Published online Jun 16, 2022. doi: 10.12998/wjcc.v10.i17.5620
Peer-review started: December 8, 2021
First decision: March 11, 2022
Revised: March 17, 2022
Accepted: April 9, 2022
Article in press: April 9, 2022
Published online: June 16, 2022
Processing time: 182 Days and 14.7 Hours
There is significant heterogeneity between gastroesophageal varices (GOV2) and isolated gastric varices (IGV1). The data on the difference between GOV2 and IGV1 are limited.
To determine the etiology, clinical profiles, endoscopic findings, imaging signs, portosystemic collaterals in patients with GOV2 and IGV1.
Medical records of 252 patients with gastric fundal varices were retrospectively collected, and computed tomography images were analyzed.
Significant differences in routine blood examination, Child–Pugh classification and MELD scores were found between GOV2 and IGV1. The incidence of peptic ulcers in patients with IGV1 (26.55%) was higher than that of GOV2 (11.01%), while portal hypertensive gastropathy was more commonly found in patients with GOV2 (22.02%) than in those with IGV1 (3.54%). Typical radiological signs of cirrhotic liver were more commonly observed in patients with GOV2 than in those with IGV1. In patients with GOV2, the main afferent vessels were via the left gastric vein (LGV) (97.94%) and short gastric vein (SGV) (39.18%). In patients with IGV1, the main afferent vessels were via the LGV (75.61%), SGV (63.41%) and posterior gastric vein (PGV) (43.90%). In IGV1 patients with pancreatic diseases, spleno-gastromental-superior mesenteric shunt (48.15%) was a major collateral vessel. In patients with fundic varices, the sizes of gastric/esophageal varices were positively correlated with afferent vessels (LGVs and PGVs) and efferent vessels (gastrorenal shunts). The size of the esophageal varices was negatively correlated with gastrorenal shunts in GOV2 patients.
Significant heterogeneity in the etiology and vascular changes between GOV2 and IGV1 is useful in making therapeutic decisions.
Core Tip: These findings highlight the differences in the etiology, clinical profiles, endoscopic findings, imaging signs, portosystemic collaterals between patients with gastroesophageal varices and patients with isolated gastric varices. Knowledge of the etiology and portosystemic collaterals in our study is helpful in making therapeutic decisions.
- Citation: Song YH, Xiang HY, Si KK, Wang ZH, Zhang Y, Liu C, Xu KS, Li X. Difference between type 2 gastroesophageal varices and isolated fundic varices in clinical profiles and portosystemic collaterals. World J Clin Cases 2022; 10(17): 5620-5633
- URL: https://www.wjgnet.com/2307-8960/full/v10/i17/5620.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i17.5620
Gastric varices (GVs) are dilated submucosal veins in the stomach and represent a type of portosystemic shunt[1-4]. GVs are a life-threatening cause of upper gastrointestinal bleeding[2,3,5-8]. According to their location, GVs are classified as gastroesophageal varices (GOVs) and isolated gastric varices (IGVs)[2,9]. GOVs are divided into GOV1 (esophageal varices extending down to the cardia or the lesser curve of the stomach) and GOV2 (esophageal varices and fundic varices)[2,9,10]. IGVs are subdivided into IGV1 (fundic varices) and IGV2 (ectopic varices located anywhere in the stomach, such as in the body, antrum or pylorus)[3,9,10]. This classification, initially described by Sarin et al[9], was helpful in understanding the natural history and management of gastric varices[2]. Physiologically, GOV1 are a continuation of esophageal varices, and their vascular alternations and therapeutic strategies are similar to those of esophageal varices[2,10], and will not be further discussed in our study. Since the incidence of IGV2 and the morbidity of IGV2-induced bleeding are much lower than those of IGV1, patients with IGV2 were not enrolled in our research. Our study focused mainly on patients with IGV1 and GOV2, the so-called fundic varices. Obviously, there are some similarities between IGV1 and GOV2. The Sarin classification does not truly describe the heterogeneity in the etiology and vascular alternation. Thus, studies should be performed to determine the etiology, clinical profiles, and imaging signs in patients with GOV2 and IGV1. However, the data are limited. To obtain a better understanding of fundic varices (GOV2 and IGV1), a large sample of patients (119 patients with GOV2, 133 patients with IGV1) was enrolled, and then the etiology, clinical profiles, endoscopic findings, imaging signs, and portosystemic collateral veins in patients with fundic varices were investigated in our study. The data in our study are helpful in making therapeutic decisions.
Further details are provided in the supplement.
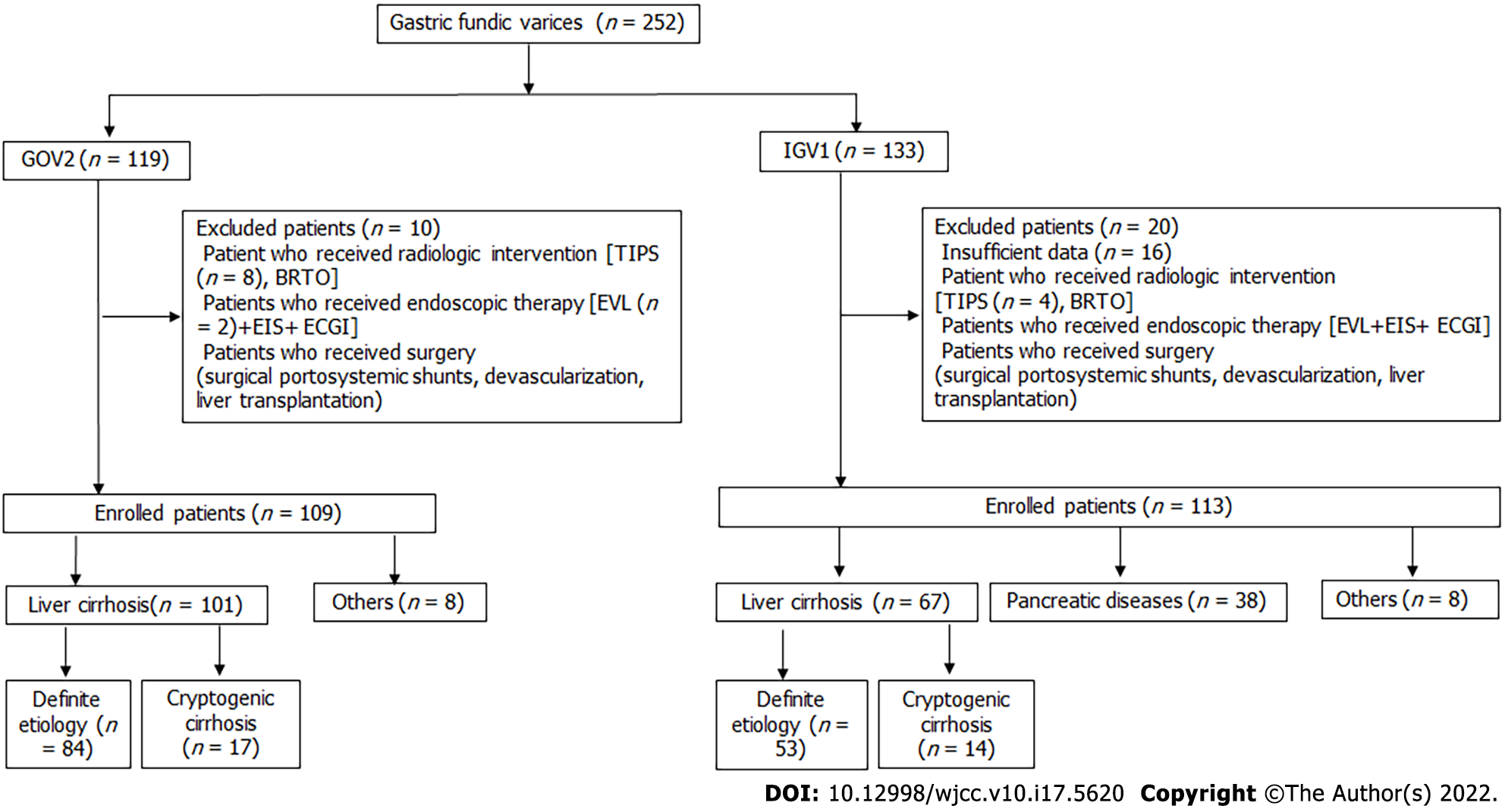
Our retrospective study was performed at Union Hospital of Huazhong University of Science and Technology (Wuhan, China). A total of 252 consecutive patients with gastric fundal varices (GOV2 and IGV1) were enrolled from October 2013 to November 2020. The inclusion criteria were as follows: (1) Patients with confirmed fundic varices after endoscopic examination; and (2) Stable hemodynamics for at least 5 d. The exclusion criteria were as follows: (1) Patients who received radiologic intervention [transjugular intrahepatic portosystemic shunt (TIPS) or balloon-occluded retrograde transvenous obliteration (BRTO)]; (2) Patients who received endoscopic therapy within 5 years [endoscopic variceal ligation (EVL), endoscopic injection sclerosis (EIS), endoscopic cyanoacrylate glue injection (ECGI)]; (3) Patients who received surgery (surgical portosystemic shunts, devascularization within 5 years); and (4) Patients who had insufficient data for further evaluation. The study was conducted according to the principles of the Declaration of Helsinki, and the protocol was approved by the ethics committee of Tongji Medical College, Huazhong University of Science and Technology (No. 2020-S216) and registered at www.chictr.org.cn (ChiCTR 2100042267).
Baseline clinical data were obtained from medical records, and then tabulated into a database. The pertinent data included etiology, age, sex, peripheral blood routine examination, biochemistry, Child–Pugh, MELD, endoscopic findings, imaging signs, and PSCV (computed tomography portal venography).
Images were acquired from one of the following CT scanners (Siemens Somatom Definition AS+, Siemens Somatom Definition, and Toshiba Aquilion ONE). Multidetector row CT portal venography (CTPV) was performed after intravenous administration of high-iodine-concentration contrast medium (iodixanol) (320 mg/mL) [Hengrui Medicine Co., Ltd., China]. All images were retrospectively and independently reviewed by two radiologists. First, cirrhotic-related radiological signs were evaluated. We assessed the following signs: the volume of esophageal/gastric varices using the regional growth method [11], the diameter of the main portal vein (1 cm distal to the junction of the splenic vein and superior mesenteric vein), splenic vein and superior mesenteric vein (1 cm proximal to the junction), portal vein thrombosis, cavernous transformation of the portal vein, gallbladder wall thickening (> 3 mm)[12-14], the longest dimension of the spleen on an axial or coronal view and the presence of ascites. Second, afferent veins and efferent veins of gastric fundal varices were determined. Third, we assessed the presence of other PSCVs, such as paraumbilical veins, intrahepatic portosystemic shunts (> 3 mm), and retroperitoneal shunts.
Continuous variables were expressed as the mean and standard deviation or median (25th-75th percentiles). Categorical variables are presented as n (%). The interobserver agreement between the two radiologists for determining radiological features was determined using kappa (κ) statistics[15-17]. The correlations of categorical or continuous variables were analyzed by Spearman’s correlation test. A P value less than 0.05 was considered to indicate statistical significance. Statistical analyses were performed using SPSS version 22.0 (IBM Inc., Armonk, NY, United States).
In this retrospective analysis, 252 consecutive patients with confirmed fundic varices were enrolled, and 30 patients were excluded (Figure 1). A total of 222 enrolled patients had liver cirrhosis (75.68%), pancreatic diseases (17.12%), and other diseases (7.21%) (Supplementary Table 1). Among patients with liver cirrhosis, the etiologies included hepatitis B/C (n = 106), alcoholic liver disease (n = 7), schistosomiasis (n = 9), autoimmune liver diseases (n = 11), cardiac cirrhosis (n = 1), Wilson diseases (n = 1), nonalcoholic fatty liver disease (n = 1), Budd-Chiari syndrome (n = 1) and cryptogenic cirrhosis (n = 31). Based on the Sarin classification, they were divided into the GOV2 group (109 patients) and IGV1 group (113 patients). Both GOV2 and IGV1 were primarily caused by liver cirrhosis (Supplementary Table 1). As shown in Supplementary Table 1, the results revealed that the constituent ratio of underlying diseases in cirrhotic patients with GOV2 was similar to that of IGV1 patients with liver cirrhosis. Importantly, the percentage of pancreatic diseases in the IGV1 group was greater than that in GOV2 patients.
Demographic data, laboratory tests (peripheral blood routine examination and biochemistry) and endoscopic findings of enrolled patients were determined, and the results are shown in Table 1. First, demographic data showed that the median age of the patients was 53 years old, and male patients were more frequently affected than female patients. No differences in sex or age were observed between the GOV2 group and IGV1 group. Second, the results of peripheral blood routine examination demonstrated that the values of erythrocytes, leukocytes and platelets were lower in GOV2 patients than of those in IGV1 patients. Additionally, among patients with IGV1, the values of erythrocytes, leukocytes and platelets were lower in cirrhotic patients than in patients with pancreatic diseases. Third, the biochemical parameters of the enrolled patients were also evaluated. No differences were observed in biomarkers of liver damage (ALT, AST) and cholestasis (ALP, γGT) between GOV2 patients and IGV1 patients. Biomarkers of liver synthetic ability (albumin, INR and cholesterol) in GOV2 patients were inferior to those of IGV1 patients (Table 1). As expected, biomarkers of liver damage, cholestasis and liver synthetic ability in cirrhotic patients with IGV1 were inferior to those of IGV1 patients resulting from pancreatic diseases. Fourth, the Child–Pugh classification and MELD score, the parameters for the prognosis of chronic liver disease, were calculated. The results showed that the percentage of Child–Pugh class A in GOV1 patients was lower than that of IGV2 caused by liver cirrhosis or pancreatic diseases. Moreover, MELD scores in GOV1 patients were higher than those in IGV1 patients. Finally, endoscopic findings were assessed. The incidence of peptic ulcers in patients with IGV1 (26.55%) was higher than that in GOV2 patients (11.01%); portal hypertensive gastropathy (PHG) was more commonly observed in patients with GOV2 (22.02%) than in those with IGV2 (3.54%). Interestingly, in cirrhotic patients, a lower incidence of peptic ulcers and a higher incidence of PHG were found in GOV2 than in IGV1.
| Variables | Total (222) | GOV2 (109) | IGV1 (113) | P value | IGV1 (113) | ||||||
| Liver cirrhosis (67) | Pancreatic diseases (38) | Others (8) | P value | ||||||||
| Gender, n (M/F) | 128/94 | 70/39 | 58/55 | 0.052 | 33/34 | 22/16 | 3/5 | 0.497 | |||
| Age, yr | 53.52 ± 12.20 | 53.00 ± 11.92 | 54.03 ± 12.51 | 0.532 | 55.61 ± 10.75 | 50.16 ± 14.74 | 59.13 ± 11.38 | 0.047 | |||
| Peripheral blood routine examination | |||||||||||
| Erythrocytes (1012/L) | 3.32 ± 0.78 | 3.20 ± 0.73 | 3.44 ± 0.81 | 0.022 | 3.47 ± 0.86 | 3.33 ± 0.75 | 3.83 ± 0.62 | 0.315 | |||
| Leukocyte (109/L) | 3.94 (2.56-5.62) | 3.28 (2.00-5.32) | 4.42 (3.29-5.86) | 0.001 | 3.85 (2.75-5.52) | 5.08 (3.79-7.61) | 4.50 (4.27-5.86) | 0.034 | |||
| Platelet (109/L) | 87.50 (57.00-148.75) | 70.00 (49.00-137.00) | 113.00 (73.00-156.00) | 0.001 | 84.00 (59.00-129.00) | 147.00 (96.00-181.00) | 117.00 (115.00-187.00) | < 0.001 | |||
| Biochemistry | |||||||||||
| ALT (U/L) | 28.00 (18.00-41.00) | 28.00 (20.00-39.25) | 27.00 (14.00-44.00) | 0.403 | 34.00 (21.00-48.00) | 18.00 (10.00-32.00) | 12.00 (7.00-23.00) | < 0.001 | |||
| AST (U/L) | 33.00 (22.00-49.00) | 35.00 (24.75-51.00) | 31.00 (19.00-47.50) | 0.095 | 41.00 (25.00-62.00) | 19.00 (15.00-31.00) | 20.00 (12.00-24.00) | < 0.001 | |||
| ALP (U/L) | 84.00 (64.00-126.00) | 85.00 (67.75-119.25) | 82.00 (59.00-128.00) | 0.515 | 92.00 (62.00-139.00) | 71.00 (51.00-113.00) | 82.00 (49.00-100.00) | 0.163 | |||
| γGT (U/L) | 35.00 (19.00-75.00) | 36.50 (20.00-75.00) | 33.00 (16.00-78.50) | 0.523 | 46.00 (25.00-84.00) | 21.00 (11.00-60.00) | 19.00 (8.00-23.00) | 0.001 | |||
| T-Bil (µmol/L) | 18.20 (13.00-26.00) | 19.70 (14.83-29.45) | 16.70 (10.65-24.05) | 0.003 | 18.00 (13.00-26.70) | 13.90 (9.80-19.40) | 12.50 (10.20-16.70) | 0.045 | |||
| Albumin (g/L) | 34.73 ± 6.43 | 33.54 ± 5.87 | 35.93 ± 6.77 | 0.007 | 34.64 ± 7.06 | 37.54 ± 5.87 | 39.40 ± 6.09 | 0.046 | |||
| INR | 1.22 (1.10-1.38) | 1.27 (1.14-1.44) | 1.18 (1.06-1.31) | 0.001 | 1.22 (1.08-1.39) | 1.12 (1.04-1.20) | 1.04 (0.99-1.43) | 0.021 | |||
| Cholesterol | 3.37 (2.68-4.15) | 3.14 (2.54-4.04) | 3.64 (2.82-4.35) | 0.036 | 3.55 (2.82-4.27) | 3.75 (2.96-4.77) | 3.43 (2.30-7.95) | 0.607 | |||
| Child-Pugh classification (A/B/C) | 113/70/4 | 48/49/1 | 65/21/3 | < 0.001 | 35/14/3 | 26/7/0 | 4/0/0 | 0.467 | |||
| MELD | 10.00 (8.00-11.00) | 10.00 (8.00-12.50) | 9.00 (7.00-11.00) | 0.006 | 10.00 (8.00-11.00) | 8.00 (7.00-9.00) | 7.00 (6.50-10.00) | 0.007 | |||
| Endoscopic findings | |||||||||||
| Portal hypertensive gastropathy | 28/222 (12.61%) | 24/109 (22.02%) | 4/113 (3.54%) | < 0.001 | 4/67 (5.97%) | 0 | 0 | / | |||
| Peptic ulcer | 42/222 (18.92%) | 12/109 (11.01%) | 30/113 (26.55%) | 0.003 | 26/67 (38.81%) | 4/38 (10.53%) | 0 | 0.001 | |||
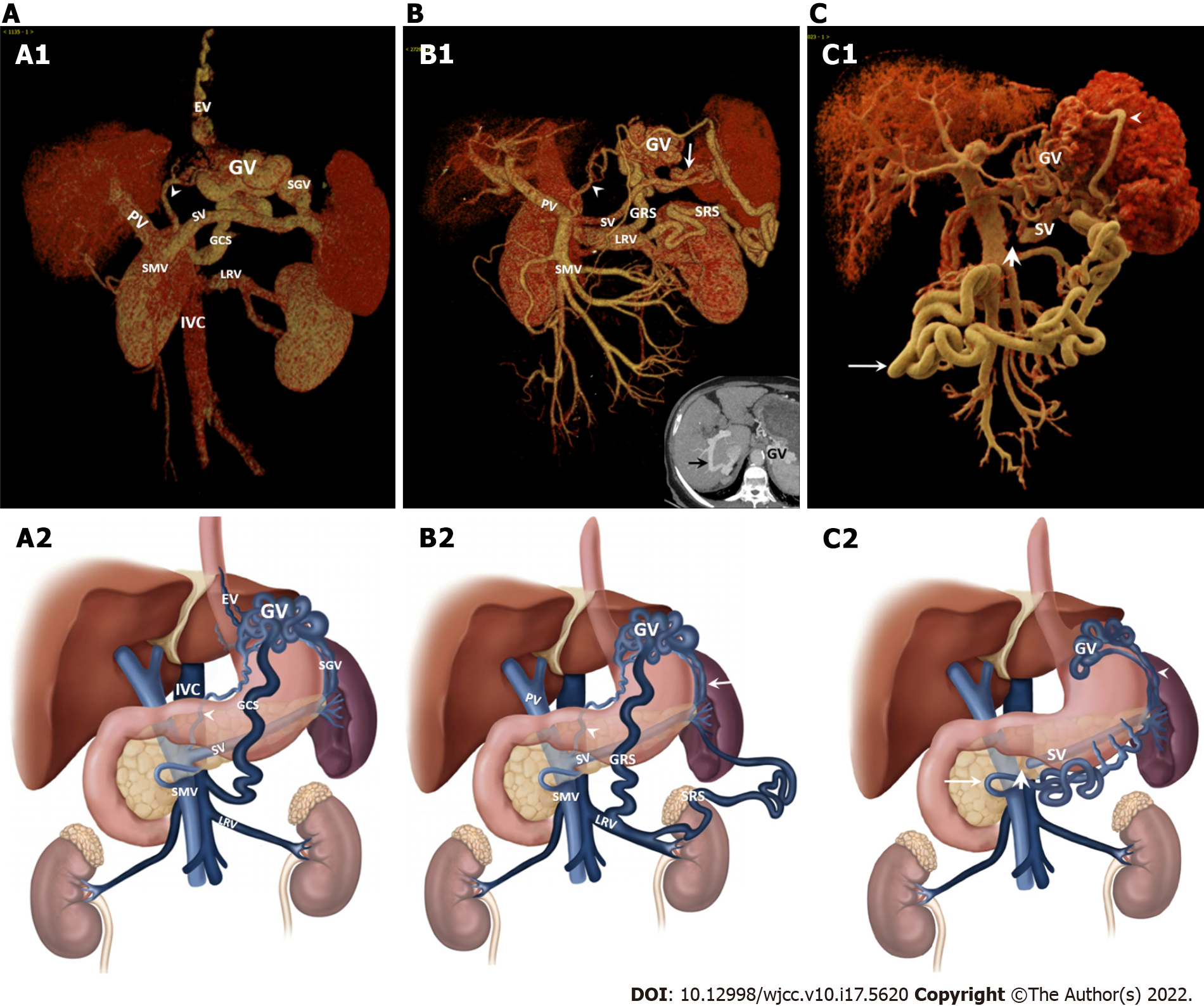
Radiological signs and portosystemic collateral vessels (PSCVs) were determined in patients with fundic varices using multidetector computed tomography (MDCT). Unfortunately, 43 cases were excluded because the patients had not received contrast CT scans or the image data were not obtained. First, typical radiological signs of liver cirrhosis were evaluated. Our study revealed gallbladder wall thickening in 42.07% of patients, ascites in 44.69% of cases, portal vein thrombosis in 18.99% of cases, and cavernous transformation of the portal vein in 11.73% of cases (Table 2). Importantly, the above radiologic signs were more commonly observed in patients with GOV2 than in those with IGV1. Moreover, the diameters of the main portal vein (PV), splenic vein and superior mesenteric vein (SMV) and the longest dimension of the spleen in the GOV2 group were larger than those in the IGV1 group. The mean volume of GVs in cirrhotic patients with IGV1 (10.00 mL) was larger than that of GOV2 (2.39 mL) patients and IGV1 patients caused by pancreatic diseases (4.12 mL). Second, afferent veins of GVs were reviewed. In patients with GOV2, gastric varices were principally supplied by the left gastric vein (LGV) (97.94%) and short gastric vein (SGV) (39.18%); in patients with IGV1, afferent veins of GVs were LGV (75.61%), SGV (63.41%) and posterior gastric vein (PGV) (43.90%). Third, efferent veins of gastric varices were also investigated. In patients with GOV2, gastric varices were drained by esophageal and para-esophageal varices (100%, data not shown), splenorenal shunts (11.34%) and gastrorenal shunts (21.65%); in patients with IGV1, efferent veins of cirrhotic patients with IGV1 were splenorenal shunts (14.00%) and gastrorenal shunts (78.00%) (Figure 2A and B). Interestingly, in IGV1 patients with pancreatic diseases, the splenogastromental-superior mesenteric shunt (48.15%) was a major collateral vessel due to splenic vein occlusion (Figure 2C). Finally, other PSCVs were assessed. Paraumbilical vein patency was more common in the GOV2 group (38.14%) than the IGV1 group (8.54%) (Table 2). A similar pattern was also observed in retroperitoneal shunts. Obvious intrahepatic portosystemic shunts were infrequent.
| Variables | Total (179) | GOV2 (97) | IGV1 (82) | P value | IGV1 (82) | |||
| Liver cirrhosis (50) | Pancreatic diseases (27) | Others (5) | P value | |||||
| Gallbladder wall thickening1 | 69/164 (42.07%) | 51/92 (55.43%) | 18/72 (25.00%) | < 0.001 | 10/46 (21.74%) | 7/21 (33.33%) | 1/5 (20.00%) | 0.678 |
| The longest dimension of spleen (cm) | 14.06 (12.44-15.93) | 15.74 ± 2.97 | 13.30 ± 2.25 | < 0.001 | 13.50 ± 2.30 | 12.90 ± 1.62 | 13.55 ± 4.91 | 0.529 |
| Ascites | 80/179 (44.69%) | 61/97 (62.89%) | 19/82 (23.17%) | < 0.001 | 13/50 (26.00%) | 6/27 (22.22%) | 0 | 0.583 |
| Portal vein thrombosis | 34/179 (18.99%) | 30/97 (30.93%) | 4/82 (4.88%) | < 0.001 | 2/50 (4.00%) | 2/27 (7.41%) | 0 | 0.697 |
| Cavernous transformation of portal vein | 21/179 (11.73%) | 18/97 (18.56%) | 3/82 (3.66%) | < 0.001 | 1/50 (2.00%) | 2/27 (7.41%) | 0 | 0.405 |
| The volume of gastric varices (mL) | 3.35 (1.62-8.55) | 2.39 (1.35-4.81) | 5.60 (2.35-15.68) | < 0.001 | 10.00 (3.14-21.50) | 4.12 (2.72-6.35) | 1.24 (0.63-8.17) | 0.005 |
| The diameter of main portal vein (mm) | 14.71 (12.19-16.59) | 15.17 (13.44-17.21) | 13.65 ± 2.76 | < 0.001 | 13.08 ± 2.40 | 14.38 ± 3.21 | 15.14 ± 2.29 | 0.066 |
| The diameter of splenic vein (mm) | 10.14 (8.21-12.38) | 11.00 (9.17-13.61) | 9.08 ± 2.43 | < 0.001 | 8.92 (7.19-10.67) | 8.14 (5.18-10.95) | 9.98 (9.42-12.37) | 0.312 |
| The diameter of superior mesenteric vein (mm) | 11.78 (10.09-13.44) | 12.63 (10.77-13.90) | 11.06 ± 2.11 | < 0.001 | 11.00 ± 2.02 | 11.23 ± 2.16 | 10.73 ± 3.07 | 0.845 |
| Afferent veins of gastric varices | ||||||||
| Left gastric vein | 157/179 (87.71%) | 95/97 (97.94%) | 62/82 (75.61%) | < 0.001 | 39/50 (78.00%) | 21/27 (77.78%) | 2/5 (40.00%) | 0.194 |
| Short gastric vein | 90/179 (50.28%) | 38/97 (39.18%) | 52/82 (63.41%) | 0.001 | 30/50 (60.00%) | 22/27 (81.48%) | 0 | 0.002 |
| Posterior gastric vein | 60/179 (33.52%) | 24/97 (24.74%) | 36/82 (43.90%) | 0.007 | 25/50 (50.00%) | 9/27 (33.33%) | 2/5 (40.00%) | 0.381 |
| Efferent veins of gastric varices | ||||||||
| Splenorenal shunt | 20/179 (11.17%) | 11/97 (11.34%) | 9/82 (10.98%) | 0.939 | 7/50 (14.00%) | 1/27 (3.70%) | 1/5 (20.00%) | 0.212 |
| Gastrorenal shunt | 65/179 (36.31%) | 21/97 (21.65%) | 44/82 (53.66%) | < 0.001 | 39/50 (78.00%) | 4/27 (14.81%) | 1/5 (20.00%) | < 0.001 |
| Other portosystemic collateral vessels | ||||||||
| Spleno-gastroomental-superior mesenteric shunt | 17/179 (9.50%) | 1/97 (1.03%) | 16/82 (19.51%) | < 0.001 | 3/50 (6.00%) | 13/27 (48.15%) | 0 | < 0.001 |
| Paraumbilical vein patency | 44/179 (24.58%) | 37/97 (38.14%) | 7/82 (8.54%) | < 0.001 | 7/50 (14.00%) | 0 | 0 | / |
| Intrahepatic portosystemic shunts | 7/179 (3.91%) | 4/97 (4.12%) | 3/82 (3.66%) | 1.000 | 3/50 (6.00%) | 0 | 0 | / |
| Retroperitoneal shunt | 52/179 (29.05%) | 37/97 (38.14%) | 15/82 (18.29%) | 0.004 | 6/50 (12.00%) | 8/27 (29.63%) | 1/5 (20.00%) | 0.131 |
To provide useful reference information for the management of gastric varices, the relationship among different PSCVs should be illustrated. First, we determined the correlation between the volumes of varices and PSCVs. In patients with GOV2, the volume of the gastric varices was positively correlated with afferent veins (the maximum diameter of the LGV and PGV) (Table 3). In addition, the volume of GVs was associated with efferent veins (the maximum diameter of the gastrorenal shunt). Interestingly, the volume of esophageal varices was negatively correlated with the gastrorenal shunt diameter (Table 3), which revealed a negative correlation between the two major divisions of efferent veins. Second, the correlation between afferent veins and efferent veins was evaluated in patients with GOV2. Only a positive correlation between the maximum diameter of the PGV and the maximum diameter of the gastrorenal shunt was found (Table 3). Third, we demonstrated no correlation among gastric varices with other PSCVs (intrahepatic portosystemic shunt, paraumbilical vein patency and retroperitoneal shunt) (Table 3). Finally, the results showed no correlation of the main portal vein with afferent/efferent veins of the GV, except for the diameter of the PGV (Table 3).
| Variables | The volume of varices (mL) | The diameter of main portal vein (mm) | Afferent veins | Efferent veins | Other portosystemic collateral vessels | |||||||
| The volume of EVs (mL) | The volume of GVs (mL) | Maximum diameter of LGV (mm) | Maximum diameter of PGV (mm) | Maximum diameter of SGV (mm) | Maximum diameter of SRS (mm) | Maximum diameter of GRS (mm) | Intrahepatic portosystemic shunts | Paraumbilical vein patency | Retroperitoneal shunt | |||
| Maximum diameter of LGV (mm) | Correlation coefficient | 0.411 | 0.372 | 0.052 | / | 0.099 | 0.307 | -0.374 | -0.018 | 0.061 | 0.081 | -0.039 |
| P value | 0.000 | 0.000 | 0.632 | / | 0.654 | 0.061 | 0.258 | 0.940 | 0.556 | 0.437 | 0.711 | |
| n | 92 | 90 | 88 | / | 23 | 38 | 11 | 20 | 95 | 95 | 95 | |
| Short gastric vein | Correlation coefficient | 0.139 | 0.066 | 0.013 | 0.132 | -0.038 | / | -0.179 | 0.268 | 0.046 | 0.022 | 0.065 |
| P value | 0.182 | 0.532 | 0.903 | 0.204 | 0.859 | / | 0.598 | 0.241 | 0.655 | 0.831 | 0.524 | |
| n | 94 | 92 | 90 | 95 | 24 | 38 | 11 | 21 | 97 | 97 | 97 | |
| Posterior gastric vein | Correlation coefficient | -0.055 | 0.378 | -0.239 | 0.199 | / | 0.100 | 0.298 | 0.520 | 0.121 | -0.106 | 0.091 |
| P value | 0.600 | 0.000 | 0.023 | 0.053 | / | 0.550 | 0.373 | 0.016 | 0.236 | 0.302 | 0.377 | |
| n | 94 | 92 | 90 | 95 | 24 | 38 | 11 | 21 | 97 | 97 | 97 | |
| Maximum diameter of splenorenal shunt (mm) | Correlation coefficient | 0.200 | -0.155 | -0.533 | -0.374 | 1.000 | 0.000 | / | / | / | -0.418 | 0.000 |
| P value | 0.580 | 0.650 | 0.139 | 0.258 | / | 1.000 | / | / | / | 0.200 | 1.000 | |
| n | 10 | 11 | 9 | 11 | 2 | 7 | / | 1 | 11 | 11 | 11 | |
| Maximum diameter of gastrorenal shunt (mm) | Correlation coefficient | -0.518 | 0.755 | -0.434 | -0.018 | 0.745 | 0.576 | / | / | 0.332 | -0.113 | -0.238 |
| P value | 0.023 | 0.000 | 0.072 | 0.940 | 0.013 | 0.082 | / | / | 0.141 | 0.625 | 0.298 | |
| n | 19 | 20 | 18 | 20 | 10 | 10 | 1 | / | 21 | 21 | 21 | |
The correlations among PSCVs in patients with IGV1 are shown in Table 4. First, the correlations between the volumes of gastric varices and efferent/afferent veins were determined, and the results showed that the volume of gastric varices was positively correlated with afferent veins (the maximum diameter of LGV and posterior gastric vein) and efferent veins (the maximum diameter of gastrorenal shunt). Second, the correlation between afferent veins and efferent veins was evaluated in patients with IGV1. The results revealed a positive correlation between the main afferent vessel (the diameter of gastrorenal shunts (GRS) and efferent veins (LGV, SGV and PGV)) (Table 4). Third, the results showed no correlations between major divisions of efferent/afferent veins and other portosystemic collateral vessels (intrahepatic portosystemic shunt, paraumbilical vein patency and retroperitoneal shunt). Finally, a negative correlation of the main portal vein with efferent veins (the gastrorenal shunt) was observed (Table 4).
| Variables | The volume of gastric varices (mL) | The diameter of main portal vein (mm) | Afferent veins | Efferent veins | Other portosystemic collateral vessels | |||||||
| Maximum diameter of LGV (mm) | Maximum diameter of SGV (mm) | Maximum diameter of PGV) (mm) | Maximum diameter of splenorenal shunt (mm) | Maximum diameter of gastrorenal shunt (mm) | Spleno-gastroomental-superior mesenteric shunt | Intrahepatic portosystemic shunts | Paraumbilical vein patency | Retroperitoneal shunt | ||||
| Maximum diameter of LGV (mm) | Correlation coefficient | 0.405 | 0.241 | / | 0.167 | 0.143 | 0.543 | 0.366 | 0.259 | -0.211 | -0.121 | 0.153 |
| P value | 0.001 | 0.065 | / | 0.291 | 0.506 | 0.266 | 0.036 | 0.042 | 0.100 | 0.350 | 0.236 | |
| n | 59 | 59 | / | 42 | 24 | 6 | 33 | 62 | 62 | 62 | 62 | |
| Maximum diameter of SGV (mm) | Correlation coefficient | 0.212 | 0.113 | 0.167 | / | 0.328 | -0.600 | 0.421 | 0.223 | 0.007 | -0.014 | 0.083 |
| P value | 0.135 | 0.432 | 0.291 | / | 0.215 | 0.285 | 0.026 | 0.111 | 0.963 | 0.919 | 0.559 | |
| n | 51 | 51 | 42 | / | 16 | 5 | 28 | 52 | 52 | 52 | 52 | |
| Posterior gastric vein | Correlation coefficient | 0.047 | 0.007 | 0.257 | -0.317 | / | 0.548 | -0.091 | -0.064 | 0.220 | 0.082 | 0.090 |
| P value | 0.682 | 0.949 | 0.044 | 0.022 | / | 0.127 | 0.555 | 0.571 | 0.047 | 0.467 | 0.422 | |
| n | 78 | 79 | 62 | 52 | 36 | 9 | 44 | 82 | 82 | 82 | 82 | |
| Maximum diameter of PGV (mm) | Correlation coefficient | 0.667 | 0.012 | 0.143 | 0.328 | / | 1.000 | 0.506 | 0.093 | -0.053 | -0.111 | -0.315 |
| P value | 0.000 | 0.946 | 0.506 | 0.215 | / | 0.000 | 0.014 | 0.588 | 0.758 | 0.521 | 0.061 | |
| n | 34 | 33 | 24 | 16 | / | 3 | 23 | 36 | 36 | 36 | 36 | |
| Splenorenal shunt | Correlation coefficient | 0.175 | -0.008 | 0.093 | -0.128 | 0.102 | / | -0.131 | 0.024 | -0.068 | 0.172 | 0.036 |
| P value | 0.126 | 0.945 | 0.472 | 0.365 | 0.555 | / | 0.395 | 0.830 | 0.541 | 0.122 | 0.750 | |
| n | 78 | 79 | 62 | 52 | 36 | 9 | 44 | 82 | 82 | 82 | 82 | |
| Maximum diameter of Splenorenal shunt (mm) | Correlation coefficient | 0.667 | -0.083 | 0.543 | -0.600 | 1.000 | / | -0.500 | -0.621 | / | 0.104 | -0.414 |
| P value | 0.071 | 0.831 | 0.266 | 0.285 | 0.000 | / | 0.667 | 0.074 | / | 0.791 | 0.268 | |
| n | 8 | 9 | 6 | 5 | 3 | / | 3 | 9 | 9 | 9 | 9 | |
| Gastrorenal shunt | Correlation coefficient | 0.173 | -0.245 | -0.123 | -0.148 | -0.062 | -0.456 | / | -0.419 | 0.177 | 0.102 | -0.141 |
| P value | 0.129 | 0.029 | 0.340 | 0.296 | 0.718 | 0.217 | / | 0.000 | 0.112 | 0.364 | 0.205 | |
| n | 78 | 79 | 62 | 52 | 36 | 9 | 44 | 82 | 82 | 82 | 82 | |
| Maximum diameter of gastrorenal shunt (mm) | Correlation coefficient | 0.735 | -0.242 | 0.366 | 0.421 | 0.506 | -0.500 | / | 0.043 | 0.018 | 0.149 | -0.037 |
| P value | 0.000 | 0.123 | 0.036 | 0.026 | 0.014 | 0.667 | / | 0.782 | 0.909 | 0.333 | 0.814 | |
| n | 44 | 42 | 33 | 28 | 23 | 3 | / | 44 | 44 | 44 | 44 | |
| Spleno-gastroomental-superior mesenteric shunt | Correlation coefficient | -0.025 | 0.314 | 0.259 | 0.223 | 0.093 | -0.621 | 0.043 | / | -0.096 | -0.150 | 0.245 |
| P value | 0.826 | 0.005 | 0.042 | 0.111 | 0.588 | 0.074 | 0.782 | / | 0.391 | 0.177 | 0.027 | |
| n | 78 | 79 | 62 | 52 | 36 | 9 | 44 | / | 82 | 82 | 82 | |
Although the incidence of bleeding from GVs is relatively low, bleeding is more severe and is associated with higher mortality[2,3,18]. In this study, 222 patients with fundic varices were enrolled, and the etiology, clinical profiles, imaging signs, and PSCVs were determined in patients with IGV1 and GOV2. The primary cause of fundic varices was liver cirrhosis. Left-side portal hypertension (LSPH) occurs as a result of narrowing and obstruction of the splenic vein secondary to pancreatitis, pancreatic cancer, and pancreatic pseudocysts, which usually results in the formation of isolated fundal varices[19]. Gastric varices were frequently supplied by LGVs, SGVs and PGVs; major efferent veins included esophageal varices, gastrorenal shunts, and splenorenal shunts. These findings were consistent with previous studies[1,3,4,20-22].
Obviously, there is substantial heterogeneity between IGV1 and GOV2. Liver cirrhosis is a major cause of GOV2, and the major etiologies of IGV1 include liver cirrhosis and pancreatic diseases. Cytopenia was frequently observed in patients with GOV2 compared with IGV1, which revealed that hypersplenism occurred more commonly in patients with GOV2. The constituent ratio of underlying diseases contributed to the difference in routine blood examination. In addition, cirrhotic patients with GOV2 had higher rates of hypersplenism than cirrhotic patients with IGV1. Simultaneously, abnormal liver function was more commonly observed in patients with GOV2. Normal liver function was observed in most of the patients with LSPH. The discrepancy between GOV2 and IGV1 was attributed to the constituent ratio of underlying diseases. Interestingly, PHG was more commonly observed in patients with GOV2 than in IGV1 patients. PHG, a complication of portal hypertension, is associated with portal venous pressure[23-25]. Patients with IGV1 have large gastrorenal shunts, so portal venous pressure in patients with IGV1 was lower than that in patients with GOV2[23,25]. In addition, the degree of liver dysfunction was correlated with the severity of PHG in cirrhotic patients[24]. High portal venous pressure and liver dysfunction resulted in a higher incidence of PHG in patients with GOV2. Interestingly, the incidence of peptic ulcers in patients with IGV1 was higher than that in GOV2 patients; HP infection, the use of NSAIDs, gastric mucosal blood flow, gastric mucosal barrier, epithelial renewal, and mucosa defense mechanisms are involved in ulcer formation. In patients with IGV1, gastrorenal shunts increased gastric submucosal shunting of blood away from the gastric mucosa, leading to reduced perfusion and accelerated ulcer formation[26,27].
Typical CT features of liver cirrhosis include morphologic changes of the liver, portal vein enlargement, portal venous thrombosis, cavernous transformation, splenomegaly, regenerative nodule, PSCVs, and ascites. Typical radiological signs were more commonly observed in patients with GOV2 than in those with IGV1. In addition to the constituent ratio of underlying diseases, the distinction between cirrhotic patients with GOV2 and IGV1 contributed to the differences in radiological signs. The afferents to GVs come from the LVG, SGV and PGV; GVs enter systemic veins through esophageal and paraesophageal varices, gastrorenal shunts, splenorenal shunts, etc. In patients with IGV1 caused by pancreatic diseases, fundic varices were supplied by SGV and PGV. More importantly, we first found that the splenogastromental-superior mesenteric shunt is a major collateral vessel.
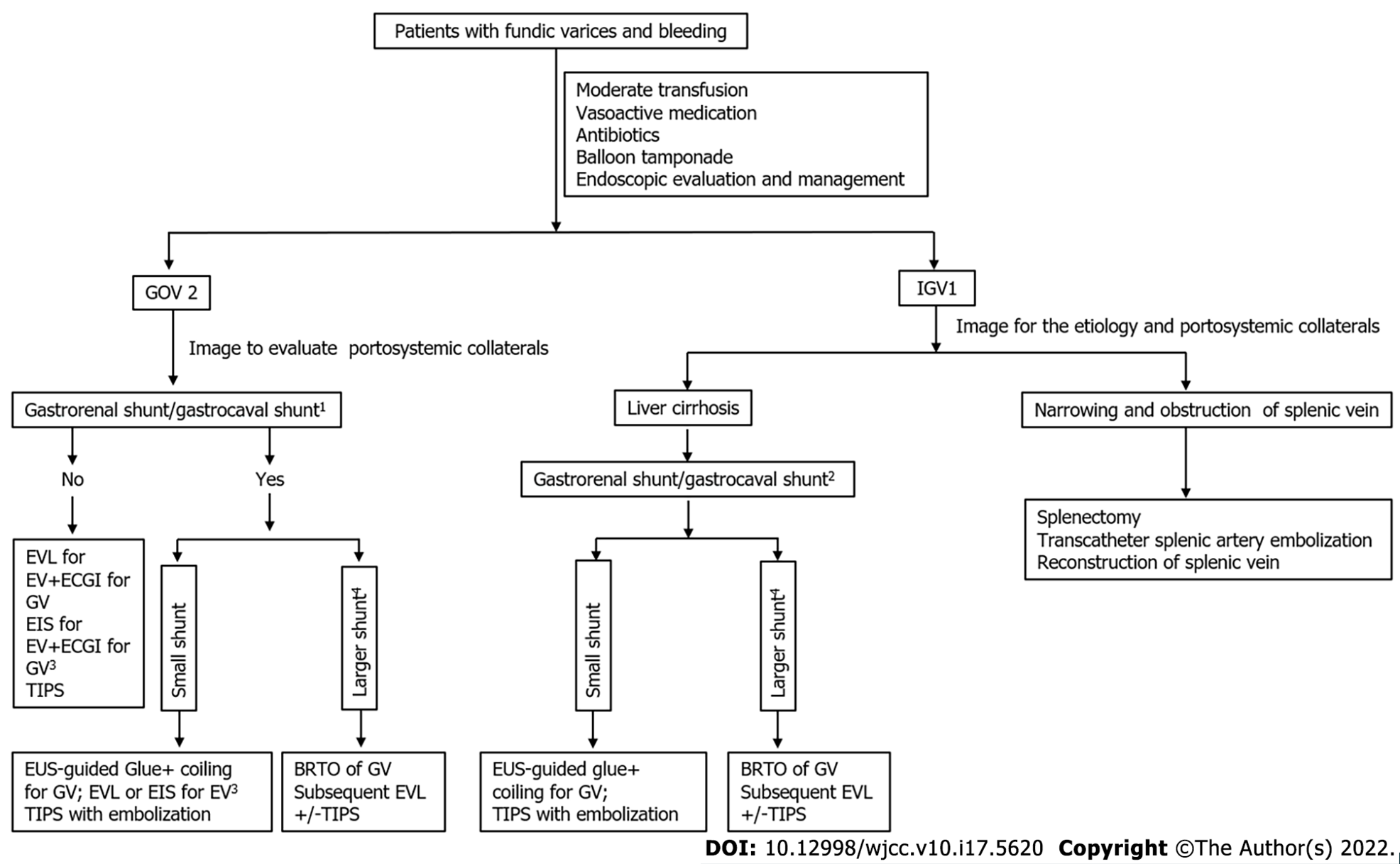
We first found that the size of varices was positively correlated with efferent/afferent vessels in patients with GOV2 or IGV1; in patients with GOV2, the size of esophageal varices was negatively correlated with gastrorenal shunt. When patients have gastrorenal shunts or gastrocaval shunts, endoscopic glue injection might result in distal systemic thromboembolic events, such as pulmonary embolism, acute kidney injury, obliteration of splenic or portal vein[3,4]. Thus, it is important to determine whether patients with gastric fundal varices have gastrorenal shunts. Our study showed that gastric varices drain mainly into the inferior vena cava via gastrorenal shunts or direct gastrocaval shunts in IGV1 caused by liver cirrhosis. Importantly, our research revealed that the size of esophageal varices was negatively correlated with the gastrorenal shunt diameter in patients with GOV2. This result indicated that gastrorenal shunts probably occurred in GOV2 patients with small esophageal varices. All these results indicated that gastrorenal shunts or gastrocaval shunts occurred frequently in GOV2 patients with small esophageal varices and IGV1 patients with liver cirrhosis. Thus, endoscopic glue injection should not be performed in these patients (Figure 3). For patients with large shunts, cardiofundal GVs with lower portal pressures reduced the efficacy of TIPS in bleeding control. Additionally, a large GRS or gastrocaval shunt increased the risks of TIPS (hepatic encephalopathy and hepatic ischemia)[3]. BRTO with subsequent EVL/EIS or TIPS should be considered for the management of gastric varices in these patients (Figure 3). For patients with small shunts, endoscopic ultrasound-guided glue coil placement and glue injection and TIPS with embolization are preferred strategies (Figure 3). In IGV patients with splenic vein obstruction, splenectomy and transcatheter splenic artery embolization are good therapeutic choices (Figure 3). In addition, the correlation of PSCVs with clinical profiles was determined in fundic varices; unfortunately, no correlation was found between PSCVs and clinical profiles (Supplementary Tables 2 and 3).
Our study had several limitations. First, it was a single center retrospective study, not a prospective, randomized, multicenter study. Second, the hepatic venous pressure gradient (HVPG) was not determined in our study. Fortunately, HVPG measurement is a valuable method to evaluate the severity of portal hypertension, predict outcomes, and guide therapeutic decisions. Our conclusions are reliable without HVPG measurement because clinical profiles and imaging findings are our research priorities. Finally, follow-up data could not be provided since the retrospective study involved a 7-year span.
These findings highlight the differences in the etiology, clinical profiles, endoscopic findings, imaging signs, and portosystemic collaterals between patients with GOV2 and patients with IGV1. Our study would be helpful in making therapeutic decisions. Further studies should be performed to confirm our conclusion based on large samples, and follow-up data should be provided based on the development of suitable therapeutic strategies in the future.
There is significant heterogeneity between gastroesophageal varices (GOV2) and isolated gastric varices (IGV1). The data on the difference between GOV2 and IGV1 are limited.
The Sarin classification does not truly describe the heterogeneity in the etiology and vascular alternation. Thus, studies should be performed to determine the etiology, clinical profiles, and imaging signs in patients with GOV2 and IGV1.
The Sarin classification does not truly describe the heterogeneity in the etiology To obtain a better understanding of fundic varices (GOV2 and IGV1), a large sample of patients (119 patients with GOV2, 133 patients with IGV1) was enrolled, and then the etiology, clinical profiles, endoscopic findings, imaging signs, and portosystemic collateral veins in patients with fundic varices were investigated in our study. The data in our study are helpful in making therapeutic decisions.
The authors retrospectively collected the medical records of 252 patients with gastric fundal varices, and analyzed computed tomography images.
Significant differences in the etiology, blood routine examination, liver function, the incidence of peptic ulcer and the morbidity of portal hypertensive gastropathy were found between GOV2 and IGV1. Typical radiological signs of liver cirrhosis were more commonly observed in patients with GOV2 compared with IGV1. Spleno-gastroomental-superior mesenteric shunt was a major collateral vessel of IGV1 patients caused by the obstruction of the splenic vein. Gastro-renal shunt or gastrocaval shunt occurred in GOV2 patients with small size of esophageal varices and IGV1 patients caused by liver cirrhosis
These findings highlight the differences in the etiology, clinical profiles, endoscopic findings, imaging signs, portosystemic collaterals between patients with GOV2 and patients with IGV1. Knowledge of the etiology and portosystemic collaterals in our study is helpful in making therapeutic decisions.
A multicenter study should be performed to determine the differences in the etiology, clinical profiles, endoscopic findings, imaging signs, portosystemic collaterals between patients with GOV2 and patients with IGV1. A prospective RCT study should be performed to determine therapeutic interventions for patients with GOV2 or IGV1.
We thank Mr. Hai-Tao Shang and Tao Bai (Union Hospital, Tongji Medical College, Huazhong University of Science and Technology) for helpful suggestions in statistical analysis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kao JT, Taiwan; Yankol Y, United States S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Sharma M, Rameshbabu CS. Collateral pathways in portal hypertension. J Clin Exp Hepatol. 2012;2:338-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 2. | Garcia-Pagán JC, Barrufet M, Cardenas A, Escorsell A. Management of gastric varices. Clin Gastroenterol Hepatol. 2014;12:919-28.e1; quiz e51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 3. | Henry Z, Patel K, Patton H, Saad W. AGA Clinical Practice Update on Management of Bleeding Gastric Varices: Expert Review. Clin Gastroenterol Hepatol. 2021;19:1098-1107.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 4. | Philips CA, Ahamed R, Rajesh S, George T, Mohanan M, Augustine P. Beyond the scope and the glue: update on evaluation and management of gastric varices. BMC Gastroenterol. 2020;20:361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Tripathi D, Ferguson JW, Therapondos G, Plevris JN, Hayes PC. Review article: recent advances in the management of bleeding gastric varices. Aliment Pharmacol Ther. 2006;24:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Mishra SR, Sharma BC, Kumar A, Sarin SK. Primary prophylaxis of gastric variceal bleeding comparing cyanoacrylate injection and beta-blockers: a randomized controlled trial. J Hepatol. 2011;54:1161-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 142] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 7. | Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1229] [Cited by in RCA: 1210] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 8. | Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1441] [Article Influence: 180.1] [Reference Citation Analysis (3)] |
| 9. | Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology. 1992;16:1343-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 847] [Article Influence: 25.7] [Reference Citation Analysis (42)] |
| 10. | Ryan BM, Stockbrugger RW, Ryan JM. A pathophysiologic, gastroenterologic, and radiologic approach to the management of gastric varices. Gastroenterology. 2004;126:1175-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 224] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 11. | Ahmed R, Kiyosue H, Maruno M, Matsumoto S, Mori H. Coexistence of "extra-gastric afferent-efferent direct connection" with gastric varices: CT evaluation and clinical significance. Abdom Radiol (NY). 2019;44:2699-2707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Kan X, Ye J, Rong X, Lu Z, Li X, Wang Y, Yang L, Xu K, Song Y, Hou X. Diagnostic performance of Contrast-enhanced CT in Pyrrolizidine Alkaloids-induced Hepatic Sinusoidal Obstructive Syndrome. Sci Rep. 2016;6:37998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Yang XQ, Ye J, Li X, Li Q, Song YH. Pyrrolizidine alkaloids-induced hepatic sinusoidal obstruction syndrome: Pathogenesis, clinical manifestations, diagnosis, treatment, and outcomes. World J Gastroenterol. 2019;25:3753-3763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (3)] |
| 14. | Liu F, Rong X, Guo H, Xu D, Liu C, Meng L, Yang X, Guo T, Kan X, Song Y. Clinical characteristics, CT signs, and pathological findings of Pyrrolizidine alkaloids-induced sinusoidal obstructive syndrome: a retrospective study. BMC Gastroenterol. 2020;20:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Li X, Yang X, Xu D, Li Q, Kong X, Lu Z, Bai T, Xu K, Ye J, Song Y. Magnetic Resonance Imaging Findings in Patients With Pyrrolizidine Alkaloid-Induced Hepatic Sinusoidal Obstruction Syndrome. Clin Gastroenterol Hepatol. 2017;15:955-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Guo T, Li X, Yang X, Kong X, Liu H, Bai T, Xu K, Ye J, Song Y. Gadoxetic Acid-Enhanced Hepatobiliary-Phase Magnetic Resonance Imaging for Pyrrolizidine Alkaloid-Induced Hepatic Sinusoidal Obstruction Syndrome and Association with Liver Function. Sci Rep. 2019;9:1231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Lei P, Zhang L, Han P, Zheng C, Tong Q, Shang H, Yang F, Hu Y, Li X, Song Y. Liver injury in patients with COVID-19: clinical profiles, CT findings, the correlation of the severity with liver injury. Hepatol Int. 2020;14:733-742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 18. | Mishra SR, Chander Sharma B, Kumar A, Sarin SK. Endoscopic cyanoacrylate injection vs beta-blocker for secondary prophylaxis of gastric variceal bleed: a randomised controlled trial. Gut. 2010;59:729-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 19. | Köklü S, Coban S, Yüksel O, Arhan M. Left-sided portal hypertension. Dig Dis Sci. 2007;52:1141-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 132] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 20. | Zhao LQ, He W, Ji M, Liu P, Li P. 64-row multidetector computed tomography portal venography of gastric variceal collateral circulation. World J Gastroenterol. 2010;16:1003-1007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Moubarak E, Bouvier A, Boursier J, Lebigot J, Ridereau-Zins C, Thouveny F, Willoteaux S, Aubé C. Portosystemic collateral vessels in liver cirrhosis: a three-dimensional MDCT pictorial review. Abdom Imaging. 2012;37:746-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Nardelli S, Riggio O, Gioia S, Puzzono M, Pelle G, Ridola L. Spontaneous porto-systemic shunts in liver cirrhosis: Clinical and therapeutical aspects. World J Gastroenterol. 2020;26:1726-1732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (3)] |
| 23. | Watanabe K, Kimura K, Matsutani S, Ohto M, Okuda K. Portal hemodynamics in patients with gastric varices. A study in 230 patients with esophageal and/or gastric varices using portal vein catheterization. Gastroenterology. 1988;95:434-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 24. | Bayraktar Y, Balkanci F, Uzunalimoglu B, Gokoz A, Koseoglu T, Batman F, Gurakar A, Van Thiel DH, Kayhan B. Is portal hypertension due to liver cirrhosis a major factor in the development of portal hypertensive gastropathy? Am J Gastroenterol. 1996;91:554-558. [PubMed] |
| 25. | Iwao T, Toyonaga A, Oho K, Sakai T, Tayama C, Masumoto H, Sato M, Nakahara K, Tanikawa K. Portal-hypertensive gastropathy develops less in patients with cirrhosis and fundal varices. J Hepatol. 1997;26:1235-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (1)] |
| 26. | Saad RJ, Chey WD. Peptic ulcer disease in patients with chronic liver disease: looking beyond bugs and drugs. Gastrointest Endosc. 2005;62:357-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Venkatesh PG, Parasa S, Njei B, Sanaka MR, Navaneethan U. Increased mortality with peptic ulcer bleeding in patients with both compensated and decompensated cirrhosis. Gastrointest Endosc. 2014;79:605-14.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |