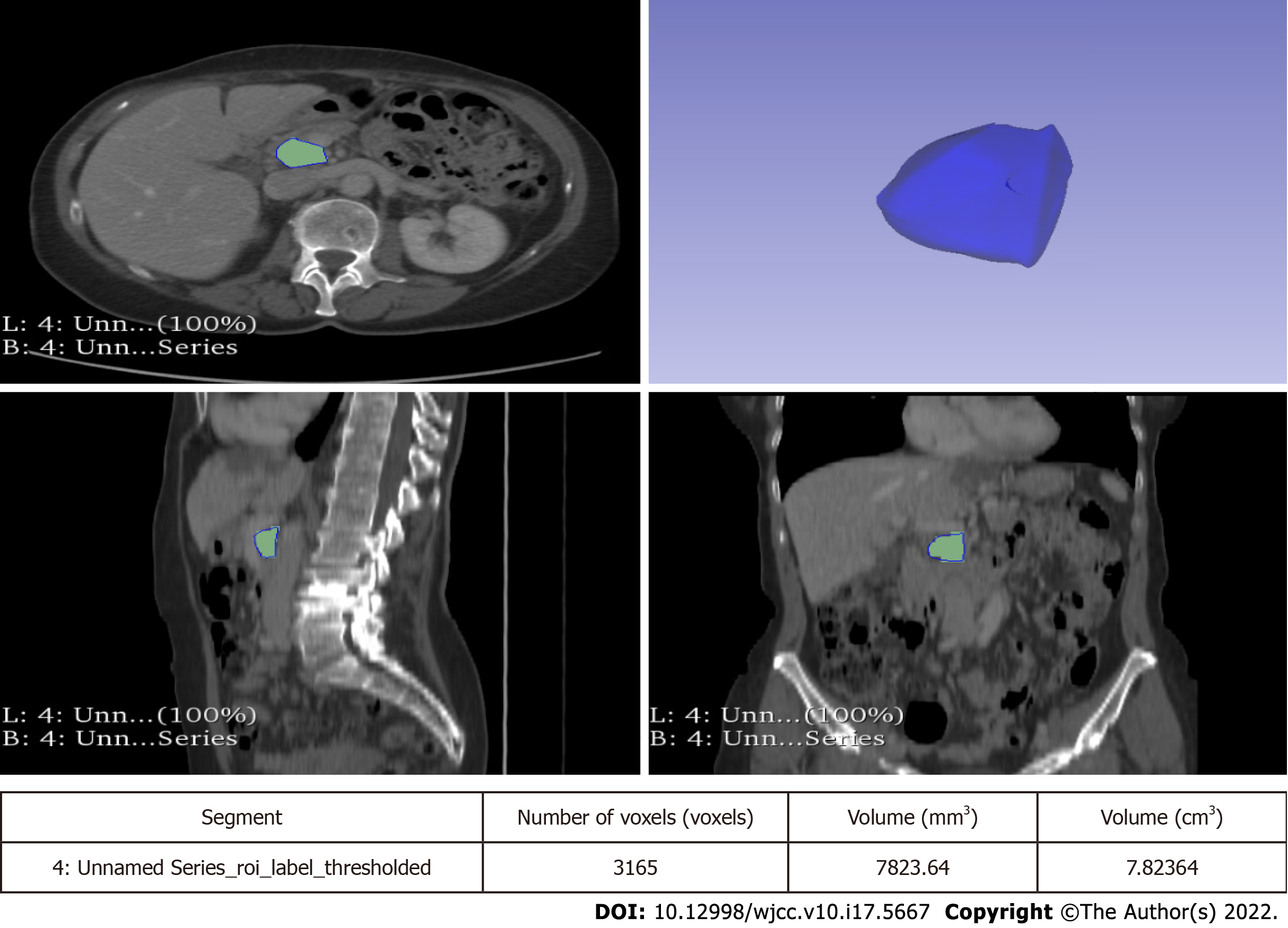
Lesions were measured on both CT and MRI images along the three major diameters (antero-posterior, latero-lateral, cranio-caudal). Volume measurement was obtained by manual segmentation with 3D Slicer software version 4.10.2 (available at https://slicer.org). Manual segmentation is a widely validated and reliable method for volume measurement in both abdominal radiology and in other fields of research[12-15]. The volume of the entire cyst was determined by manually drawing a region of interest along the edge of the neoplasm on each consecutive slice covering the whole lesion. Therefore, a three-dimensional (3D) volume of interest was finally obtained with the calculated value expressed in cm3. More particularly, the tumour contours were individually outlined slice-by-slice by two radiologists (at least 5 years of experience) and then reviewed by a senior radiologist (more than 10 years of experience). Any disagreement between the readers was discussed until a final consensus was generated. An example of segmentation is reported in Figure 4.
