Published online May 6, 2022. doi: 10.12998/wjcc.v10.i13.4242
Peer-review started: October 21, 2021
First decision: December 17, 2021
Revised: January 4, 2022
Accepted: March 15, 2022
Article in press: March 15, 2022
Published online: May 6, 2022
Processing time: 190 Days and 15.2 Hours
BCR-ABL1 fusion gene is associated with a poor prognosis and a high incidence in central nervous system (CNS) leukemia. CNS invasion which detected at the initial diagnosis is commonly with bone marrow infiltration. It is uncommon for the leukemia cells to be located primarily in the CNS without bone marrow involvement.
We here report the rare initial presentation of CNS-restricted BCR-ABL-positive acute lymphoblastic leukemia in a 30-year-old female patient who clinically manifested with leukemic meningitis, with no involvement in peripheral blood or bone marrow. Identification of abnormal phenotypes of blast cells, and BCR-ABL1 rearrangement in the cerebrospinal fluid alone established the diagnosis of primary CNS-isolated acute lymphocytic leukemia. The patient received a combination of intrathecal therapy and high-dose chemotherapy. But the benefits of the treatments were short-lived and she experienced recurrence.
Flow cytometry in combination with molecular genetic analysis improved diagnostic accuracy. New approaches that may enhance the efficacy of the existing therapies and cure CNS leukemia are required.
Core Tip: We report a rare newly diagnosed case in a female patient with BCR-ABL-positive leukemia cells primarily located in the arachnoid surface and the subarachnoid space, clinically manifesting as leukemic meningitis, without blood and bone marrow involvement. Given the rarity of this specific presentation of B cell-acute lymphocytic leukemia, the diagnosis and treatment approach are challenging.
- Citation: Chen Y, Lu QY, Lu JY, Hong XL. Primary isolated central nervous system acute lymphoblastic leukemia with BCR-ABL1 rearrangement: A case report. World J Clin Cases 2022; 10(13): 4242-4248
- URL: https://www.wjgnet.com/2307-8960/full/v10/i13/4242.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i13.4242
Acute leukemia (AL) is a malignancy hematologic disease of bone marrow (BM)-derived immature cells, which most often involves the BM and peripheral blood but may also manifest in extramedullary tissue. Acute lymphocytic leukemia (ALL) patients appear to have a higher incidence of central nervous system (CNS) disease than acute myelogenous leukemia. CNS leukemia can be present at the initial diagnosis concurrent with bone marrow involvement, but it also can develop at any time during the natural course of AL, even after years of complete remission, as isolated CNS relapse[1]. In a few cases, leukemia cells are isolated to the CNS primarily without involvement of bone marrow. However, these cases are all non-lymphoid leukemia[2-4]. We here report a rare case of newly diagnosed BCR-ABL-positive ALL primarily located in the superficial arachnoid and the subarachnoid space, clinically manifesting as leukemic meningitis, without peripheral blood or bone marrow involvement.
A 30-year-old Chinese woman presented with 2 mo history of unsteadiness while walking starting in May 2020.
A mo later, she presented with progressive difficulty in walking and both lower limbs dragging.
She denied a history of fever, wasting and night sweats.
She had no personal or family history of malignancy.
On physical examination, no lymphadenopathy or organomegaly was detected. The myodynamia of left distant and proximate limb were grade 5- and grade 3+ respectively. The myodynamia of right distant and proximate limb were grade 5- and grade 4- respectively. Meningeal stimulation sign was negative. Cranial nerve examination and sensory examination was within normal limits.
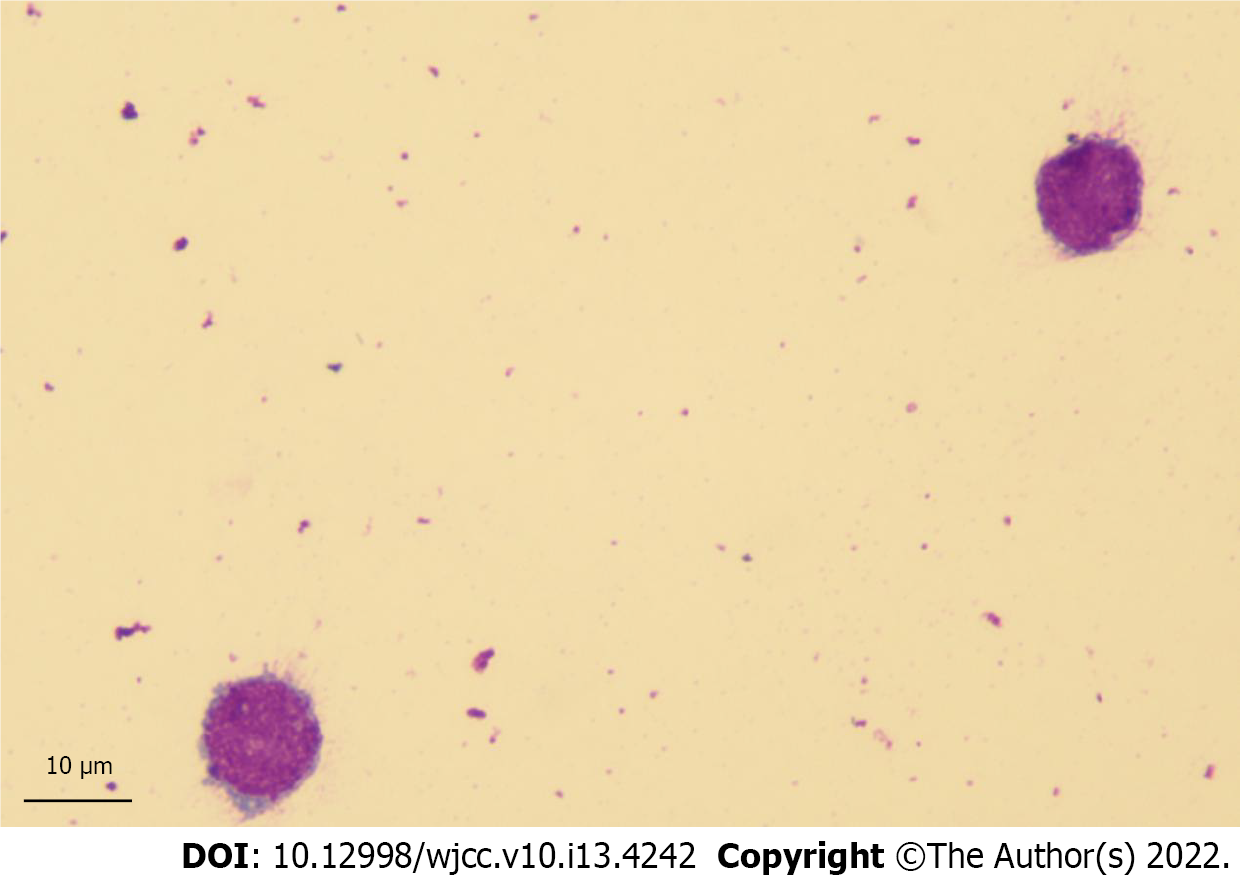
Complete blood counts showed white blood cells 4.08 × 109/L (normal: 4-10 × 109/L), hemoglobin 134 g/L (normal: 110-160 g/L), and platelets 280 × 109/L (normal: 100-300 × 109/L). Peripheral blood smears were negative. Lumbar puncture demonstrated increased opening pressure (220 mm H2O) and was hypercellular with numerous large malignant cells in cerebrospinal fluid (CSF) (Figure 1). As central nervous system leukemia was suspected, the patient referred to our hematology department for further evaluation.
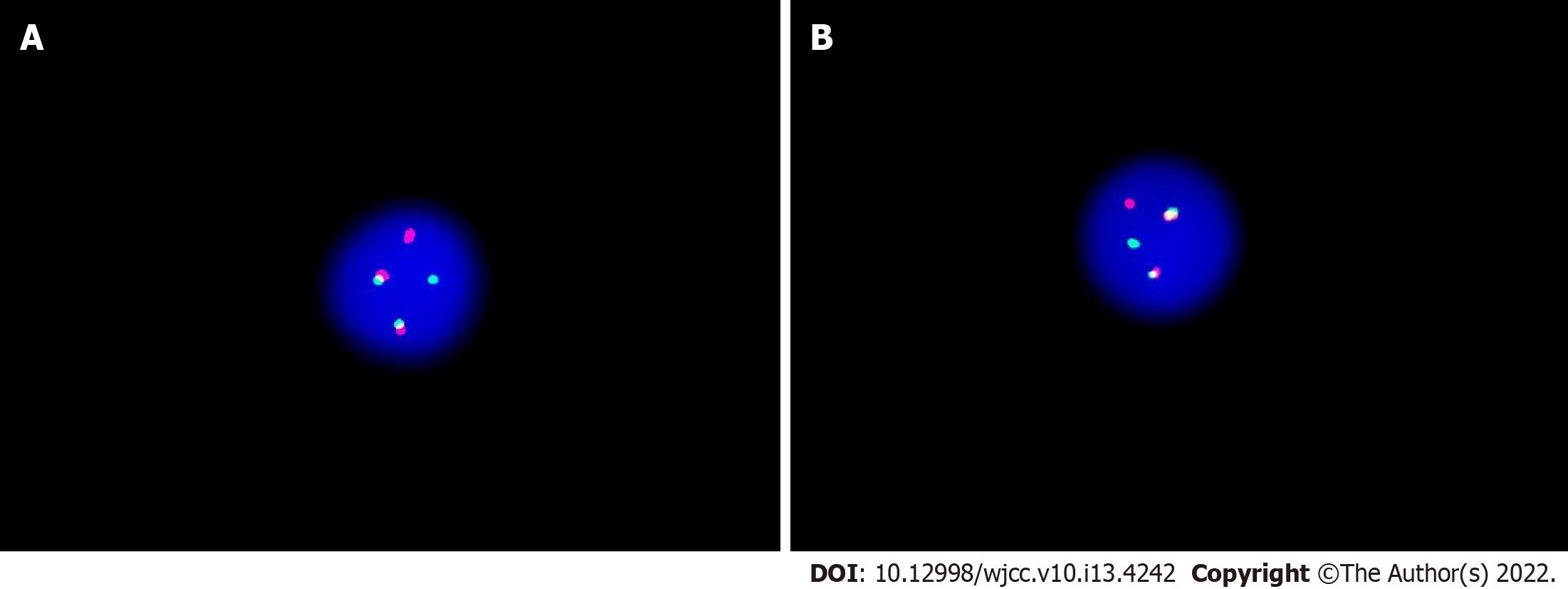
Flow cytometry of lumbar puncture indicated an aberrant lymphoblast population (92.8%); expression of cells of differentiation (CD) 19, CD10, CD34, human leukocyte antigen D related, CD13, and cCD79a; and absence of CD33, CD117, CD20, CD7, CD15, and cytoplasmic myeloperoxidase (Figure 2). Immunophenotype was consistent with pre-B cell-ALL. Polymerase chain reaction (PCR) screening covered 56 transcripts of myeloid fusion proteins and 15 transcripts of lymphoblastic fusion proteins, and showed the presence of BCR-ABL1 chimeric oncogene, encoding proteins of 190 kDa (p190) in CSF. This was quantified by real-time reverse transcriptase-PCR assay and found to be 24.061% (Table 1). Fluorescence in situ hybridization from the CSF revealed that 89.5% of the 200 cells screened had BCR-ABL fusion signals (Figure 3). Next-generation sequencing for BCR-ABL1 kinase domain mutation of CSF malignant cells did not show any mutations.
| Test | Result |
| BCR-ABL1(p190) | Positive |
| BCR-ABL1(p210) | Negative |
| BCR-ABL1 gene copy number | 18580 |
| ABL1 gene copy number | 77222 |
| BCR-ABL1/ABL1 | 24.061% |
Bone marrow biopsies were performed several times, and were normocellular by flow cytometry and cytological analysis. No genetic mutations were found in BM by next-generation sequencing (NGS). PCR screening of the same transcripts with CNS was negative and indicated absence of BCR-ABL1 (p190).
There were no obvious abnormalities on brain and spine magnetic resonance imaging (MRI). Positron emission tomography-computed tomography (PET-CT) scans demonstrated no hypermetabolic involvement in other areas. There was no evidence of occuping diseases.
Primary isolated central nervous system acute lymphoblastic leukemia with BCR-ABL1 rearrangement.
The patient initially received chemotherapy on July 23, 2020. Treatment consisted of methotrexate (3.5 g/m2) on day 1 plus cytarabine (2 g/m2) on day 2 and was combined with triple intrathecal chemotherapy including methotrexate 10 mg, cytarabine 50 mg and dexamethasone 5mg twice a week. After the treatments for 3 wk, she was free from symptoms. CSF cytology, flow cytometry, and BCR-ABL1 (p190) transcripts remained positive. She then underwent three cycles of methotrexate (3.5 g/m2) in combination with cytarabine (2 g/m2) twice daily on days 2 and 3, with repeating treatment every 3 wk and seven courses of intrathecal chemotherapy. CSF cytology became negative, but the disease remained detectable by flow cytometry in September 30, 2020. Due to financial reasons, the patient discontinued treatment.
She returned 4 mo later reporting the same symptoms with positive CSF cytology. Peripheral blood and bone marrow continued to be negative by flow cytometry. The patient received dasatinib (140 mg/d) in combination with steroids and intrathecal agents, but dasatinib was discontinued after 4 wk due to hematologic toxicity. After myelosuppression recovery, CSF cytology remained positive. The patient rejected further systemic drugs and only accepted intrathecal triple therapy intermittently. The patient is currently alive, with positive CSF cytology in September 2021.
BCR-ABL1 fusion is associated with a higher incidence of CNS leukemia than other B cell precursor-ALL[5]. Here, we report a case of BCR-ABL1 positive for ALL that was primarily isolated to CNS with no involvement in blood or bone marrow. Given the rarity of this condition, no standard diagnostic assessment or treatment is currently available. The most valuable diagnostic procedure was the detection of aberrant cells in the CSF. PET-CT and MRI can exclude brain parenchyma and spinal cord occupancy. Laboratory techniques including molecular biology, molecular genetics, and flow cytometry in combination with cytology improved diagnostic accuracy in the ALL with unique presentation[6-8]. In the present case, identification of abnormal phenotype of blast cells, and BCR-ABL1 rearrangement in the CSF alone established the diagnosis of primary CNS-isolated ALL.
Because this is a CNS-restricted disease, treatment protocols were based on the diagnosis of primary CNS lymphoma. The patient showed a clinical response to systemic and intrathecal chemotherapy. High-dose chemotherapy with stem-cell rescue was strongly recommended, but she declined. Unfortunately, the benefits of the treatments were short-lived and she experienced recurrence.
CSF blast cells did not show any BCR-ABL1 kinase domain mutation by sequencing. Imatinib does not penetrate the CNS well[9]. Dasatinib has been reported to exhibit better penetration into the CSF than imatinib in in vitro studies[10]. However, the use of a higher dasatinib dosage (140 mg/d) was unable offer a proper control of the patient’s CNS leukemia. Other case reports also presented that dasatinib failed to prevent CNS progression[11-13]. Factors except for ABL kinase domain mutations might influence dasatinib efficacy in the CNS, such as dose intensity of dasatinib, lack of protein binding, and comedications known to decrease dasatinib plasma concentrations, but data are limited in this regard to come to a conclusion.
The mechanisms fostering primary CNS leukemia are unknown. CNS involvement is a difficult problem in the treatment of ALL. In order to develop novel treatment programmers, it is essential to investigate the molecular mechanisms of CNS leukemia.
Recent studies have shown that anti-CD19 chimeric antigen receptor (CAR) T cell effectively and safely eliminate leukemia cells in the CNS[14,15]. Some patients who received no further therapy for CNS leukemia after CAR-T cells infusion experienced sustained remission for more than 5 mo. Cellular therapies may be able to cross blood-brain barrier in the brain. But systematic studies with larger amount of patients are needed to evaluate the superiority of CAR-T cells in CNS leukemia.
CSF examination is the most useful laboratory test in the diagnosis of the rare primary CNS-isolated ALL. Flow cytometry in combination with PCR/NGS analyses improved diagnostic accuracy and facilitated subsequent analysis of minimal residual disease. Current CNS leukemia treatment strategies are not specific and associated with long-term toxicity. CAR-T cell therapy is a new and promising approach that may improve the efficacy of the existing therapies and eliminate CNS leukemia cells.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Hematology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ghimire R, Nepal; Trifan A, Romania S-Editor: Guo XR L-Editor: A P-Editor: Guo XR
| 1. | Deak D, Gorcea-Andronic N, Sas V, Teodorescu P, Constantinescu C, Iluta S, Pasca S, Hotea I, Turcas C, Moisoiu V, Zimta AA, Galdean S, Steinheber J, Rus I, Rauch S, Richlitzki C, Munteanu R, Jurj A, Petrushev B, Selicean C, Marian M, Soritau O, Andries A, Roman A, Dima D, Tanase A, Sigurjonsson O, Tomuleasa C. A narrative review of central nervous system involvement in acute leukemias. Ann Transl Med. 2021;9:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 2. | Liu H, Guinipero TL, Schieffer KM, Carter C, Colace S, Leonard JR, Orr BA, Kahwash SB, Brennan PJ, Fitch JR, Kelly B, Magrini VJ, White P, Wilson RK, Mardis ER, Cottrell CE, Boué DR. De novo primary central nervous system pure erythroid leukemia/sarcoma with t(1;16)(p31;q24) NFIA/CBFA2T3 translocation. Haematologica. 2020;105:e194-e197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Celebisoy N, Bayam FE, Cağirgan S, Hekimgil M. Primary central nervous system leukemia presenting with an isolated oculomotor palsy. J Clin Neurosci. 2008;15:1144-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Park C, Suh D, Ramey A, Heston C, Abromowitch M. Isolated central nervous system primary acute monoblastic leukemia presenting as papilledema. Pediatr Blood Cancer. 2016;63:2256-2257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Sanchez R, Ayala R, Alonso RA, Martínez MP, Ribera J, García O, Sanchez-Pina J, Mercadal S, Montesinos P, Martino R, Barba P, González-Campos J, Barrios M, Lavilla E, Gil C, Bernal T, Escoda L, Abella E, Amigo ML, Moreno MJ, Bravo P, Guàrdia R, Hernández-Rivas JM, García-Guiñón A, Piernas S, Ribera JM, Martínez-López J. Clinical characteristics of patients with central nervous system relapse in BCR-ABL1-positive acute lymphoblastic leukemia: the importance of characterizing ABL1 mutations in cerebrospinal fluid. Ann Hematol. 2017;96:1069-1075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Bromberg JE, Breems DA, Kraan J, Bikker G, van der Holt B, Smitt PS, van den Bent MJ, van't Veer M, Gratama JW. CSF flow cytometry greatly improves diagnostic accuracy in CNS hematologic malignancies. Neurology. 2007;68:1674-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 166] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 7. | Gaipa G, Cazzaniga G, Valsecchi MG, Panzer-Grümayer R, Buldini B, Silvestri D, Karawajew L, Maglia O, Ratei R, Benetello A, Sala S, Schumich A, Schrauder A, Villa T, Veltroni M, Ludwig WD, Conter V, Schrappe M, Biondi A, Dworzak MN, Basso G. Time point-dependent concordance of flow cytometry and real-time quantitative polymerase chain reaction for minimal residual disease detection in childhood acute lymphoblastic leukemia. Haematologica. 2012;97:1582-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 8. | Wood B, Wu D, Crossley B, Dai Y, Williamson D, Gawad C, Borowitz MJ, Devidas M, Maloney KW, Larsen E, Winick N, Raetz E, Carroll WL, Hunger SP, Loh ML, Robins H, Kirsch I. Measurable residual disease detection by high-throughput sequencing improves risk stratification for pediatric B-ALL. Blood. 2018;131:1350-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 155] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 9. | Takayama N, Sato N, O'Brien SG, Ikeda Y, Okamoto S. Imatinib mesylate has limited activity against the central nervous system involvement of Philadelphia chromosome-positive acute lymphoblastic leukaemia due to poor penetration into cerebrospinal fluid. Br J Haematol. 2002;119:106-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 107] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Porkka K, Koskenvesa P, Lundán T, Rimpiläinen J, Mustjoki S, Smykla R, Wild R, Luo R, Arnan M, Brethon B, Eccersley L, Hjorth-Hansen H, Höglund M, Klamova H, Knutsen H, Parikh S, Raffoux E, Gruber F, Brito-Babapulle F, Dombret H, Duarte RF, Elonen E, Paquette R, Zwaan CM, Lee FY. Dasatinib crosses the blood-brain barrier and is an efficient therapy for central nervous system Philadelphia chromosome-positive leukemia. Blood. 2008;112:1005-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 312] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 11. | Papageorgiou SG, Pappa V, Economopoulou C, Tsirigotis P, Konsioti F, Ionnidou ED, Chondropoulos S, Vasilatou D, Papageorgiou E, Economopoulos T, Dervenoulas J. Dasatinib induces long-term remission in imatinib-resistant Philadelphia chromosome-positive acute megakaryoblastic leukemia but fails to prevent development of central nervous system progression. Leuk Res. 2010;34:e254-e256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Frigeri F, Arcamone M, Luciano L, Di Francia R, Pane F, Pinto A. Systemic dasatinib fails to prevent development of central nervous system progression in a patient with BCR-ABL unmutated Philadelphia chromosome-positive leukemia. Blood. 2009;113:5028-5029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Kiran PK, Badarke GV, Suresh CN, Srinivas BJ, Naik R. Isolated central nervous system blast crisis in a case of chronic myeloid leukemia on dasatinib. South Asian J Cancer. 2018;7:170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | He X, Xiao X, Li Q, Jiang Y, Cao Y, Sun R, Jin X, Yuan T, Meng J, Ma L, Lu W, Lyu C, Liu K, Zhao M. Anti-CD19 CAR-T as a feasible and safe treatment against central nervous system leukemia after intrathecal chemotherapy in adults with relapsed or refractory B-ALL. Leukemia. 2019;33:2102-2104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Rheingold SR, Chen LN, Maude SL, Aplenc R, Barker C, Barrett DM, Callahan C, Cebry K, Kulikovskaya I, Lacey SF. Efficient trafficking of chimeri antigen receptor (CAR)-modified T cells to CSF and induction of durable CNS remissions in children with CNS/combined relapsed/refractory ALL. Blood. 2015;58:434-442. [RCA] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |