Published online Jun 16, 2022. doi: 10.12998/wjcc.v10.i17.5595
Peer-review started: November 23, 2021
First decision: January 22, 2022
Revised: January 30, 2022
Accepted: April 3, 2022
Article in press: April 3, 2022
Published online: June 16, 2022
Processing time: 197 Days and 16.2 Hours
Glioblastoma (GBM) is one of the most common and aggressive primary malignant brain tumors with severe symptoms and a poor prognosis. Leptomeningeal dissemination (LMD) is a serious complication of GBM that often results in dire outcomes. There is currently no effective treatment.
To estimate the clinical outcomes of combination therapy in GBM patients with LMD
A retrospective analysis was conducted using data collected from GBM patients diagnosed with LMD from January 2012 to December 2019 at our institution. All these patients had received at least one cycle of a combination therapy consisting of intrathecal methotrexate (MTX) and systemic chemotherapy. Clinical and pathological data were analyzed to explore the outcome of GBM patients with LMD and to determine the most effective treatment.
Twenty-six patients were enrolled in this study. The median time from GBM diagnosis to LMD development was 9.3 mo (range: 2-59 mo). The median overall survival of LMD patients from diagnosis to after receiving systemic chemotherapy in combination with intrathecal MTX was 10.5 mo (range: 2-59 mo). In the Cox univariate analysis, gross resection of tumor (P = 0.022), Karnofsky performance status (KPS) > 60 (P = 0.002), and Ommaya reservoir implant (P < 0.001) were correlated with survival. Multivariate analysis showed that KPS > 60 (P = 0.037) and Ommaya reservoir implant (P = 0.014) were positive factors correlated with survival. Myelotoxicity and gastrointestinal reactions were the common toxicities of this combination therapy. According to Common Terminology Criteria of Adverse Events 4.03, most of the patients presented with toxicity less than grade 3.
Intrathecal MTX administration combined with systemic chemotherapy is a potentially effective treatment for patients with GBM and LMD, with mild treatment-related side effects.
Core Tip: Glioblastoma (GBM) patients with leptomeningeal dissemination (LMD) have severe symptoms and poor prognosis. We investigated the use of intrathecal methotrexate in combination with systemic chemotherapy in terms of effectivity and patient outcome. We showed the potential effectivity of this treatment and that KPS > 60, gross resection of the brain tumor, and the Ommaya reservoir implantation are positive prognostic factors for patients with LMD. We believe that our study gives evidence systemetic treatment is potentially effective in GBM patients with LMD.
- Citation: Kang X, Chen F, Yang SB, Wang YL, Qian ZH, Li Y, Lin H, Li P, Peng YC, Wang XM, Li WB. Intrathecal methotrexate in combination with systemic chemotherapy in glioblastoma patients with leptomeningeal dissemination: A retrospective analysis. World J Clin Cases 2022; 10(17): 5595-5605
- URL: https://www.wjgnet.com/2307-8960/full/v10/i17/5595.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i17.5595
Glioblastoma (GBM) is one of the most malignant brain tumors with a median overall survival (OS) of 14.6 mo despite treatment with surgery, radiotherapy, and temozolomide (TMZ)[1,2]. The survival time for patients with GBM was shown to increase after treatment with a combination of tumor-treating fields, but only to 20.9 mo[3]. Leptomeningeal dissemination (LMD) occurs when glioma cells invade the cerebrospinal fluid (CSF) and the leptomeninges. GBM patients with LMD often showed a worse prognosis than those with progression of parenchymal disease and had a median survival of 2-5 mo[4-6]. LMD was initially considered a rare complication of gliomas, but the incidence seems to be higher than the estimated rate of 4%, reaching 25% in postmortem neuropathological studies[4,7,8]. Recent studies indicated that LMD incidence is increasing, possibly due to the improvement in survival rates and survival time of GBM patients[9,10]. Diagnosis of LMD involves either only enhanced magnetic resonance imaging (MRI) or MRI along with positive morphology of CSF cells.
Intrathecal injection of methotrexate (MTX) is a potentially effective method for treating glioma with LMD. Our team found that MTX can inhibit the growth of GBM cells by downregulating the Ras/MAPK/Myc/CD47 signaling pathway[11]. However, an intrathecal injection of MTX was insufficient because it is ineffective on tumor cells in the brain parenchyma. Thus, a combined systemic treatment is needed. TMZ and its combination regimen with etoposide combined with a platinum chemotherapy regimen are optional systemic treatments. We used intrathecal MTX in combination with systemic chemotherapy as a treatment regimen for patients with LMD. This study aimed to estimate the clinical outcomes of combination therapy in GBM patients with LMD.
This study was approved by the Medical Ethics Committee of Beijing Tiantan Hospital, Capital Medical University. Between January 2012 and December 2019, intrathecal MTX in combination with systemic chemotherapy was administered to 26 patients with GBM with LMD in our institution. The patient cohort had a median age of 43 years (range: 18-61 years). Their GBM diagnosis was confirmed by specialized neuropathologists according to the 2016 World Health Organization classification of brain tumors.
Diagnosis of LMD was performed as explained above. Concomitant chemoradiotherapy followed by adjuvant TMZ chemotherapy was administered according to standard protocols[1,2].
Data on sex, date of birth, date of initial glioma diagnosis, date of LMD diagnosis, Karnofsky Performance Status (KPS) score at LMD diagnosis, molecular pathologic analysis, initial and subsequent LMD treatments, first CSF results after LMD diagnosis, hematological toxicity of treatment, and date of death or last follow-up were collected for each patient.
Each treatment cycle lasted for 28 d. MTX was intrathecally injected 2-3 times during each cycle, with a single dose of 10 mg once a week. A TMZ regimen was the first choice until after 6 cycles of TMZ systemic chemotherapy had been completed. A dose of 150-200 mg/m2/d TMZ was administered over 5 d every 28 d. An etoposide and carboplatin (EC) regimen was used in patients in whom the previous TMZ regimen failed in less than 6 mo. Carboplatin AUC5 was administered once every 28 d, and etoposide was administered at 100 mg/m2/d, for 3 d every 28 d. A TMZ and cisplatin (TP) combinatorial regimen was used in patients whose previous TMZ regimen had failed for over 6 mo. Cisplatin was administered at 30 mg/m2/d, 3 d every 28 d, and TMZ was administered at 150-200 mg/m2/d, 5 d every 28 d. Bevacizumab (BEV) was administered to patients with severe brain edema after they showed poor reactions to conventional brain edema treatment. BEV was administered at 5 mg/kg, 1 day every 28 d. Chemotherapy dosage and interval were adjusted according to chemotherapy principles. Either only MRI or MRI along with CSF morphology was reviewed every 2 mo. If the treatments were evaluated as effective, then patients would continue for no more than 8 cycles. If the treatment was ineffective, then the patient was changed to another combined chemotherapy regimen. This process was carried on until either the tumor progressed, the patients gave up treatment, or the patient died.
The time from patients’ initial GBM diagnosis to their death, time from GBM diagnosis to LMD diagnosis, time from LMD diagnosis to death or last follow-up, and OS (recorded until January 1, 2021) were evaluated using the Kaplan–Meier method. The comparison between patients’ characteristics was assessed using a log-rank test. Univariate Cox regression models were applied to assess the effect of the covariates of interest on the time-to-event endpoint. A P value of < 0.05 was considered significant for all analyses. All computations were carried out in SPSS 23.0.
Twenty-six patients with GBM developed LMD and were treated at our institution.
Among the 26 patients included in the analysis, 16 (61.5%) were men and 10 (38.5%) were women, with a median age of 43 years (range: 16-61 years). Most patients had supratentorial primary tumor locations; only two had infratentorial tumors (in the cerebellum). Total gross resection was carried out for 7 patients’ tumors (26.9%). Eighteen tumors (69.2%) that were within 1 cm of the ventricular system or that had infiltrated the ventricular system were opened during the initial surgery. Tumor samples from all patients were sent for molecular pathology tests, namely immunohistochemistry or next-generation sequencing. The patterns of treatment after GBM diagnosis and before LMD diagnosis are provided in Table 1.
| Variable | N (%) |
| Patient | 26 (100) |
| Age at GBM diagnosis: median (range) | 43 (18-61) |
| Sex | |
| Female | 10 (38.5) |
| Male | 16 (61.5) |
| Location | |
| Infratentorial (cerebellum) | 2 (7.7) |
| Supratentorial | 24 (92.3) |
| Extent of resection of GBM at diagnosis | |
| Gross total | 7 (26.9) |
| Non-gross total | 19 (73.1) |
| Communicating with the ventricle at time of GBM diagnosisa | |
| Yes | 18 (69.2) |
| No | 8 (30.8) |
| Concurrent radiation + TMZ after GBM diagnosis | |
| Yes | 26 (100) |
| No | 0 (0) |
| Adjuvant TMZ cycles for GBM: Median (range) | 7 (1-20) |
| < 7 | 15 (57.7) |
| ≥ 7 | 11 (42.3) |
| Molecular pathology, positive testb | |
| MGMT methylation | 5 (19.2) |
| IDH1 mutation | 1 (3.8) |
| TERT C228T mutation | 8 (30.8) |
Twenty-six of these LMD patients had 4 types of clinical symptoms: headache (46.2%), backache (15.4%), lower extremity weakness (11.5%), and visual changes (3.8%). Only 6 patients (23.1%) were asymptomatic upon diagnosis, needing diagnosis to be made through routine examination. With the progress of the disease, most patients appeared to have intracranial hypertension syndrome, severe headache, progressive cognitive impairment, cranial nerve damage, ataxia, and other symptoms of brain and spinal cord injury. The details are presented in Table 2.
| Variable | N (%) |
| Patient | 26 (100) |
| Time from GBM diagnosis to develop of LMD (months) | |
| Median (range) | 9.3 (0.7-41.4) |
| KPS at LMD diagnosis | |
| ≤ 60 | 12 (46.2) |
| > 60 | 14 (53.8) |
| Common presenting symptoms | |
| Headache | 12 (46.2) |
| None | 6 (23.1) |
| Backache | 4 (15.4) |
| Lower extremity weakness | 3 (11.5) |
| Visual changes | 1 (3.8) |
| MRI positive characteristics | |
| Subarachnoid and ventricular Spinal cord | 10 (38.5)16 (61.5) |
| CSF cytology for malignant cells | |
| Yes | 13 (50) |
| No | 13 (50) |
| The content of total protein in the CSF (mg/fL)a | |
| Median (range) | 149.2 (21.6-1600.3) |
| 15-45 | 2 (7.7) |
| > 45 | 21 (80.8) |
| Ommaya reservoir implant | |
| Yes | 20 (76.9) |
| No | 6 (23.1) |
| Intrathecal injection chemotherapy | |
| MTX | 26 (100) |
| Systemic chemotherapy | |
| TMZ | 5 (19.2) |
| TMZ + DDP | 8 (30.8) |
| vp-16 + CBP | 13 (50) |
| Bevacizumab | |
| Yes | 8 (28.6) |
| No | 18 (71.4) |
| Cycles of intrathecal injection and systemic chemotherapy | |
| Median (range) | 4 (1-8) |
| < 4 | 13 (50) |
| ≥ 4 | 13 (50) |
| Gastrointestinal toxicity (grade)b | |
| 1 | 17 (65.4) |
| 2 | 7 (26.9) |
| 3 | 2 (7.7) |
| Myelotoxicity | |
| < 3 | 19 (73.1) |
| 3-4 | 7 (26.9) |
The median time from GBM surgery to diagnosis of LMD was 9.4 mo (range: 0.7–41.4 mo). One patient (3.8%) was diagnosed with LMD (spinal cord metastasis) at the time of initial GBM diagnosis, and the remaining 25 patients were diagnosed after surgery.
All 26 patients had positive MRI findings. Leptomeningeal tumor enhancement was found in the brain around the contours of the gyri and sulci or in multiple nodular deposits in the subarachnoid space, cerebellar folia, and the cortical surface. When these are observed in the spinal cord as linear or nodular enhancements along the surface, a conclusive diagnosis of LMD can be made.
All 26 patients underwent a single lumbar puncture for CSF analysis. Only half these samples (13, 50%) were positive for malignant cells in the cytologic examination. Twenty-one patients (80.8%) had total CSF protein levels over the normal range.
For both the convenience of intrathecal chemotherapy and to avoid lumbar puncture-related metastasis, 20 patients (76.9%) accepted an Ommaya reservoir implant. According to the chemotherapy plan mentioned above, 5 patients used TMZ as systemic chemotherapy, 8 accepted TMZ+DDP (TP), and 13 were treated with vp-16 + CBP (EC). The median number of chemotherapy cycles was 4 (range: 1-8). Four patients changed their systemic chemotherapy plan after first-line failure. One patient accepted vemurafenib therapy because of a BRAF mutation.
The significant treatment-related side effects were gastrointestinal toxicity and myelotoxicity. According to the Common Terminology Criteria of Adverse Events version 4.03, 24 patients (92.3%) had grade 1-2 gastrointestinal side effects, whereas 19 patients (73.1%) had grade 1-2 myelotoxicity.
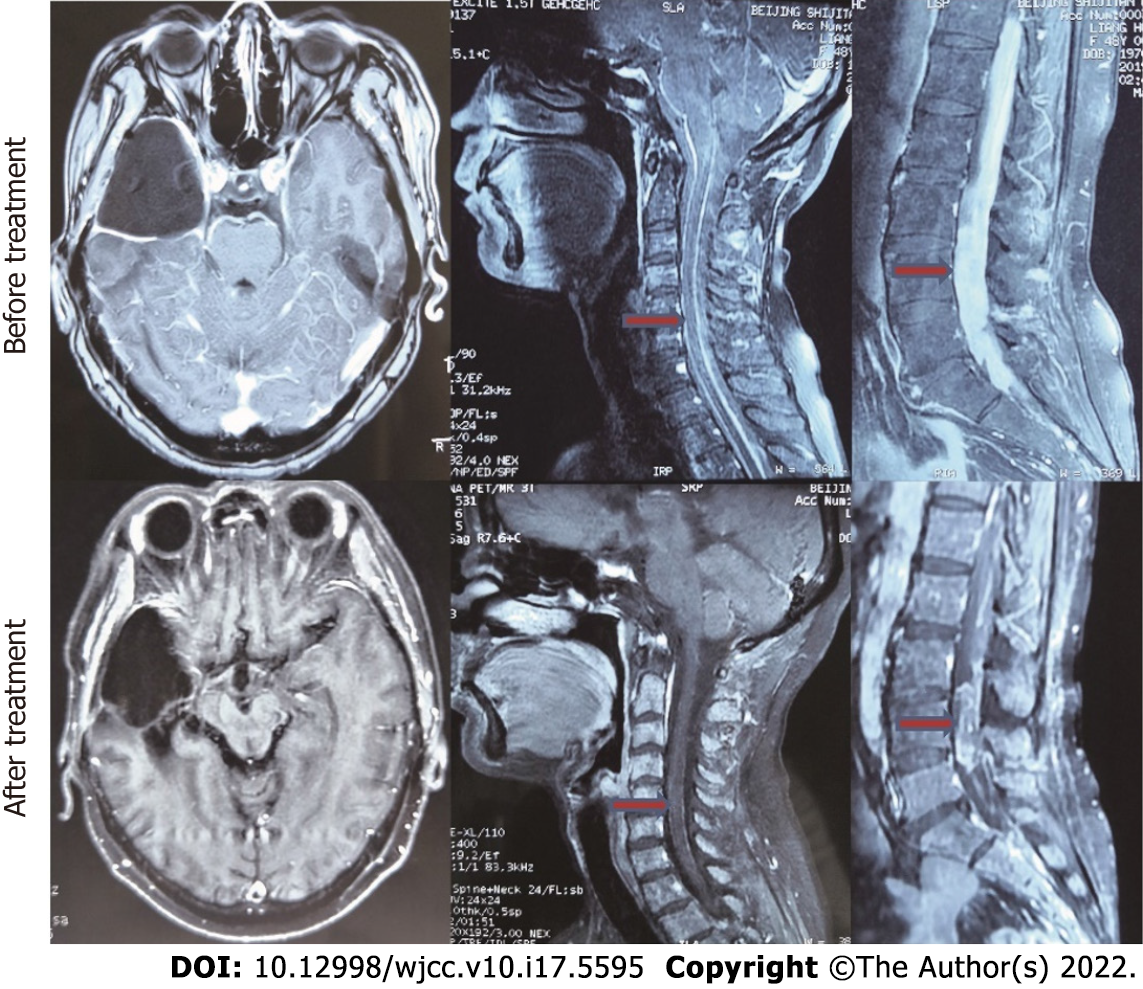
At the last follow-up, 6 patients were still alive. The median OS for all patients from the date of GBM diagnosis was 27.8 mo. The median survival time from diagnosis of GBM to LMD was 9.4 mo (range: 2-59 mo). The median survival from the diagnosis of LMD was 10.5 mo (Figure 1A). Ten patients showed improvement in neurological symptoms and imaging. The image of a typical case is shown in Figure 2. Eight patients had stable disease, whereas treatment was not effective in the remaining 8 patients.
Univariate analysis showed that the median OS from the diagnosis of LMD was significantly different between those with KPS > 60 and KPS ≤ 60 (16 mo vs 9 mo, P = 0.002), Ommaya reservoir implant or no implant (15 mo vs 6 mo, P < 0.001), and gross total resection of the tumor or not [median 24.7, 95%CI (15.1, 34.3) vs 10.9, 95%CI (8.0, 13.7), P = 0.022] (Figure 1B-D). MGMT methylation (P = 0.187), communicating with the ventricle at time of GBM diagnosis (P = 0.778), total protein in CSF (P = 0.321), and BEV use (P = 0.085) had no significant outcome association (Table 3).
| Covariate | χ2 | P value |
| Sex | < 0.001 | 0.99 |
| Extent of resection of GBM at diagnosis | 5.236 | 0.022 |
| Communicating with the ventricle at time of GBM diagnosis | 0.08 | 0.778 |
| MGMT methylation | 1.743 | 0.187 |
| TERT C228T mutation | 0.811 | 0.368 |
| Adjuvant TMZ cycles for GBM | 0.153 | 0.695 |
| Bevacizumab | 2.963 | 0.085 |
| KPS at the time of LMD diagnosis | 9.192 | 0.002 |
| Total protein in the CSF | 0.986 | 0.321 |
| Ommaya reservoir implant | 12.701 | < 0.001 |
| CSF cytology | 3.28 | 0.07 |
Multivariate analysis showed that OS from diagnosis of LMD was positively associated with KPS > 60 (P = 0.037) and the Ommaya reservoir implant (P = 0.014) (Table 4).
| Covariate | HR (95%CI) | P value |
| Extent of resection of GBM at diagnosis | 0.485 (0.126, 1.871) | 0.293 |
| KPS at the time of LMD diagnosis | 0.338 (0.122, 0.935) | 0.037 |
| Ommaya reservoir implant | 0.212 (0.062, 0.729) | 0.014 |
LMD in patients with GBM is a serious complication with adverse outcomes. There is no consensus on the treatment for LDM. Disease progression or treatment-related complications, such as intrathecal treatment leading to bleeding and infection after ventricular-abdominal shunt, can sometimes lead to fatal results[6,12]. Considering the multifocal nature of LMD, surgical treatment is not appropriate. Palliative radiotherapy is the most used treatment that can relieve symptoms and slightly improve survival[13,14]. Completed clinical trials have explored the application of a variety of single-use intrathecal chemotherapeutics, including topotecan, MTX, and cytarabine. Although the safety evaluation is satisfactory, none of the single-use drugs have been shown to significantly improve the survival rate of LMD patients[15]. Most intrathecal drugs are single use for LMD patients[A1]; Scott et al[16] reported that concurrent intrathecal MTX and liposomal cytarabine for solid tumors that developed LMD showed a median non-GBM OS of 30.2 wk, thereby demonstrating a possible strategy of multidrug intrathecal chemotherapy. Single-use BEV or BEV in combination with irinotecan showed inconsistent clinical benefits[4,17,18]. Targeted therapy can be used in selected cases with sensitive mutations, but it is not widely used due to the insufficient detection or the low sensitivity of glioma mutations[19-21]. Although Chimeric Antigen Receptor T-Cell Immunotherapy therapy for IDH wild-type MGMT-methylated GBM combined with LMD has shown encouraging effects and no related side effects[22], it is difficult to find a suitable target. An immunosuppressive microenvironment and subsequent toxicity limits immunotherapy.
Chemotherapy is one of the main treatment methods for brain tumors. Multiple chemotherapeutic regimens have been investigated, both single or combination treatments (TMZ, lomustine, irinotecan, and BEV)[4,17]. No significant effect was achieved with the intrathecal injection chemotherapy of different drugs (MTX or cytarabine)[5,23]. Based on our team’s previous research on the use of MTX in the treatment of gliomas and on the combined therapeutic effect of chemotherapy on recurrent gliomas, we combined an intrathecal MTX injection with systemic chemotherapy. The results showed that the current OS improved compared to that obtained in previous studies[4-6]. To the best of our knowledge, this clinical study has the largest number of patients receiving treatment for GBM and LMD by intrathecal MTX combined with systemic chemotherapy showing good clinical research conclusions.
Previous studies have shown that MGMT promoter methylation status can be used as an indicator for prognosis in newly diagnosed GBM patients. It was also proposed as a risk factor of LMD development in glioma patients[24]. The suspected mechanisms include the increase in the survival of patients subjected to MGMT methylation treatment. MGMT status has no correlation with OS for GBM after LMD diagnosis. Therefore, for patients diagnosed with LMD, MGMT methylation status should not determine whether TMZ treatment should be used.
BEV has been suggested to promote the development of LMD[18], but available data remain conflicting. Considering the high cost of the BEV and the fact that BEV was not approved by the FDA in China until this year, all the patients in this cohort did not use BEV when GBM was first diagnosed. We only used BEV in patients with severe brain edema and in those who did not respond to conventional dehydration treatment. The results showed that the use of BEV had a negative effect on the OS of patients with LMD but had a favorable effect on relieving intracranial hypertension.
Some studies have shown that ventricular opening during surgery or tumor invasion of the ventricle system may be one of the main factors causing LMD[7,25]. In our study, 18 patients with LMD (69.2%) showed communication with the ventricle at GBM diagnosis, and this result is consistent with what was obtained in other studies. When it comes to the relationship between ventricular opening and OS of LMD patients, there was no significant difference between the two groups, possibly due to the small sample sizes. Further verification is needed.
Ommaya reservoir implants have been widely used to treat LDM in different cancers. It can avoid the injury caused by a lumbar puncture and reduce the corresponding risks. In this study, Ommaya reservoir implant was a positive factor for the OS of LMD patients. In addition to intrathecal MTX administration, we also used the Ommaya reservoir as a simple device for external ventricular drainage at the end stage of the disease, which can sometimes alleviate the symptoms of intracranial hypertension.
In this study, the MRI [A2] abnormalities of the brain and spinal cord were used to diagnose LMD. However, only 13 patients had positive results on a CSF morphological examination. Generally, the CSF morphology test should be combined with another test result, and with CSF flow cytometry if necessary. Considering that our CSF morphology result is a single lumbar puncture test before an intrathecal MTX injection, the presence of false negatives is possible.
The CSF protein content is a clinical characteristic of patients with brain tumors. Of [A3] 23 patients, 21 showed increased levels of CSF proteins in this study, and the protein content of CSF decreased in effectively treated patients. Therefore, we supposed that CSF protein content can be used as a marker for disease diagnosis and as a treatment efficiency evaluator.
Our results showed that total resection of the brain tumor at initial diagnosis and KPS ≥ 60 at the time of LMD diagnosis are good prognostic factors, and this conclusion is similar to that obtained in other studies[4].
In the study, some patients showed improvement in clinical symptoms, and partial remission was observed with imaging. Considering the lack of a unified evaluation standard, it is impossible to evaluate the curative effect. Therefore, this study only takes OS as the main endpoint and evaluation standard of the curative effect.
Firstly, this is a single-center retrospective study with a small sample size. Secondly, it is a single-arm study that lacks a control group. Nevertheless, this retrospective study aimed to preliminarily evaluate the efficacy and safety of intrathecal MTX in combination with systemic chemotherapy in GBM patients with LMD. Promising outcomes have been obtained. Based on this result, a prospective study of combination therapy in GBM patients with LMD is ongoing.
LMD is a lethal outcome among patients with glioma and is showing an increasing incidence rate. It remarkably reduces patients’ OS. Intrathecal MTX combined with systemic chemotherapy is a potentially effective therapy for GBM patients with LMD. KPS > 60, gross resection of the brain tumor, and the Ommaya reservoir implant are positive prognostic factors for patients with LMD.
Glioblastoma (GBM) is one of the most common and aggressive primary malignant brain tumors with severe symptoms and a poor prognosis. Leptomeningeal dissemination (LMD) is a serious complication of GBM that often results in dire outcomes. There is currently no effective treatment.
Looking for a potential effective methods in GBM patients with LMD.
To estimate the clinical outcomes of intrathecal MTX combination with systemic therapy in GBM patients with LMD.
A retrospective analysis was conducted using data collected from GBM patients diagnosed with LMD from January 2012 to December 2019 at our institution. Clinical and pathological data were analyzed to explore the clinical outcome of GBM patients with LMD.
Twenty-six patients were enrolled in this study. The median time from GBM diagnosis to LMD development was 9.3 mo (range: 2-59 mo). The median overall survival of LMD patients from diagnosis to after receiving systemic chemotherapy in combination with intrathecal MTX was 10.5 mo (range: 2-59 mo). In the Cox univariate analysis, gross resection of tumor (P = 0.022), Karnofsky Performance Status (KPS) > 60 (P = 0.002), and Ommaya reservoir implant (P < 0.001) were correlated with survival. Multivariate analysis showed that KPS > 60 (P = 0.037) and Ommaya reservoir implant (P = 0.014) were positive factors correlated with survival. Myelotoxicity and gastrointestinal reactions were the common toxicities of this combination therapy. According to Common Terminology Criteria of Adverse Events 4.03, most of the patients presented with toxicity less than grade 3.
Intrathecal MTX administration combined with systemic chemotherapy is a potentially effective treatment for patients with GBM and LMD, with mild treatment-related side effects.
This retrospective study aimed to preliminarily evaluate the efficacy and safety of intrathecal MTX in combination with systemic chemotherapy in GBM patients with LMD. Promising outcomes have been obtained. Based on this result, a prospective study of combination therapy in GBM patients with LMD is ongoing.
The authors sincerely thank all the patients and their families for their participation in the present study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ata F, Qatar; Lang F, United States S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14033] [Cited by in RCA: 15808] [Article Influence: 790.4] [Reference Citation Analysis (0)] |
| 2. | Stupp R, Hegi ME, Gilbert MR, Chakravarti A. Chemoradiotherapy in malignant glioma: standard of care and future directions. J Clin Oncol. 2007;25:4127-4136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 383] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 3. | Stupp R, Taillibert S, Kanner A, Read W, Steinberg D, Lhermitte B, Toms S, Idbaih A, Ahluwalia MS, Fink K, Di Meco F, Lieberman F, Zhu JJ, Stragliotto G, Tran D, Brem S, Hottinger A, Kirson ED, Lavy-Shahaf G, Weinberg U, Kim CY, Paek SH, Nicholas G, Bruna J, Hirte H, Weller M, Palti Y, Hegi ME, Ram Z. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA. 2017;318:2306-2316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1175] [Cited by in RCA: 1752] [Article Influence: 219.0] [Reference Citation Analysis (0)] |
| 4. | Mandel JJ, Yust-Katz S, Cachia D, Wu J, Liu D, de Groot JF, Yung AW, Gilbert MR. Leptomeningeal dissemination in glioblastoma; an inspection of risk factors, treatment, and outcomes at a single institution. J Neurooncol. 2014;120:597-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Noh JH, Lee MH, Kim WS, Lim DH, Kim ST, Kong DS, Nam DH, Lee JI, Seol HJ. Optimal treatment of leptomeningeal spread in glioblastoma: analysis of risk factors and outcome. Acta Neurochir (Wien). 2015;157:569-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Autran D, Barrie M, Matta M, Monserrat C, Campello C, Petrirena G, Boucard C, Padovani L, Loundou A, Appay R, Graillon T, Dufour H, Figarella-Branger D, Chinot O, Tabouret E. Leptomeningeal Gliomatosis: A Single Institution Study of 31 Patients. Anticancer Res. 2019;39:1035-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Alatakis S, Malham GM, Thien C. Spinal leptomeningeal metastasis from cerebral glioblastoma multiforme presenting with radicular pain: case report and literature review. Surg Neurol. 2001;56:33-7; discussion 37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Krause Molle Z, Gierga K, Turowski B, Steiger HJ, Cornelius JF, Rapp M, Sabel M, Kamp MA. 5-ALA-Induced Fluorescence in Leptomeningeal Dissemination of Spinal Malignant Glioma. World Neurosurg. 2018;110:345-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Narayana A, Golfinos JG, Fischer I, Raza S, Kelly P, Parker E, Knopp EA, Medabalmi P, Zagzag D, Eagan P, Gruber ML. Feasibility of using bevacizumab with radiation therapy and temozolomide in newly diagnosed high-grade glioma. Int J Radiat Oncol Biol Phys. 2008;72:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | de Groot JF, Fuller G, Kumar AJ, Piao Y, Eterovic K, Ji Y, Conrad CA. Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice. Neuro Oncol. 2010;12:233-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 386] [Cited by in RCA: 416] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 11. | Bai Y, Zhong XS, Li WB. Glioblastoma progression is inhibited by methotrexate via RAS/MEK/ERK/MYC/CD47 signaling pathways. Electron J Metab Nutr Cancer. 2018;5:144-150. |
| 12. | Saito R, Kumabe T, Jokura H, Yoshimoto T. Fatal hemorrhage after radiochemotherapy for leptomeningeal dissemination of glioma: report of two cases. Surg Neurol. 2002;57:46-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Karaca M, Andrieu MN, Hicsonmez A, Guney Y, Kurtman C. Cases of glioblastoma multiforme metastasizing to spinal cord. Neurol India. 2006;54:428-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Fiorentino A, Caivano R, Chiumento C, Cozzolino M, Fusco V. Radiotherapy and bevacizumab for intramedullary and leptomenigeal metastatic glioblastoma: a case report and review of the literature. Int J Neurosci. 2012;122:691-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Birzu C, Tran S, Bielle F, Touat M, Mokhtari K, Younan N, Psimaras D, Hoang-Xuan K, Sanson M, Delattre JY, Idbaih A. Leptomeningeal Spread in Glioblastoma: Diagnostic and Therapeutic Challenges. Oncologist. 2020;25:e1763-e1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Scott BJ, van Vugt VA, Rush T, Brown T, Chen CC, Carter BS, Schwab R, Fanta P, Helsten T, Bazhenova L, Parker B, Pingle S, Saria MG, Brown BD, Piccioni DE, Kesari S. Concurrent intrathecal methotrexate and liposomal cytarabine for leptomeningeal metastasis from solid tumors: a retrospective cohort study. J Neurooncol. 2014;119:361-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Burger MC, Zeiner PS, Jahnke K, Wagner M, Mittelbronn M, Steinbach JP. Addition of Anti-Angiogenetic Therapy with Bevacizumab to Chemo- and Radiotherapy for Leptomeningeal Metastases in Primary Brain Tumors. PLoS One. 2016;11:e0155315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Ruff MW, Kizilbash SH. Glioblastoma with bilateral extraocular muscle infiltration preceded by evidence of vascular tropism. J Clin Neurosci. 2019;61:277-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Leaver KE, Zhang N, Ziskin JL, Vogel H, Recht L, Thomas RP. Response of metastatic glioma to vemurafenib. Neurooncol Pract. 2016;3:268-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Burger MC, Ronellenfitsch MW, Lorenz NI, Wagner M, Voss M, Capper D, Tzaridis T, Herrlinger U, Steinbach JP, Stoffels G, Langen KJ, Brandts C, Senft C, Harter PN, Bähr O. Dabrafenib in patients with recurrent, BRAF V600E mutated malignant glioma and leptomeningeal disease. Oncol Rep. 2017;38:3291-3296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 21. | Woo PYM, Lam TC, Pu JKS, Li LF, Leung RCY, Ho JMK, Zhung JTF, Wong B, Chan TSK, Loong HHF, Ng HK. Regression of BRAF V600E mutant adult glioblastoma after primary combined BRAF-MEK inhibitor targeted therapy: a report of two cases. Oncotarget. 2019;10:3818-3826. [PubMed] |
| 22. | Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, Ostberg JR, Blanchard MS, Kilpatrick J, Simpson J, Kurien A, Priceman SJ, Wang X, Harshbarger TL, D'Apuzzo M, Ressler JA, Jensen MC, Barish ME, Chen M, Portnow J, Forman SJ, Badie B. Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. N Engl J Med. 2016;375:2561-2569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1255] [Cited by in RCA: 1346] [Article Influence: 149.6] [Reference Citation Analysis (0)] |
| 23. | Zhao KH, Zhang C, Bai Y, Li Y, Kang X, Chen JX, Yao K, Jiang T, Zhong XS, Li WB. Antiglioma effects of cytarabine on leptomeningeal metastasis of high-grade glioma by targeting the PI3K/Akt/mTOR pathway. Drug Des Devel Ther. 2017;11:1905-1915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Brandes AA, Tosoni A, Franceschi E, Sotti G, Frezza G, Amistà P, Morandi L, Spagnolli F, Ermani M. Recurrence pattern after temozolomide concomitant with and adjuvant to radiotherapy in newly diagnosed patients with glioblastoma: correlation With MGMT promoter methylation status. J Clin Oncol. 2009;27:1275-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 290] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 25. | Grabb PA, Albright AL, Pang D. Dissemination of supratentorial malignant gliomas via the cerebrospinal fluid in children. Neurosurgery. 1992;30:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 102] [Article Influence: 3.1] [Reference Citation Analysis (0)] |