All statistical analyses were performed using SPSS Statistics version 26.0 (IBM, Armonk, NY, United States). Continuous variables were expressed as medians and interquartile ranges (IQR) while categorical variables were presented as percentages. Descriptive statistics was used to compare the characteristics of patients who developed worrisome features or high-risk stigmata with that of patients who did not: continuous variables were compared using the Mann-Whitney U-test while categorical variables were compared with a Yate’s χ2 test or a Fisher’s exact test (depending on the sample size). A two-sided P value < 0.05 was considered as significant.
The primary outcomes of our study were to assess the volume growth rate of BD-IPMNs without worrisome features or high-risk stigmata at baseline and to evaluate if a higher volume growth rate correlates with the development of worrisome features or high-risk stigmata. The secondary outcomes were the following: (1) To evaluate the impact of measuring volume dimension vs flat dimension (axial, coronal and longitudinal diameters); (2) To evaluate the impact of measuring volume growth rate vs diameter growth rate; and (3) To test the ability of first-year volume growth rate to predict the development of worrisome features or high-risk stigmata.
Overall, 148 patients were identified. Five patients had worrisome features or high-risk stigmata at baseline and were excluded from the study. An additional 45 patients were excluded as they had unsatisfactory or incomplete follow-up information. Therefore, a total of 98 patients were included in our study. Baseline and demographic characteristics of the patients and cysts are reported in Table 1.
| Characteristics | n = 98 | |
| Males (%) | 33 (33.7) | |
| Females (%) | 65 (66.3) | |
| Median age at diagnosis (IQR) | 69 (63-75) | |
| Familial history (%) | 9 (9.2) | |
| History of diabetes (%) | 16 (16.3) | |
| Diabetes onset during follow-up (%) | 0 (0.0) | |
| Median follow-up [mo (IQR)] | 40.5 (24-72) | |
| Former smokers (%) | 14 (14.3) | |
| Active smokers (%) | 8 (8.1) | |
| Never smoked (%) | 76 (77.6) | |
| Multifocal | 57 (58.2) | |
| Largest cyst localization | Head (%) | 44 (44.9) |
| Isthmus/body (%) | 40 (40.8) | |
| Tail (%) | 14 (14.3) | |
| Median cyst size | a [mm (IQR)] | 9 (6-15) |
| b [mm (IQR)] | 10 (6.75-16) | |
| c [mm (IQR)] | 10 (6-16) | |
| Volume [cm3 (IQR)] | 0.54 (0.17-1.65) | |
| EUS | 19 (19.4) | |
| Median time to EUS (IQR) | 6 (0-31.5) | |
| FNA (%) | 9 (47.4) | |
| Cyst fluid amylases [IU/L (IQR)], n = 5 | 289 (69.25-1725.25) | |
| Cyst fluid CEA [IU/L (IQR)], n = 6 | 166 (8.55-24062.75) | |
| Serum CA19.9 [IU/L (IQR)], n = 25 | 25.2 (6.1-58.7) | |
| Serum CEA [IU/L (IQR)], n = 22 | 2.15 (1.27-2.92) | |
After a 40.5-mo median follow-up (IQR 24-72), 10 patients (10.2%) developed worrisome features: 6 patients had cysts larger diameter > 3 cm; 3 patients had increased serum CA19.9 (> 37 IU/L); 1 patient had MPD ≥ 5 mm. No patients developed high-risk stigmata. Baseline cyst dimensions, both diameter and volume, were the only patient baseline characteristics which were significantly associated with the development of worrisome features (Table 2). Two of the patients who developed worrisome features underwent surgical resection. They both had a pancreaticoduodenectomy and their main characteristics are resumed in Table 3.
| Non-worrisome features (n = 88) | Worrisome features (n = 10) | P value | ||
| Males (%) | 31 (35.2) | 2 (20.0) | 0.540234 | |
| Females (%) | 57 (64.8) | 8 (80.0) | 0.540234 | |
| Median age at diagnosis (IQR) | 70 (63.5-74.75) | 67 (59.75-76.25) | 0.58232 | |
| Familial history (%) | 8 (9.1) | 1 (10.0) | 0.628786 | |
| History of diabetes (%) | 14 (15.9) | 2 (20.0) | 0.904665 | |
| Diabetes onset during follow-up (%) | 0 (0.0) | 0 (0.0) | - | |
| Median follow-up [mo (IQR)] | 37.5 (23.25-71) | 69.5 (46.5-94) | 0.01828 | |
| Former smokers (%) | 11 (12.5) | 3 (30.0) | 0.306885 | |
| Active smokers (%) | 8 (9.1) | 0 (0.0) | 0.738111 | |
| Never smoked (%) | 69 (78.4) | 7 (70.0) | 0.83833 | |
| Multifocal | 52 (59.1) | 5 (50.0) | 0.830551 | |
| Largest cyst localization | Head (%) | 39 (44.3) | 5 (50.0) | 0.994538 |
| Isthmus/body (%) | 35 (39.8) | 5 (50.0) | 0.776364 | |
| Tail (%) | 14 (15.9) | 0 (0.0) | 0.746616 | |
| Median cyst size | a [mm (IQR)] | 9 (6-13) | 19 (11.25-21.25) | 0.0035 |
| b [mm (IQR)] | 10 (6-15) | 18 (10.75-20.75) | 0.00652 | |
| c [mm (IQR)] | 9.5 (6-15) | 22.5 (12-27.65) | 0.00424 | |
| EUS | 14 (15.9) | 5 (50.0) | 0.030618 | |
| Median time to EUS (IQR) | 3 (0-18.25) | 29 (5.5-69.5) | 0.22628 | |
| Serum CA19.9 [IU/L (IQR)], n = 25 | 6.2 (5.6-24.2) | 23.8 (6.2-37.9) | 0.1556 | |
| Serum CEA [IU/L (IQR)], n = 22 | 2.15 (1.28-2.93) | 2.15 (1.55-2.75) | 0.93624 | |
| Sex, age | Final cyst volume (cm3) | Growth rate (cm3/yr) | MPD (mm) | Location | CA19.9 (IU/L) | Time to surgery (mo) | Histopathology | |
| Patient A | F, 68 | 28.73 | 33.91 | < 5 | Isthmus | 33.2 | 85 | LGD |
| Patient B | F, 74 | 14.70 | 24.27 | < 5 | Head | 22.5 | 65 | LGD |
Baseline median cyst sizes were 9 mm (IQR 6-15) × 10 mm (IQR 6.75-16) × 10 mm (IQR 6-16). Measurements are intended to be sagittal (antero-posterior) × transversal (latero-lateral) × coronal (cranio-caudal). Baseline cyst volume was 0.54 cm3 (IQR 0.17-1.65). Cysts at baseline were significantly larger in patients who developed worrisome features, as reported in Table 4. Cyst sizes and growth after follow-up are resumed in Tables 5-7. Particularly, at the end of the follow-up, patients who developed worrisome features had a median cyst volume of 10.17 cm3 (IQR 2.72-19.36), with a median growth of 4.01 cm3 (IQR 0.83-13.87). Both volume and cyst volume growth were significantly higher in patients who developed worrisome features (P = 0.0005 and P < 0.00001, respectively). Indeed, in patients who developed worrisome features, cyst volume grew faster than in patients who did not (1.12 cm3/year, IQR 0.28-2.65 vs 0 cm3/year, IQR -0.02-0.17, P = 0.0001). Diameter size also grew more in patients who developed worrisome features but this growth was not always significant across all three measurement planes.
| All (n = 98) | Non-worrisome features (n = 88) | Worrisome features (n = 10) | P value | |
| a [mm (IQR)] | 9 (6-15) | 9 (6-13) | 19 (11.25-21.25) | 0.0035 |
| b [mm (IQR)] | 10 (6.75-16) | 10 (6-15) | 18 (10.75-20.75) | 0.00652 |
| c [mm (IQR)] | 10 (6-16) | 9.5 (6-15) | 22.5 (12-27.65) | 0.00424 |
| volume [cm3 (IQR)] | 0.54 (0.17-1.65) | 0.45 (0.15-1.28) | 5.28 (1.10-6.30) | 0.00222 |
| All (n = 98) | Non-worrisome features (n = 88) | Worrisome features (n = 10) | P value | |
| a [mm (IQR)] | 9 (6-16) | 9 (6-13.75) | 23 (13.5-27.5) | 0.00288 |
| b [mm (IQR)] | 12 (7-18) | 11 (7-16.75) | 28 (19.25-37.5) | 0.00054 |
| c [mm (IQR)] | 11 (7-18) | 10.5 (6.25-16) | 29 (15.75-38.75) | 0.0003 |
| volume [cm3 (IQR)] | 0.58 (1.76-2.02) | 0.52 (0.17-1.63) | 10.17 (2.72-19.36) | 0.0005 |
| All (n = 98) | Non-worrisome features (n = 88) | Worrisome features (n = 10) | P value | |
| a [mm (IQR)] | 0 (-0.25-2) | 0 (-0.75-1.75) | 2 (-0.5-8.75) | 0.0601 |
| b [mm (IQR)] | 0 (0-3) | 0 (0-2) | 10 (-0.25-14.25) | 0.01016 |
| c [mm (IQR)] | 0 (0-3) | 0 (-1-2) | 5 (3.25-14) | 0.00008 |
| Volume [cm3 (IQR)] | 0.02 (-0.01-0.70) | 0 (-0.03-0.24) | 4.01 (0.83-13.87) | < 0.00001 |
| Volume increased (%) | 28 (28.6) | 18 (20.4) | 10 (100.0) | < 0.00001 |
| Volume unchanged or reduced (%) | 70 (71.4) | 70 (79.6) | 0 (0.0) | < 0.00001 |
| All (n = 98) | Non-worrisome features (n = 88) | Worrisome features (n = 10) | P value | |
| a [mm/yr (IQR)] | 0 (-0.04-0.73) | 0 (-0.11-0.6) | 0.63 (-0.11-1.275) | 0.14156 |
| b [mm/yr (IQR)] | 0 (0-1.33) | 0 (0-1.09) | 1.55 (-0.53-3.52) | 0.0394 |
| c [mm/yr (IQR)] | 0 (0-0.99) | 0 (-0.29-0.71) | 1.96 (0.64-2.47) | 0.00152 |
| Volume [cm3/yr (IQR)] | 0 (-0.01-0.27) | 0 (-0.02-0.17) | 1.12 (0.28-2.65) | 0.0001 |
Seventy-four patients had at least 3 follow-up timepoints, of which at least two were 12 mo apart and another one later in time. In this subgroup of patients, followed for a median time of 56.5 mo (IQR 30-80.25), 10 developed worrisome features. The first-year volume growth rate was higher in patients who developed worrisome features (P = 0.00634). The first-year diameter growth rate was also higher in patients with worrisome features at the end of follow-up but differences with patients who did not end up with worrisome features were not always significant. Data regarding this group of patients are reported in Table 8.
| All (n = 74) | Non-worrisome features (n = 64) | Worrisome features (n = 10) | P value | |
| a [mm (IQR)] | 0 (0-0.56) | 0 (0-0.5) | 0.47 (-0.11-1.1) | 0.22628 |
| b [mm (IQR)] | 0 (0-1.13) | 0 (0-0.14) | 0.4 (-1.12-2.81) | 0.64552 |
| c [mm (IQR)] | 0 (0-0.94) | 0 (0-0.74) | 1.4 (0-2.47) | 0.00932 |
| Volume [cm3 (IQR)] | 0 (-0.02-0.18) | 0 (-0.04-0.08) | 0.46 (0-1.62) | 0.00634 |
| Volume increased (%) | 35 (47.3) | 28 (43.7) | 7 (70.0) | 0.227942 |
| Volume unchanged or reduced (%) | 39 (52.7) | 36 (56.3) | 3 (30.0) | 0.227942 |
Pancreatic cystic neoplasms are often detected incidentally in patients who undergo abdomen CT scans or MRI for various indications[16-18]. IPMNs are the most frequently diagnosed pancreatic cystic neoplasms[1] and they come in several variants: main duct IPMN or type 1 (MD-IPMN), branch duct IPMN or type 2 (BD-IPMN), and mixed type[19]. IPMNs have a malignancy progression cumulative risk of 7%-24% in 10 years and percentages vary on an individual risk basis[2]. In particular, MD-IPMN may reach a malignancy risk of 60%, while in BD-IPMN the risk remains lower than 25% in both surgical and non-surgical series[20-22].
Current guidelines agree on surgically treating MD-IPMN and MT-IPMN and monitoring most BD-IPMN (CT scan, MRI, EUS)[3-5]. BD-IPMN follow-up may vary on the basis of some radiological characteristics of the cysts and on clinical and biochemical parameters. In particular, International Guidelines outlined some “high-risk stigmata” and “worrisome features”[4]. The former guide the physician to a surgical approach, while the latter require a tighter follow-up.
As already mentioned, the baseline characteristic which is considered most important by the scientific community is the dimension of the cyst of larger diameter and only the International Guidelines by Tanaka et al[4] include the cyst diameter growth rate as a worrisome feature. What is more, it is not always easy to identify which is the larger diameter especially when comparing different methods such as CT scan, MRI and EUS. In this study, we tried to find a new parameter that is easy to use and that brings more information about the evolutionary potential of BD-IPMNs: we identified it in the volume of the cysts. Cyst volume is a parameter with high reproducibility and low inter-observer variability[9] and in our opinion may allow the diagnostician to overcome some issues related to the flat measurement of the cyst.
In this study, we assess the diameter and volume growth rate of BD-IPMNs and evaluate its correlation with the development of worrisome features. We reviewed both CT and MRI images: many previous studies confirmed that diagnostic performance of contrast-enhanced CT and MRI is comparable without significant differences[23-25]. We retrospectively analysed the evolution of BD-IPMNs of 98 patients, who were referred to our tertiary referral centre for pancreatic diseases. Worrisome features appeared in 10.8% of patients, while no-one had high-risk stigmata, over a median 40.5-mo follow-up time. These data are in line with the current literature[26,27].
In our cohort, patients who developed worrisome features had larger cysts at baseline. In this group, cysts were significantly larger if we considered both diameters (for all diameters, P = 0.0035, P = 0.00652, P = 0.00424, respectively) and volume (P = 0.00222). Patients who developed worrisome features had a median baseline cyst volume of 5.8 cm3 and a final cyst volume of 10.17 cm3. These findings are in line with a previous study in which CT and MRI techniques were used to measure IPMNs volume: a volume > 10 cm3 was associated with malignancy[28]. However, this study did not assess volume changes over time but only a spot volume measurement.
In the group of patients who developed worrisome features, diameter growth rate was significantly higher if we considered latero-lateral and cranio-caudal diameters (1.55 mm/year vs 0.0 mm/year, P = 0.0394, and 1.96 mm/year vs 0.0 mm/year, P = 0.00152, respectively), but not antero-posterior diameters (0.63 mm/year vs 0.0 mm/year, P = 0.14156). In a recent study by Kolb et al[7], 188 patients were followed-up for a median 55-mo period and 12 out of all patients developed worrisome features. They measured cysts diameter on both the axial and coronal plans on X and Y axis and cyst growth rate was higher in patients who developed worrisome features (axial X 2.84 mm/year vs 0.23 mm/year, P < 0.001; axial Y 1.02 mm/year vs 0.02 mm/year, P = 0.033; coronal X 1.21 mm/year vs 0.19 mm/year, P = 0.001, coronal Y 1.56 mm/year vs 0.00 mm/year, P < 0.001). The larger population and the longer median follow-up period may have contributed to improving statistical significance in their work. A similar result was obtained in a previous surgical cohort (4.1 mm/year vs 1.0 mm/year, P = 0.001)[6].
The peculiarity of our work was the measurement of cyst volume over time. In our cohort, total growth and growth rate were significantly higher in patients who developed worrisome features if compared to patients who did not (P < 0.00001 and P = 0.0001, respectively). To our knowledge, this is the first study in which baseline volume and particularly volume total growth and growth rate are correlated with development of worrisome features in patients with BD-IPMN.
Beyond the raw data of annual growth, we set out to identify a parameter that allows the early prediction of development of worrisome features in the medium-long term: we found out that patients who developed worrisome features had a higher first-year cyst volume growth if compared with patients who did not (0.46 cm3/year vs 0.0 cm3/year, P = 0.00634). These data are notable if we consider that the diameter growth rate was not significantly different between the two groups, except for the cranio-caudal one (P = 0.22628, P = 0.64552, P = 0.00932). To our knowledge, this is a unique result as this is the first study to prove that cyst volume growth in the first year predicts development of worrisome features in patients with BD-IPMN without worrisome features at baseline. This could be an important tool to add to the current knowledge to improve the management of low-risk IPMNs.
In summary, as opposed to diameter growth, the cyst volume growth (total, annual and first-year growth) was consistently greater in patients who developed worrisome features. The diameter growth rate was also significantly correlated in some cases, but not of all diameters at once, and not always of the largest diameter. Therefore, the flat growth rate could be misinterpreted if it is not the largest diameter that grows, or when the cysts have an irregular shape which is hard to measure in a reproducible way. The volume measurement could overcome these issues. In fact, the volume has a unique size instead of diameters that are virtually endless. For these reasons, volume measurement could be more comparable than diameters. Recently, Pandey et al[9] demonstrated that measurement of volume is feasible and reproducible and can be considered as an alternative to diameter measurement. In their work, they used both a manual and a semiautomatic technique of measurement while we used a manual technique.
This study has some limitations. First, the retrospective design could not allow us to set pre-determined timepoints of imaging follow-up. We solved this problem by assessing growth rate rather than the total growth. Moreover, we could not assess the presence of atypia or dysplasia as histology was not available for our patients unless they underwent surgery. Third, we could not carry out a proper statistical analysis involving the characteristics of the cyst fluid as FNA was not routinely performed in our patients. However, our study was meant to search for characteristics that predict the development of worrisome features so clinical, radiological and biochemical parameters were sufficient for our purposes. Finally, since BD-IPMNs grow very slowly[29,30], a possible criticism could be addressed to the length of our cohort follow-up median time (40.5 mo vs 55 mo in the study by Kolb et al[7]). It must be said that small increases in diameter can result in bigger volume variations so a shorter follow-up is justified. Indeed, the first-year volume growth was superior to diameter growth in predicting the development of worrisome features in our cohort. Anyway, a prospective extension of our study is ongoing to confirm our results.
Our study confirms that BD-IPMN higher cyst growth rate is associated with a higher risk of developing worrisome features. Moreover, we proved that cyst volume growth rate is also related to the emergence of worrisome features and that the cyst volume increase in the first year of follow-up is an early predictor of the development of worrisome features. More specifically, our data show that in the first year of follow-up, volume measurement is more accurate than diameter alone for risk stratification. This suggests that measuring cyst volume routinely could be a useful tool to monitor low-risk IPMNs. Further studies, comprehensive of a larger pool of patients and a longer follow-up time are needed to corroborate these data and to understand whether our findings could influence routine clinical practice.
Branch duct-intraductal papillary mucinous neoplasms (BD-IPMNs) are the most common pancreatic cystic tumours. Cyst diameter growth rate is one of the parameters that current guidelines take into account to predict the development of malignant features in patients with BD-IPMN. However, to date, there are no studies investigating the correlation between cyst volume growth rate and the risk of BD-IPMNs malignant degeneration.
The optimal surveillance protocol for BD-IPMNs has not been established as there is lack of agreement on when follow-up should be intensified or interrupted mainly due to the slow growth rate of BD-IPMNs. We propose a more precise tool for the measurement of BD-IPMNs which allows an early prediction of the development of worrisome features.
The primary objective of our research was to assess the volume growth rate of BD-IPMNs without worrisome features or high-risk stigmata at baseline and to evaluate its correlation with the development of worrisome features or high-risk stigmata during follow-up. We also aimed to evaluate the impact of measuring volume vs diameter growth rate and to test the ability of first-year volume growth rate to predict the development of worrisome features or high-risk stigmata.
We measured diameter in CT-scans and MRI on three planes, while we calculated the volume by manual segmentation: the volume of the cyst was determined by drawing a region of interest along the edge of the neoplasm on each consecutive slice covering the whole lesion; therefore, a three-dimensional volume of interest was finally obtained with the calculated value expressed in cm3. Changes in size over time and development of worrisome features or high-risk stigmata were measured.
Ninety-eight patients were evaluated across a 40.5-mo median follow-up time, of which 10 developed worrisome features, while none developed high-risk stigmata. Baseline volume was larger, and volume and first-year volume growth rate were higher in patients who developed worrisome features than in patients who did not. Baseline diameter was larger in patients who developed worrisome features. Diameter growth rate was higher as well but the difference did not always reach statistical significance.
The measurement of baseline volume and of its variation over time is a reliable tool for the follow-up of BD-IPMNs. Particularly, in the first year of BD-IPMNs follow-up, volume measurement is more accurate than diameter alone for risk stratification.
We suggest that measuring cyst volume routinely could be a useful tool to monitor low-risk IPMNs. Larger cohorts of patients and a longer follow-up time are needed to corroborate these data and to understand whether our findings could influence routine clinical practice.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Società Italiana Di Gastroenterologia Ed Endoscopia Digestiva.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Jin ZD, China; Kang KJ, South Korea S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Gong ZM
| 1. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2439] [Article Influence: 487.8] [Reference Citation Analysis (3)] |
| 2. | Choi SH, Park SH, Kim KW, Lee JY, Lee SS. Progression of Unresected Intraductal Papillary Mucinous Neoplasms of the Pancreas to Cancer: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2017;15:1509-1520.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 3. | Vege SS, Ziring B, Jain R, Moayyedi P; Clinical Guidelines Committee; American Gastroenterology Association. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:819-822; quize12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 759] [Article Influence: 75.9] [Reference Citation Analysis (1)] |
| 4. | Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, Salvia R, Shimizu Y, Tada M, Wolfgang CL. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 1156] [Article Influence: 144.5] [Reference Citation Analysis (1)] |
| 5. | European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 2018;67:789-804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1006] [Cited by in RCA: 895] [Article Influence: 127.9] [Reference Citation Analysis (1)] |
| 6. | Kang MJ, Jang JY, Kim SJ, Lee KB, Ryu JK, Kim YT, Yoon YB, Kim SW. Cyst growth rate predicts malignancy in patients with branch duct intraductal papillary mucinous neoplasms. Clin Gastroenterol Hepatol. 2011;9:87-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 7. | Kolb JM, Argiriadi P, Lee K, Liu X, Bagiella E, Gupta S, Lucas AL, Kim MK, Kumta NA, Nagula S, Sarpel U, DiMaio CJ. Higher Growth Rate of Branch Duct Intraductal Papillary Mucinous Neoplasms Associates With Worrisome Features. Clin Gastroenterol Hepatol. 2018;16:1481-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Jang JY, Park T, Lee S, Kim Y, Lee SY, Kim SW, Kim SC, Song KB, Yamamoto M, Hatori T, Hirono S, Satoi S, Fujii T, Hirano S, Hashimoto Y, Shimizu Y, Choi DW, Choi SH, Heo JS, Motoi F, Matsumoto I, Lee WJ, Kang CM, Han HS, Yoon YS, Sho M, Nagano H, Honda G, Kim SG, Yu HC, Chung JC, Nagakawa Y, Seo HI, Yamaue H. Proposed Nomogram Predicting the Individual Risk of Malignancy in the Patients With Branch Duct Type Intraductal Papillary Mucinous Neoplasms of the Pancreas. Ann Surg. 2017;266:1062-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 9. | Pandey P, Pandey A, Varzaneh FN, Ghasabeh MA, Fouladi D, Khoshpouri P, Shao N, Zarghampour M, Hruban RH, Canto M, O'Broin-Lennon AM, Kamel IR. Are pancreatic IPMN volumes measured on MRI images more reproducible than diameters? Eur Radiol. 2018;28:2790-2800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Kim SY, Lee JM, Kim SH, Shin KS, Kim YJ, An SK, Han CJ, Han JK, Choi BI. Macrocystic neoplasms of the pancreas: CT differentiation of serous oligocystic adenoma from mucinous cystadenoma and intraductal papillary mucinous tumor. AJR Am J Roentgenol. 2006;187:1192-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Ciprani D, Morales-Oyarvide V, Qadan M, Hank T, Weniger M, Harrison JM, Rodrigues C, Horick NK, Mino-Kenudson M, Ferrone CR, Warshaw AL, Lillemoe KD, Fernández-Del Castillo C. An elevated CA 19-9 is associated with invasive cancer and worse survival in IPMN. Pancreatology. 2020;20:729-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Zhang X, Zhong L, Zhang B, Zhang L, Du H, Lu L, Zhang S, Yang W, Feng Q. The effects of volume of interest delineation on MRI-based radiomics analysis: evaluation with two disease groups. Cancer Imaging. 2019;19:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Granata V, Fusco R, Barretta ML, Picone C, Avallone A, Belli A, Patrone R, Ferrante M, Cozzi D, Grassi R, Izzo F, Petrillo A. Radiomics in hepatic metastasis by colorectal cancer. Infect Agent Cancer. 2021;16:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 14. | Nazari M, Shiri I, Zaidi H. Radiomics-based machine learning model to predict risk of death within 5-years in clear cell renal cell carcinoma patients. Comput Biol Med. 2021;129:104135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 15. | Sepehri S, Tankyevych O, Iantsen A, Visvikis D, Hatt M, Cheze Le Rest C. Accurate Tumor Delineation vs. Rough Volume of Interest Analysis for 18F-FDG PET/CT Radiomics-Based Prognostic Modeling inNon-Small Cell Lung Cancer. Front Oncol. 2021;11:726865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Laffan TA, Horton KM, Klein AP, Berlanstein B, Siegelman SS, Kawamoto S, Johnson PT, Fishman EK, Hruban RH. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 724] [Cited by in RCA: 658] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 17. | Lee KS, Sekhar A, Rofsky NM, Pedrosa I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol. 2010;105:2079-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 436] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 18. | Pezzilli R, Buscarini E, Pollini T, Bonamini D, Marchegiani G, Crippa S, Belfiori G, Sperti C, Moletta L, Pozza G, De Nucci G, Manes G, Mandelli ED, Casadei R, Ricci C, Alicante S, Vattiato C, Carrara S, Di Leo M, Fabbri C, Giovanelli S, Barresi L, Tacelli M, Mirante VG, Conigliaro R, Antonini F, Macarri G, Frulloni L, De Marchi G, Sassatelli R, Cecinato P, Del Vecchio Blanco G, Galli A, Pezzullo A, Fantin A, Graffeo M, Frego M, Stillittano D, Monica F, Germanà B, Capurso G, Quartini M, Veneroni L, Cannizzaro R, Falconi M. Epidemiology, clinical features and diagnostic work-up of cystic neoplasms of the pancreas: Interim analysis of the prospective PANCY survey. Dig Liver Dis. 2020;52:547-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Klibansky DA, Reid-Lombardo KM, Gordon SR, Gardner TB. The clinical relevance of the increasing incidence of intraductal papillary mucinous neoplasm. Clin Gastroenterol Hepatol. 2012;10:555-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 20. | Perri G, Marchegiani G, Frigerio I, Dervenis CG, Conlon KC, Bassi C, Salvia R. Management of Pancreatic Cystic Lesions. Dig Surg. 2020;37:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Pergolini I, Sahora K, Ferrone CR, Morales-Oyarvide V, Wolpin BM, Mucci LA, Brugge WR, Mino-Kenudson M, Patino M, Sahani DV, Warshaw AL, Lillemoe KD, Fernández-Del Castillo C. Long-term Risk of Pancreatic Malignancy in Patients With Branch Duct Intraductal Papillary Mucinous Neoplasm in a Referral Center. Gastroenterology. 2017;153:1284-1294.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 181] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 22. | Crippa S, Capurso G, Cammà C, Fave GD, Castillo CF, Falconi M. Risk of pancreatic malignancy and mortality in branch-duct IPMNs undergoing surveillance: A systematic review and meta-analysis. Dig Liver Dis. 2016;48:473-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 23. | Choi SY, Kim JH, Yu MH, Eun HW, Lee HK, Han JK. Diagnostic performance and imaging features for predicting the malignant potential of intraductal papillary mucinous neoplasm of the pancreas: a comparison of EUS, contrast-enhanced CT and MRI. Abdom Radiol (NY). 2017;42:1449-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Liu H, Cui Y, Shao J, Shao Z, Su F, Li Y. The diagnostic role of CT, MRI/MRCP, PET/CT, EUS and DWI in the differentiation of benign and malignant IPMN: A meta-analysis. Clin Imaging. 2021;72:183-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Huynh T, Ali K, Vyas S, Dezsi K, Strickland D, Basinski T, Chen DT, Jiang K, Centeno B, Malafa M, Klapman JB, Hodul PJ, Jeong D, Permuth JB. Comparison of imaging modalities for measuring the diameter of intraductal papillary mucinous neoplasms of the pancreas. Pancreatology. 2020;20:448-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Canto MI, Almario JA, Schulick RD, Yeo CJ, Klein A, Blackford A, Shin EJ, Sanyal A, Yenokyan G, Lennon AM, Kamel IR, Fishman EK, Wolfgang C, Weiss M, Hruban RH, Goggins M. Risk of Neoplastic Progression in Individuals at High Risk for Pancreatic Cancer Undergoing Long-term Surveillance. Gastroenterology. 2018;155:740-751.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 305] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 27. | Ciprani D, Weniger M, Qadan M, Hank T, Horick NK, Harrison JM, Marchegiani G, Andrianello S, Pandharipande PV, Ferrone CR, Lillemoe KD, Warshaw AL, Bassi C, Salvia R, Fernández-Del Castillo C. Risk of malignancy in small pancreatic cysts decreases over time. Pancreatology. 2020;20:1213-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Murayama S, Kimura W, Hirai I, Takasu N, Takeshita A, Moriya T. Volumetric and morphological analysis of intraductal papillary mucinous neoplasm of the pancreas using computed tomography and magnetic resonance imaging. Pancreas. 2011;40:876-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Crippa S, Bassi C, Salvia R, Malleo G, Marchegiani G, Rebours V, Levy P, Partelli S, Suleiman SL, Banks PA, Ahmed N, Chari ST, Fernández-Del Castillo C, Falconi M. Low progression of intraductal papillary mucinous neoplasms with worrisome features and high-risk stigmata undergoing non-operative management: a mid-term follow-up analysis. Gut. 2017;66:495-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 152] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 30. | Lee BS, Nguyen AK, Tekeste TF, Chang K, Girgis A, Adeyemo M, Hanna MS, Yao JF, Kwok KK, Giap AQ, Hunt GC, Chaya CT, Kao KT, Attam R, Ko A, Pio JR, Tovar S, Lim BS. Long-term follow-up of branch-duct intraductal papillary mucinous neoplasms with No change in first 5 Years of diagnosis. Pancreatology. 2021;21:144-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |