Published online Jun 16, 2022. doi: 10.12998/wjcc.v10.i17.5566
Peer-review started: August 10, 2021
First decision: November 17, 2021
Revised: December 16, 2021
Accepted: April 21, 2022
Article in press: April 21, 2022
Published online: June 16, 2022
Processing time: 302 Days and 19.3 Hours
Liver fibrosis is a common pathway of liver injury and is a feature of most chronic liver diseases. Fibrosis progression varies markedly in patients with hepatitis C virus (HCV). Liver stiffness has been recommended as a parameter of fibrosis progression/regression in patients with HCV.
To investigate changes in liver stiffness measured by transient elastography (TE) in a large, racially diverse cohort of United States patients with chronic hepatitis C (CHC).
We evaluated the differences in liver stiffness between patients treated with direct-acting antiviral (DAA) therapy and untreated patients. Patients had ≥ 2 TE measurements and no prior DAA exposure. We used linear regression to measure the change in liver stiffness between first and last TE in response to treatment, controlling for age, sex, race, diabetes, smoking status, human immunodeficiency virus status, baseline alanine aminotransferase, and baseline liver stiffness. Separate regression models analyzed the change in liver stiffness as measured by kPa, stratified by cirrhosis status.
Of 813 patients, 419 (52%) initiated DAA treatment. Baseline liver stiffness was 12 kPa in 127 (16%). Median time between first and last TE was 11.7 and 12.7 mo among treated and untreated patients, respectively. There was no significant change in liver stiffness observed over time in either the group initiating DAA treatment (0.016 kPa/month; CI: -0.051, 0.084) or in the untreated group (0.001 kPa/mo; CI: -0.090, 0.092), controlling for covariates. A higher baseline kPa score was independently associated with decreased liver stiffness.
DAA treatment was not associated with a differential change in liver stiffness over time in patients with CHC compared to untreated patients.
Core Tip: We evaluated changes in liver stiffness measured by transient elastography (TE) in a large, racially diverse cohort of United States patients with chronic hepatitis C (CHC). We retrospectively evaluated differences in liver stiffness between patients treated with direct-acting antiviral (DAA) therapy and untreated patients. Our study shows a higher baseline kPa score was independently associated with decreased liver stiffness, and that differences in liver stiffness may be observed on serial TE measurements in patients with higher baseline scores, irrespective of treatment effect. DAA treatment was not associated with a differential change in liver stiffness over time in patients with CHC compared to untreated patients.
- Citation: Mezina A, Krishnan A, Woreta TA, Rubenstein KB, Watson E, Chen PH, Rodriguez-Watson C. Longitudinal assessment of liver stiffness by transient elastography for chronic hepatitis C patients. World J Clin Cases 2022; 10(17): 5566-5576
- URL: https://www.wjgnet.com/2307-8960/full/v10/i17/5566.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i17.5566
Chronic hepatitis C (CHC) infection, affecting an estimated 71.1 million people worldwide, is a major cause of liver cirrhosis [1,2]. The degree of liver fibrosis is a chief determinant of complications related to end-stage liver disease and is associated with the risk of hepatic decompensation, hepatocellular carcinoma, and mortality[3]. The level of CHC-related hepatic fibrosis is detected through histology by the meta-analysis of histological data in viral hepatitis (METAVIR) scoring system (F0 indicating no fibrosis to F4 indicating cirrhosis)[4]. The temporal evolution of fibrosis in patients with CHC is non-linear, with variable progression observed between different stages of disease specified by METAVIR fibrosis scores[5]. The degree of progression of liver fibrosis is further influenced by variables including age, gender, alcohol consumption, insulin resistance, high body mass index, and co-infection with hepatitis B virus (HBV) or human immunodeficiency virus (HIV)[6-9]. Histological assessment of the liver is considered the optimal approach to evaluate changes in fibrosis over time and remains the gold standard for the diagnosis of cirrhosis[10]. However, reliance on liver biopsy is limited by the intra- and interobserver variability of fibrosis scoring, the sampling variability, and finally, the potential for complications from an invasive procedure, which renders serial histopathological assessments impractical in routine clinical care[11-13].
Transient elastography (TE) has become a widely used, noninvasive ultrasound-based technique to measure liver stiffness and accurately assess severe fibrosis and cirrhosis without the morbidity and mortality associated with liver biopsy. TE is validated in patients with CHC with 89% sensitivity and 91% specificity for the diagnosis of cirrhosis[14]. Achieving sustained virological response (SVR) after treatment of CHC has been shown to reduce the rate of liver fibrosis progression and even partially reverse fibrosis as assessed by histology as well as TE[15-17]. Eradication of HCV is associated with a reduction in all-cause and liver-related mortality and a lower risk of progression to cirrhosis[15,18,19]. However, despite achieving SVR, some patients will develop progressively higher liver stiffness measurements over time, and up to 60% of those with cirrhosis at the time of treatment with direct-acting antiviral agents (DAA) will continue to exhibit liver stiffness measurements in the cirrhotic range, increasing the risk for hepatic decompensation and adverse clinical outcomes[20-22]. Due to the broad implementation of guideline-directed treatment of CHC, understanding the factors leading to differential changes in liver stiffness will be integral to refining clinical decisions regarding management and surveillance for complications of end-stage liver disease[17,23]. Many studies utilizing TE to evaluate longitudinal changes in liver stiffness have been limited to the duration of DAA therapy or the 12-24 wk post-treatment when SVR is ascertained[24,25-27]. Other studies with long-term follow-up periods have been conducted outside of the United States and may not reflect the same racially diverse patient population and genotypic distribution of HCV, limiting the generalizability of findings[22,28].
The American Gastroenterological Association has recognized the need for large, long-term investigations of outcomes in patients with CHC treated with DAA therapy and indicated that current evidence is insufficient to recommend a universal policy of noninvasive testing of liver fibrosis in successfully treated patients[14,29]. To address this need, we performed a longitudinal, retrospective observational study investigating changes in liver stiffness measured by TE in a racially diverse cohort of United States patients with CHC.
Study design: We conducted a longitudinal retrospective study of patients with confirmed CHC infection seen at Johns Hopkins Health System (JHHS) and Kaiser Permanente Mid-Atlantic States (KPMAS) between April 1, 2014 and March 31, 2018. JHHS is an academic health system primarily serving Baltimore, MD, and the surrounding area. KPMAS is an integrated healthcare system serving more than 750000 members in a region that includes the District of Columbia, Maryland, and Virginia. The Institutional Review Board approved the study at JHHS and KPMAS.
Study population: Patients at JHHS were identified by querying the electronic health record (EHR) system for the International Classification of Disease, Ninth (ICD-9), or Tenth Revision (ICD-10) diagnosis codes for hepatitis C viral (HCV) infection or a positive HCV RNA test. At KPMAS, HCV patients were identified by positive HCV RNA, HCV genotype, ≥ 2 refills of interferon-based HCV therapy within 1 year, or positive HCV antibody test plus ≥ 1 HCV-coded visit. Included patients were at least 18 years of age, treatment naïve to DAA at the time of the first liver stiffness measurement, and had at least two liver stiffness measurements. Patients were excluded if the date of the first liver stiffness measurement was more than 30 d before the date on which our HCV definition is met. We reason that liver stiffness measurements performed fewer than 30 d before HCV recognition was likely due to clinically suspected HCV, but those performed more than 30 d before were due to other chronic liver conditions. We further excluded any patients found to have an undetectable HCV viral load within 180 d prior to the first liver stiffness measurement indicating clearance of HCV infection. The baseline was defined as the date of the first liver stiffness measurement. Treatment was defined by pharmacy dispensation dates in the EHR. Only those patients initiating treatment between the date of the first and last TE were included in the treatment group. TE results and biochemical, clinical, and demographic characteristics were extracted from the EHR. We collected data on the following: age, sex, race, diabetes, chronic kidney disease (CKD), body mass index (BMI), smoking status, alcohol consumption, HIV status, HBV status, hepatocellular carcinoma, aspartate aminotransferase (AST), alanine aminotransferase (ALT), HCV RNA viral load, bilirubin, platelet count, and TE results. The most proximal laboratory and clinical data were collected from a window that spanned 180 days prior to the baseline TE scan and up to 30 d after.
Liver stiffness measurements: Liver stiffness measurements were obtained using transient elastography (Fibroscan 502, Echosens, Paris, France) by trained operators at JHHS and KPMAS. Participants were instructed to fast for a minimum of 2 h prior to testing. The liver stiffness measurement was considered reliable only if a minimum of 10 successful acquisitions were obtained, with an interquartile range ≤ 30% of the median liver stiffness measurement and success rate ≥ 60%. The success rate was calculated as the ratio of the number of successful acquisitions over the total number of acquisitions. The result was reported as a median of liver stiffness expressed in kilopascals (kPa).
Statistical analysis: All statistical analyses were conducted using R version 3.6.0, Stata version 14 (StataCorp LP., College Station, TX, United States), and SAS version 9.4 (SAS Institute Inc., Cary, NC, United States). Descriptive statistics were computed, including counts and percentages for categorical variables, and either means and standard deviations, medians with quartiles, or medians with minimum and maximum values for continuous variables. A t-test was used to compare mean baseline liver stiffness measurements (kPa) between treatment groups. We used multivariable linear regression models to assess the association between treatment and the difference in liver stiffness measurements between first and last TE, adjusting for baseline kPa, and the covariates mentioned above. We included an interaction term between time and treatment to directly compare the change in kPa between treated and untreated subjects. This model can be considered equivalent to one with only the last TE measurement as the outcome, provided that baseline kPa (also referred to as first TE measurement) is included along with all of the same covariates[30]. The analysis was further stratified by baseline cirrhosis status (kPa ≥ 12). A P value < 0.05 was considered statistically significant.
Sensitivity analysis: We found that some patients had two liver stiffness measurements obtained in rapid succession within 7 d of one another. We chose to omit patients with only two measurements taken within 7 d because differences in liver stiffness during a brief time interval are likely to reflect measurement error rather than meaningful physiological changes in the liver. Therefore, as a sensitivity analysis, we repeated all analyses, including patients with liver stiffness measurements taken within 7 d of one another. We will describe results, both including and excluding pairs of liver stiffness measurements taken within 7 d, to assess whether these data impact our conclusions.
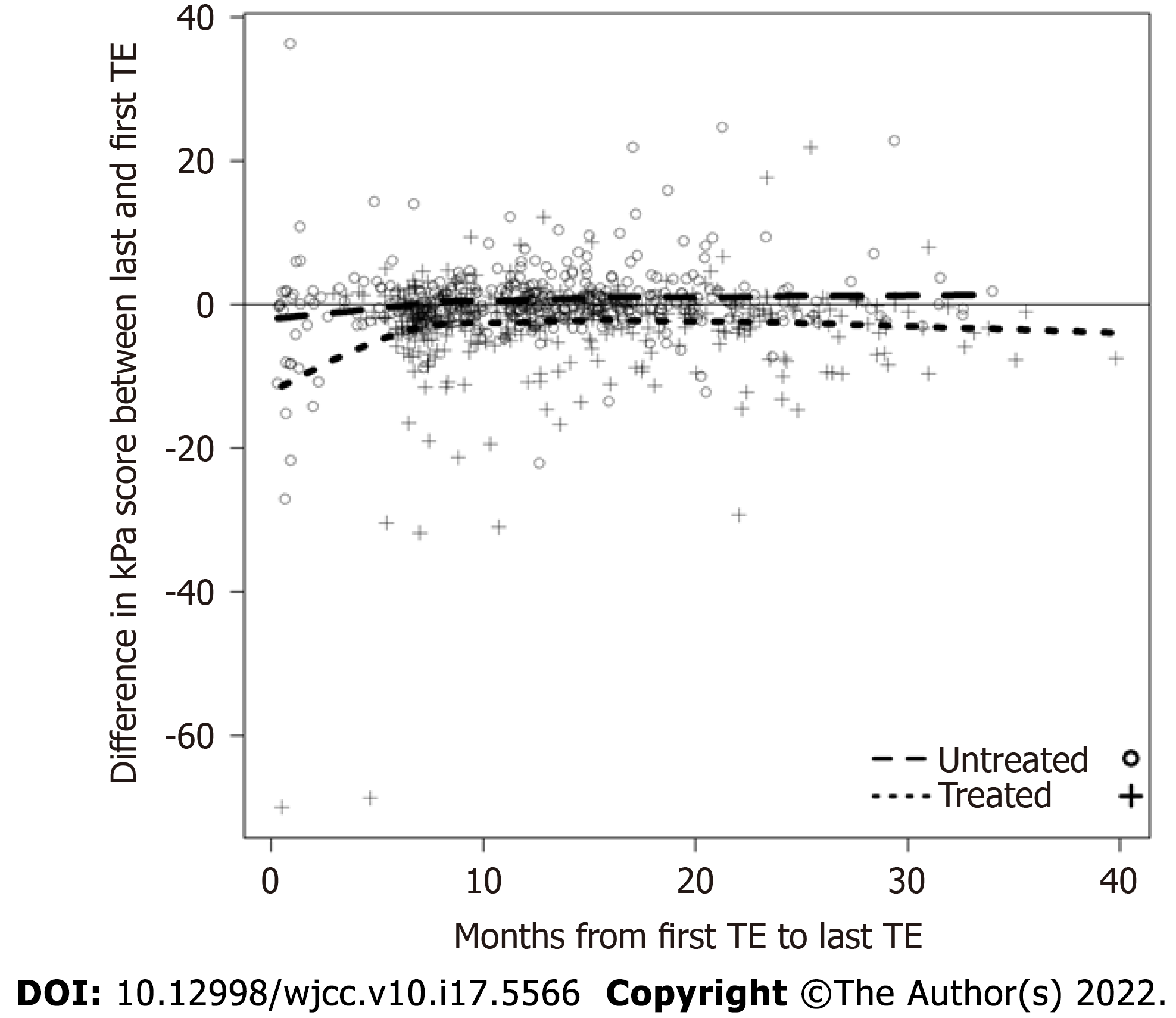
Of 813 patients, 84% were at least 50 years of age, 79% were Black, 79% were current or former smokers, 37% were coinfected with HIV, 3% were coinfected with HBV, 19% had diabetes, and 52% initiated treatment with a DAA (Table 1). The median (Q1, Q3) time between first and last liver stiffness measurement was 11.73 (7.41, 17.87) months among those initiating treatment and 12.68 (8.71, 16.94) months among those who were untreated (Table 2). 22% (n = 91) of treated subjects had a kPa score ≥ 12, compared with 9% (n = 36) of untreated patients. The mean (SD) baseline liver stiffness among treated patients was 10.69 (9.48) kPa, significantly higher compared to the mean (SD) of 7.32 (6.10) kPa among untreated patients (P < 0.001). The HCV genotype was genotype 1 in 60% of patients (comprising 91% of patients with known HCV genotype); however, HCV genotype data are not included elsewhere in our analyses because of missing data in 34% of patients. Additionally, due to missing data regarding sustained virologic response (SVR) rates in treated patients, alcohol consumption, and body mass index, these variables were also excluded from the analyses. We also omitted 38 patients (4.7%) who did not have an ALT result up to 180 d before the first liver stiffness measurement, as well as 3 patients (0.4%) with unknown race and 2 patients (0.2%) with unknown smoking status. After excluding these 43 total patients with incomplete information, 770 total patients were included in the regression models. In the multivariable linear regression model examining the change in kPa as the dependent variable, the baseline kPa score was independently associated with a reduction in liver stiffness between the first and last TE (-0.557 kPa; CI: -0.699, -0.415), controlling for age, race (Black, White, or other), sex, HIV status, smoking status, baseline ALT score, and treatment status (Table 3). We observed no significant change in liver stiffness over time among patients who were treated (0.016 kPa/mo; CI: -0.051, 0.084) or untreated (0.001 kPa/mo; CI: -0.090, 0.092); as such, there was no significant difference in the change in liver stiffness among treated patients compared to untreated patients (0.015 kPa/mo, CI: -0.106, 0.136). The difference in the kPa score between the first and last TE, stratified by treatment status, is shown in Figure 1. Next, the analysis was stratified to examine the association of these covariates with liver stiffness in patients with cirrhosis (kPa ≥ 12, n = 119) as well as non-cirrhotic patients (kPa < 12, n = 651). There was not a significant effect of DAA treatment on the change in liver stiffness over time in either cohort, and the difference in liver stiffness between treated and untreated patients was similar. A higher baseline kPa was an independent predictor of a reduction in liver stiffness measurement in both groups. Among patients without cirrhosis, age ≥ 50 was independently associated with increased liver stiffness (0.949 kPa; CI: 0.336, 1.563).
| Variables | HCV treated | HCV untreated |
| (n = 419 (51.5%)] | [n = 394 (48.5%)] | |
| Age (yr) [mean (SD)] | 56.54 (8.26) | 56.14 (9.02) |
| Male sex [n (%)] | 285 (68.02) | 234 (59.39) |
| Race [n (%)] | ||
| Black | 339 (80.91) | 303 (77.49) |
| Other | 18 (4.30) | 20 (5.12) |
| White | 62 (14.80) | 68 (17.39) |
| Smoking history | ||
| Non-smoker | 70 (16.71) | 98 (25.00) |
| Current smoker | 216 (51.55) | 183 (46.68) |
| Former smoker | 133 (31.74) | 111 (28.32) |
| Co-infections [n (%)] | ||
| HIV | 184 (43.91) | 113 (28.68) |
| HBV | 9 (2.15) | 15 (3.81) |
| HCV RNA at baseline, IU/mL [median (Q1, Q3)] | 2220000 (803000, 6606934) | 2340000 (693015, 5924209) |
| Biochemistry | ||
| AST, IU/L, mean (SD) | 57.91 (44.75) | 46.38 (31.13) |
| ALT, IU/L, mean (SD) | 56.63 (51.54) | 51.52 (59.04) |
| Bilirubin < 2 mg/dL [n (%)] | 393 (97.52) | 359 (98.09) |
| Platelets, K/uL, median (Q1, Q3) | 196.00 (155.50, 238.00) | 209.00 (170.50, 255.00) |
| Hepatocellular carcinoma [n (%)] | 4 (0.95) | 2 (0.51) |
| Baseline diabetes [n (%)] | 82 (19.57) | 73 (18.53) |
| Baseline chronic kidney disease [n (%)] | 41 (9.79) | 26 (6.60) |
| Variables | HCV treated (n = 419) | HCV untreated (n = 394) |
| Baseline liver stiffness (kPa) [mean (SD); range] | 10.69 (9.48); 1.30-75.00 | 7.32 (6.10); 0.00-75.00 |
| Change in kPa score [mean (SD)] | -2.78 (7.10) | 0.51 (5.14) |
| Months between first and last TE [median (Q1, Q3)] | 11.73 (7.41, 17.87) | 12.68 (8.71, 16.94) |
| Months between first TE and DAA start [median (Q1, Q3)] | 1.64 (0.95, 3.84) | – |
| Months between DAA end and last TE [median (Q1, Q3)] | 4.17 (3.07, 10.96) | – |
| All patients (n = 770) | Baseline kPa < 12 (n = 651) | Baseline kPa ≥ 12 (n = 119) | |||||||
| Variables | Effect estimate | 95%CI | P | Effect estimate | 95%CI | P value | Effect estimate | 95%CI | P value |
| Antiviral treatment effect | -1.821 | (-3.452, -0.191) | 0.029 | -1.487 | (-2.820, -0.154) | 0.029 | -2.071 | (-7.477, 3.335) | 0.453 |
| Baseline kPa | -0.557 | (-0.699, -0.415) | < 0.001 | -0.677 | (-0.875, -0.480) | < 0.001 | -0.600 | (-0.818, -0.383) | < 0.001 |
| Black race1 | -0.797 | (-1.775, 0.181) | 0.110 | -0.654 | (-1.362, 0.054) | 0.070 | -2.747 | (-7.304, 1.810) | 0.237 |
| Other race1 | -0.497 | (-1.901, 0.907) | 0.488 | -0.687 | (-1.893, 0.520) | 0.264 | 1.071 | (-6.423, 8.566) | 0.779 |
| Age ≥ 502 | 0.663 | (-0.165, 1.490) | 0.117 | 0.949 | (0.336, 1.563) | 0.002 | -0.204 | (-4.089, 3.682) | 0.918 |
| Female gender | -0.210 | (-0.843, 0.424) | 0.517 | -0.269 | (-0.772, 0.235) | 0.295 | 0.383 | (-3.330, 4.096) | 0.840 |
| Diabetes | 0.758 | (-0.189, 1.705) | 0.117 | 0.751 | (-0.097, 1.599) | 0.082 | 1.721 | (-2.034, 5.475) | 0.369 |
| Current smoker | 0.617 | (-0.390, 1.624) | 0.230 | 0.199 | (-0.467, 0.865) | 0.558 | 2.266 | (-3.371, 7.903) | 0.431 |
| Former smoker | -0.258 | (-1.155, 0.638) | 0.572 | -0.236 | (-0.867, 0.395) | 0.464 | -0.554 | (-6.741, 5.633) | 0.861 |
| HIV co-infected | 0.468 | (-0.230, 1.166) | 0.189 | 0.254 | (-0.286, 0.794) | 0.356 | 2.040 | (-1.292, 5.372) | 0.230 |
| Baseline ALT (per 10 units) | 0.023 | (-0.043, 0.089) | 0.499 | 0.018 | (-0.039, 0.075) | 0.532 | 0.060 | (-0.250, 0.370) | 0.705 |
| Time between first and last TE (untreated group) (mo) | 0.001 | (-0.090, 0.092) | 0.975 | -0.012 | (-0.103, 0.078) | 0.787 | 0.129 | (-0.229, 0.488) | 0.480 |
| Time between first and last TE (treated group) (mo)3 | 0.016 | (-0.051, 0.084) | 0.634 | 0.008 | (-0.044, 0.059) | 0.772 | 0.004 | (-0.215, 0.223) | 0.972 |
| Interaction of treatment and time | 0.015 | (-0.106, 0.136) | 0.807 | 0.020 | (-0.086, 0.126) | 0.711 | -0.125 | (-0.523, 0.273) | 0.537 |
| Intercept | 4.146 | (2.365, 5.927) | < 0.001 | 4.781 | (2.478, 7.084) | < 0.001 | 5.852 | (-0.994, 12.698) | 0.094 |
Sensitivity analysis results: We repeated the analyses described above with the inclusion of patients who had two liver stiffness measurements obtained within 7 d (n = 7). We did not observe a significant change over time in liver stiffness in the treated (0.017 kPa/mo; CI: -0.051, 0.086) or untreated patients (-0.027 kPa/mo; CI: -0.120, 0.066), and the rate of change was not different when comparing treated and untreated patients. Results stratified by cirrhosis status (kPa < 12 and kPa ≥ 12) were similar to the original analysis (Supplementary Table 1 and Supplementary Figure 1). Estimates for all other cova
The widespread application of TE as a noninvasive tool to estimate liver stiffness in patients with CHC has raised important questions regarding the clinical benefit of repeating TE following DAA treatment and the evolution of fibrosis on post-treatment measurements. Our retrospective longitudinal cohort study employed TE to investigate changes in liver stiffness over time in a large, racially diverse patient population with CHC infection and assess treatment response.
In our multivariable regression models, we compared the rate of change of liver stiffness over time between DAA-treated and untreated subjects, controlling for race, age, gender, diabetes, smoking status, HIV co-infection, baseline ALT, and baseline kPa that may be independently associated with liver fibrosis. Interestingly, estimated liver stiffness as measured in kPa did not improve over a median of 11.7 mo among patients treated with DAA therapy. There was no significant difference in the change in liver stiffness between treated and untreated patients when stratifying patients based on underlying cirrhosis. Patients with longer times between TE measurements did not exhibit more pronounced differences in liver stiffness.
Our findings were unexpected due to abundant clinical evidence that initiation of antiviral treatment is associated with rapid improvement in liver stiffness scores as well as long-term attenuation of liver fibrosis in patients who achieve SVR[17]. It is possible that our study was underpowered to detect a change in liver stiffness during a median follow-up time period of 4.2 mo after treatment. Since liver stiffness measurements were not always available at the initiation of DAA therapy, the effect of treatment may have been underestimated due to the inclusion of a median of 1.6 mo of untreated time in the treated group. Our study may have been underpowered to detect a modest effect of treatment on cirrhosis regression, as the analysis subsumed only 119 patients with cirrhosis at baseline. The apparent lack of improvement in hepatic stiffness among treated patients may be due to continued liver injury from other, potentially modifiable causes, including non-alcoholic fatty liver disease, alcohol use and drug use, which were not fully captured in our dataset. Despite successful treatment, a subset of patients cured of CHC, particularly those with advanced fibrosis at baseline, will exhibit a progression of liver fibrosis and are at risk of developing adverse liver-related outcomes[21,31]. Although we did not capture clinical data indicative of portal hypertension or decompensated cirrhosis in our study population, it has been postulated that decompensated cirrhosis represents an irreversible threshold that precludes regression to a noncirrhotic state[31,32].
In all analyses, higher baseline liver stiffness was independently associated with a reduction in kPa measurements between first and last TE, which is consistent with results from other investigations. In one systematic review and meta-analysis by Singh et al[17] of studies examining liver stiffness measurements after antiviral therapy, a greater absolute decrease in stiffness over time was observed among studies that included a larger proportion of patients with advanced fibrosis and cirrhosis at baseline. We found that antiviral treatment status was associated with a decline in kPa when analyzing the entire study population as well as patients without baseline cirrhosis. However, this estimated effect of treatment was independent of time, therefore, this does not represent the incremental decrease in stiffness that may be expected in successfully treated patients, and may instead reflect another confounder in our population. We cannot rule out the possibility that this observed decline in kPa is an artifact of regression towards the mean[33,34]. If there is random variability in the measurement of liver stiffness, then misclassifying patients with cirrhosis based on high baseline liver stiffness may result in observed declines in subsequent stiffness measurements that do not reflect a biological change in the liver[34,35]. We further found that treated patients have significantly higher baseline liver stiffness than untreated patients on average, which may make this effect stronger in the treated group than the untreated group. We included baseline kPa in our models to mitigate the impact of regression to the mean on the change over time in liver stiffness, but we cannot be certain that we have entirely accounted for this effect[35].
Based on our eligibility criteria, we identified 7 subjects who underwent serial TE measurements within 7 d or fewer. We performed a sensitivity analysis to investigate a potential bias introduced by our decision to exclude these subjects from the main analysis, based on the hypothesis that changes in liver stiffness observed in a short time period would be more likely to reflect measurement error than changes in inflammation or fibrosis due to DAA treatment. The sensitivity analysis demonstrated that DAA treatment did not significantly change liver stiffness when comparing treated vs untreated patients.
We found that age ≥ 50 was independently associated with increased liver stiffness, consistent with the natural history of fibrosis progression in CHC infection[36]. Baseline pre-treatment ALT levels were not predictive of a change in liver stiffness. However, other reports have identified a positive relationship between elevated ALT levels and improvement in liver stiffness over time as measured by TE, which may be partially due to a decline in necroinflammation[25,37].
Limitations of our study include missing data regarding SVR rates in patients initiating treatment with DAA. Although reported SVR rates are greater than 95% with DAA combinations, this approximation may dilute the effect of treatment on liver stiffness measurements in our study[38]. We were unable to assess the relationship between DAA treatment and liver-related outcomes in our cohort as these data were not collected as part of our study, however, achieving SVR has been demonstrated to lower the risk of liver-related morbidity and mortality[19,36,37].
The results from our study contribute to the clinical evidence regarding the real-world utility of post-treatment evaluation for fibrosis regression using TE[14,39]. We found that differences in liver stiffness may be observed on serial TE measurements in patients with higher baseline scores, irrespective of treatment effect, which suggests that false positive results on TE are not uncommon in clinical practice. The Baveno VI Consensus criteria includes a weak recommendation for obtaining two fasting TE measurements for patients undergoing screening for compensated advanced chronic liver disease[40]. Since liver stiffness measurements may be taken into account by both clinicians and patients as part of the decision to pursue a liver biopsy for definitive histopathologic assessment, and may impact clinical management with respect to variceal bleeding surveillance, we advocate that a repeat confirmatory TE measurement should be considered for patients with an elevated kPa without other clinical or radiographic signs of advanced fibrosis.
Our study underscores the imperfect characteristics of TE for the assessment of liver fibrosis and cirrhosis, compared with the gold standard metrics of hepatic venous pressure gradient measurement and liver histopathology, despite the impetus to avoid invasive testing as part of routine clinical care of patients with CHC[41]. Ultimately, the question remains whether a decline in liver stiffness meas
Liver fibrosis is a common pathway of liver injury and is a feature of most chronic liver diseases. Fibrosis progression varies markedly in patients with hepatitis C virus (HCV), and the severity of liver fibrosis is associated with the prognosis of liver disease. Liver stiffness has been recommended as a parameter of fibrosis progression/regression in patients with HCV.
To investigate the changes in liver stiffness measured by transient elastography (TE) in a large, racially diverse cohort of U.S. patients with chronic hepatitis C (CHC).
We evaluated the differences in liver stiffness between patients treated with direct-acting antiviral (DAA) therapy and untreated patients. In addition, we performed a longitudinal, retrospective observational study investigating changes in liver stiffness measured by TE in a racially diverse cohort of United States patients with CHC.
We conducted a longitudinal retrospective study of patients with confirmed CHC infection seen at Johns Hopkins Health System (JHHS) and Kaiser Permanente Mid-Atlantic States (KPMAS). Patients had ≥ 2 TE measurements and no prior DAA exposure. We used linear regression to measure the change in liver stiffness between first and last TE in response to treatment, controlling for age, sex, race, diabetes, smoking status, HIV status, baseline ALT, and baseline liver stiffness.
Of 813 patients, 84% were at least 50 years of age, 79% were Black, 79% were current or former smokers, 37% were coinfected with HIV, 3% were coinfected with HBV, 19% had diabetes, and 52% initiated treatment with a DAA. The median time between first and last TE was 11.7 and 12.7 mo among treated and untreated patients, respectively. There was no significant change in liver stiffness observed over time in either the group initiating DAA treatment (0.016 kPa/month; CI: -0.051, 0.084) or in the untreated group (0.001 kPa/month; CI: -0.090, 0.092), controlling for covariates. A higher baseline kPa score was independently associated with decreased liver stiffness.
DAA treatment was not associated with a differential change in liver stiffness over time, as measured by TE, in patients with CHC compared to untreated patients. Our study underscores the imperfect characteristics of any single noninvasive test for assessing liver fibrosis, which continues to be compared to the gold standard of liver biopsy and histopathology, despite the impetus to avoid invasive testing for CHC infection in clinical practice.
Direct-acting antiviral therapy was not associated with a differential change in liver stiffness over time in patients with CHC compared to untreated patients. Further longitudinal prospective studies are needed to evaluate the clinical utility of obtaining TE measurements in patients with CHC who have achieved sustained virologic response and assess liver fibrosis progression in diverse populations.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kishida Y S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
| 1. | Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1764] [Cited by in RCA: 1844] [Article Influence: 97.1] [Reference Citation Analysis (0)] |
| 2. | Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1493] [Cited by in RCA: 1472] [Article Influence: 184.0] [Reference Citation Analysis (0)] |
| 3. | Singh S, Fujii LL, Murad MH, Wang Z, Asrani SK, Ehman RL, Kamath PS, Talwalkar JA. Liver stiffness is associated with risk of decompensation, liver cancer, and death in patients with chronic liver diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11:1573-84.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 238] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 4. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2860] [Cited by in RCA: 3082] [Article Influence: 106.3] [Reference Citation Analysis (0)] |
| 5. | Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48:418-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 623] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 6. | Marcellin P, Asselah T, Boyer N. Fibrosis and disease progression in hepatitis C. Hepatology. 2002;36:S47-S56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 101] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Hoefs JC, Shiffman ML, Goodman ZD, Kleiner DE, Dienstag JL, Stoddard AM; HALT-C Trial Group. Rate of progression of hepatic fibrosis in patients with chronic hepatitis C: results from the HALT-C Trial. Gastroenterology. 2011;141:900-908.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Konerman MA, Mehta SH, Sutcliffe CG, Vu T, Higgins Y, Torbenson MS, Moore RD, Thomas DL, Sulkowski MS. Fibrosis progression in human immunodeficiency virus/hepatitis C virus coinfected adults: prospective analysis of 435 liver biopsy pairs. Hepatology. 2014;59:767-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Patel S, Jinjuvadia R, Patel R, Liangpunsakul S. Insulin Resistance is Associated With Significant Liver Fibrosis in Chronic Hepatitis C Patients: A Systemic Review and Meta-Analysis. J Clin Gastroenterol. 2016;50:80-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | McHutchison J, Poynard T, Afdhal N. Fibrosis as an end point for clinical trials in liver disease: a report of the international fibrosis group. Clin Gastroenterol Hepatol. 2006;4:1214-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Maharaj B, Maharaj RJ, Leary WP, Cooppan RM, Naran AD, Pirie D, Pudifin DJ. Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver. Lancet. 1986;1:523-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 462] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 12. | Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614-2618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1504] [Cited by in RCA: 1569] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 13. | Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol. 2003;39:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 596] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 14. | Lim JK, Flamm SL, Singh S, Falck-Ytter YT; Clinical Guidelines Committee of the American Gastroenterological Association. American Gastroenterological Association Institute Guideline on the Role of Elastography in the Evaluation of Liver Fibrosis. Gastroenterology. 2017;152:1536-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 138] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 15. | Poynard T, McHutchison J, Manns M, Trepo C, Lindsay K, Goodman Z, Ling MH, Albrecht J. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122:1303-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 828] [Cited by in RCA: 803] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 16. | D'Ambrosio R, Aghemo A, Fraquelli M, Rumi MG, Donato MF, Paradis V, Bedossa P, Colombo M. The diagnostic accuracy of Fibroscan for cirrhosis is influenced by liver morphometry in HCV patients with a sustained virological response. J Hepatol. 2013;59:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 118] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 17. | Singh S, Facciorusso A, Loomba R, Falck-Ytter YT. Magnitude and Kinetics of Decrease in Liver Stiffness After Antiviral Therapy in Patients With Chronic Hepatitis C: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2018;16:27-38.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 18. | Singal AG, Volk ML, Jensen D, Di Bisceglie AM, Schoenfeld PS. A sustained viral response is associated with reduced liver-related morbidity and mortality in patients with hepatitis C virus. Clin Gastroenterol Hepatol. 2010;8:280-288, 288.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 260] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 19. | Backus LI, Boothroyd DB, Phillips BR, Belperio P, Halloran J, Mole LA. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509-516.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 357] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 20. | Chekuri S, Nickerson J, Bichoupan K, Sefcik R, Doobay K, Chang S, DelBello D, Harty A, Dieterich DT, Perumalswami PV, Branch AD. Liver Stiffness Decreases Rapidly in Response to Successful Hepatitis C Treatment and Then Plateaus. PLoS One. 2016;11:e0159413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 21. | van der Meer AJ, Feld JJ, Hofer H, Almasio PL, Calvaruso V, Fernández-Rodríguez CM, Aleman S, Ganne-Carrié N, D'Ambrosio R, Pol S, Trapero-Marugan M, Maan R, Moreno-Otero R, Mallet V, Hultcrantz R, Weiland O, Rutter K, Di Marco V, Alonso S, Bruno S, Colombo M, de Knegt RJ, Veldt BJ, Hansen BE, Janssen HLA. Risk of cirrhosis-related complications in patients with advanced fibrosis following hepatitis C virus eradication. J Hepatol. 2017;66:485-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 216] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 22. | Pietsch V, Deterding K, Attia D, Ringe KI, Heidrich B, Cornberg M, Gebel M, Manns MP, Wedemeyer H, Potthoff A. Long-term changes in liver elasticity in hepatitis C virus-infected patients with sustained virologic response after treatment with direct-acting antivirals. United European Gastroenterol J. 2018;6:1188-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | AASLD-IDSA. HCV testing and linkage to care. Recommendations for testing, managing, and treating hepatitis C. [cited 2018 Nov 21] Available from: https://www.hcvguidelines.org/evaluate/when-whom. |
| 24. | Bernuth S, Yagmur E, Schuppan D, Sprinzl MF, Zimmermann A, Schad A, Kittner JM, Weyer V, Knapstein J, Schattenberg JM, Wörns MA, Galle PR, Zimmermann T. Early changes in dynamic biomarkers of liver fibrosis in hepatitis C virus-infected patients treated with sofosbuvir. Dig Liver Dis. 2016;48:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Sporea I, Lupușoru R, Mare R, Popescu A, Gheorghe L, Iacob S, Șirli R. Dynamics of liver stiffness values by means of transient elastography in patients with HCV liver cirrhosis undergoing interferon free treatment. J Gastrointestin Liver Dis. 2017;26:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Knop V, Hoppe D, Welzel T, Vermehren J, Herrmann E, Vermehren A, Friedrich-Rust M, Sarrazin C, Zeuzem S, Welker MW. Regression of fibrosis and portal hypertension in HCV-associated cirrhosis and sustained virologic response after interferon-free antiviral therapy. J Viral Hepat. 2016;23:994-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 27. | Elsharkawy A, Alem SA, Fouad R, El Raziky M, El Akel W, Abdo M, Tantawi O, AbdAllah M, Bourliere M, Esmat G. Changes in liver stiffness measurements and fibrosis scores following sofosbuvir based treatment regimens without interferon. J Gastroenterol Hepatol. 2017;32:1624-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 28. | Bachofner JA, Valli PV, Kröger A, Bergamin I, Künzler P, Baserga A, Braun D, Seifert B, Moncsek A, Fehr J, Semela D, Magenta L, Müllhaupt B, Terziroli Beretta-Piccoli B, Mertens JC. Direct antiviral agent treatment of chronic hepatitis C results in rapid regression of transient elastography and fibrosis markers fibrosis-4 score and aspartate aminotransferase-platelet ratio index. Liver Int. 2017;37:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 195] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 29. | Jacobson IM, Lim JK, Fried MW. American Gastroenterological Association Institute Clinical Practice Update-Expert Review: Care of Patients Who Have Achieved a Sustained Virologic Response After Antiviral Therapy for Chronic Hepatitis C Infection. Gastroenterology. 2017;152:1578-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 30. | Garcia-Tsao G. Regression of HCV cirrhosis: Time will tell. Hepatology. 2018;67:1651-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Labarga P, Fernandez-Montero JV, de Mendoza C, Barreiro P, Pinilla J, Soriano V. Liver fibrosis progression despite HCV cure with antiviral therapy in HIV-HCV-coinfected patients. Antivir Ther. 2015;20:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Garcia-Tsao G. Regression of HCV cirrhosis: Time will tell. Hepatology. 2018;67:1651-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Joy KP, Agha AK. Seasonal effects of administration of melatonin and 5-methoxytryptophol on ovarian activity in the catfish Heteropneustes fossilis (Bloch). J Pineal Res. 1991;10:65-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 392] [Cited by in RCA: 376] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 35. | Barnett AG, van der Pols JC, Dobson AJ. Regression to the mean: what it is and how to deal with it. Int J Epidemiol. 2005;34:215-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1027] [Cited by in RCA: 1197] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 36. | Lingala S, Ghany MG. Natural History of Hepatitis C. Gastroenterol Clin North Am. 2015;44:717-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 183] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 37. | Martinez SM, Foucher J, Combis JM, Métivier S, Brunetto M, Capron D, Bourlière M, Bronowicki JP, Dao T, Maynard-Muet M, Lucidarme D, Merrouche W, Forns X, de Lédinghen V. Longitudinal liver stiffness assessment in patients with chronic hepatitis C undergoing antiviral therapy. PLoS One. 2012;7:e47715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | Nahon P, Bourcier V, Layese R, Audureau E, Cagnot C, Marcellin P, Guyader D, Fontaine H, Larrey D, De Lédinghen V, Ouzan D, Zoulim F, Roulot D, Tran A, Bronowicki JP, Zarski JP, Leroy V, Riachi G, Calès P, Péron JM, Alric L, Bourlière M, Mathurin P, Dharancy S, Blanc JF, Abergel A, Serfaty L, Mallat A, Grangé JD, Attali P, Bacq Y, Wartelle C, Dao T, Benhamou Y, Pilette C, Silvain C, Christidis C, Capron D, Bernard-Chabert B, Zucman D, Di Martino V, Thibaut V, Salmon D, Ziol M, Sutton A, Pol S, Roudot-Thoraval F; ANRS CO12 CirVir Group. Eradication of Hepatitis C Virus Infection in Patients With Cirrhosis Reduces Risk of Liver and Non-Liver Complications. Gastroenterology. 2017;152:142-156.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 401] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 39. | Cheng PN, Chiu HC, Chiu YC, Chen SC, Chen Y. Comparison of FIB-4 and transient elastography in evaluating liver fibrosis of chronic hepatitis C subjects in community. PLoS One. 2018;13:e0206947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2011] [Cited by in RCA: 2293] [Article Influence: 229.3] [Reference Citation Analysis (3)] |
| 41. | Castera L, Pinzani M, Bosch J. Non invasive evaluation of portal hypertension using transient elastography. J Hepatol. 2012;56:696-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 236] [Article Influence: 18.2] [Reference Citation Analysis (0)] |