Published online May 6, 2022. doi: 10.12998/wjcc.v10.i13.4236
Peer-review started: October 5, 2021
First decision: January 11, 2022
Revised: January 20, 2022
Accepted: March 16, 2022
Article in press: March 16, 2022
Published online: May 6, 2022
Processing time: 206 Days and 14.4 Hours
Paravalvular leak (PVL), also known as paravalvular prosthetic regurgitation, is not a rare complication after surgical valve replacement, and it may cause varying degrees of heart failure. The transcatheter closure of PVL is technically demanding and challenging.
A 68-year-old man presented with degenerative mitral regurgitation with heart failure, New York Heart Association functional class 3. He received bioprosthetic mitral valve replacement in December 2019. PVL was noted at the location of the aorto-mitral curtain in transesophageal echocardiography without signs of endocarditis or dehiscence of the bioprosthetic valve. Transseptal transcatheter closure of the mitral PVL was performed efficiently using the EchoNavigator virtual marker and Agilis NxT steerable introducer.
This case highlights that the EchoNavigator virtual marker and Agilis NxT steerable introducer can facilitate transseptal transcatheter closure of mitral PVL by reducing the procedure time and contrast media.
Core Tip: The transcatheter closure of paravalvular leak (PVL) is technically demanding and can be time consuming. Transseptal transcatheter closure of mitral PVL can be performed efficiently using the EchoNavigator virtual marker (fusion imaging) and Agilis NxT steerable introducer.
- Citation: Hsu JC, Khoi CS, Huang SH, Chang YY, Chen SL, Wu YW. EchoNavigator virtual marker and Agilis NxT steerable introducer facilitate transseptal transcatheter closure of mitral paravalvular leak. World J Clin Cases 2022; 10(13): 4236-4241
- URL: https://www.wjgnet.com/2307-8960/full/v10/i13/4236.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i13.4236
Paravalvular leak (PVL), also known as paravalvular prosthetic regurgitation, is not a rare complication after surgical or transcatheter valve replacement, and it may cause varying degrees of heart failure or hemolysis[1,2]. Paraprosthetic jets have been reported in 10% of aortic and 15% of mitral valves after around 1 year of follow-up in transesophageal echocardiography (TEE)[2]. Surgical repair and transcatheter closure of PVL are both the treatment options. However, transcatheter closure of PVL is technically demanding and can be time consuming. We present this case to demonstrate a more efficient way to close PVL.
A 68-year-old man presented with progressive dyspnea and bilateral lower leg edema. Orthopnea was noted several days before this hospitalization.
He received mitral valve replacement with a Hancock II 29 mm bioprosthesis (Medtronic, Minneapolis, MN) in December 2019 because of degenerative mitral regurgitation with heart failure, New York Heart Association functional class 3.
The patient had not been diagnosed with any diseases.
There was no significant family history.
The patient’s temperature was 36.4°C, heart rate was 98 bpm, respiratory rate was 20 breaths per minute, blood pressure was 132/80 mmHg, and oxygen saturation in room air was 94%. His consciousness was clear and oriented. His jugular vein was engorged, and a holosystolic murmur was heard at the left sternal border. There was also bilateral lower leg pitting edema.
The complete blood count and serum creatinine were within normal limits. No hemolysis was noted from the laboratory findings.
PVL was noted at the location of the aorto-mitral curtain in TEE without signs of endocarditis or dehiscence of the bioprosthetic valve.
Severe mitral PVL with heart failure was diagnosed.
We chose to perform transseptal antegrade transcatheter closure of the PVL rather than redo surgery as the procedure is less invasive. Transapical access would be the alternative if the transseptal approach failed. EchoNavigator transesophageal echocardiographic-fluoroscopic fusion imaging software (Philips Medical Systems, Best, the Netherlands) was used during the procedure (Figure 1). PVL was well visualized at the X-plane (zero and 90°) in TEE. We used the mark function of the EchoNavigator software and easily adjusted marker 1 for the location of the PVL in the X-plane views of TEE. Virtual marker 1 then appeared in real-time fluoroscopic imaging indicating the PVL location. Transseptal puncture was done using an SL0 transseptal sheath (St Jude Medical, 8.5 Fr) and a BRK1 transseptal needle at the superior/mid-anterior fossa ovalis. We changed this to an Agilis NxT steerable introducer (Abbot Vascular, Santa Clara, CA) using Amplatzer Super Stiff wire. The fluoroscopic projection was changed to right anterior oblique to visualize the side view of the bioprosthetic valve. Through a combination of the virtual marker on the fluoroscopic screen and the TEE imaging, we adjusted the Agilis NxT steerable introducer to orient the path to the PVL. After crossing the PVL with Terumo Stiff guidewire (0.035 inches) supported by the telescoping system of a 6 Fr MP guide catheter and 5 Fr JR4 diagnostic catheter, we sequentially deployed two Amplatzer Vascular Plug II (AVP II 12 mm/10 mm; Abbot Vascular, Santa Clara, CA, United States). When a significant reduction in the PVL was noted in TEE imaging without interfering with the function of the mitral bioprosthetic valve (transmitral mean pressure gradient below 2 mmHg), we successfully released the devices one at a time. No contrast media was administered, and the whole procedure was finished within 2 h from skin to skin.
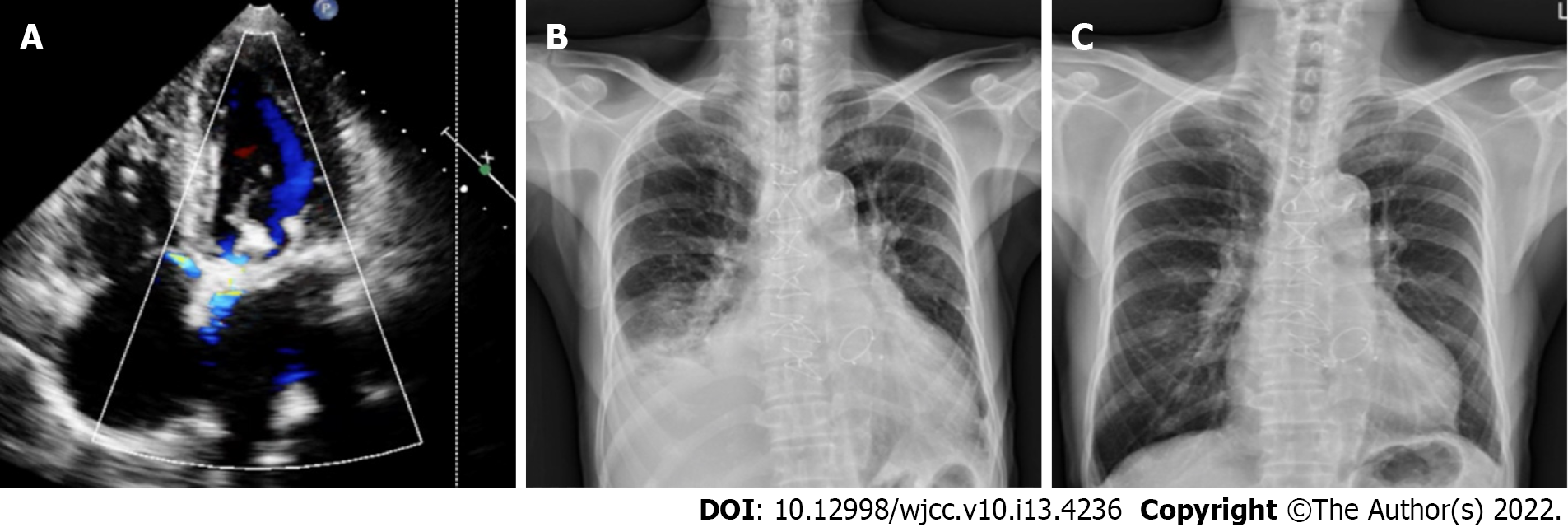
Follow-up echocardiography showed only mild residual paravalvular mitral regurgitation, and chest plain film showed improvements in heart size and bilateral pleural effusion (Figure 2). He currently leads an uneventful life without signs of heart failure, and he was appreciative to the whole team for this episode of care.
Paravalvular leak, also known as paravalvular prosthetic regurgitation, is not a rare complication after surgical valve replacement, and it may cause heart failure[1,2]. Considering the high risk of redo surgery, we planned to perform transseptal antegrade transcatheter closure of the PVL as the procedure is less invasive. The transcatheter closure of PVL has been discussed in previous studies[3,4], and the reported success rate generally ranges from 80% to 90%[5,6]. The clinical success rates and 5-year overall survival rates of transcatheter closure and surgical treatment of PVL are similar, however fewer in-hospital major adverse events have been reported with transcatheter closure[7]. We chose transseptal access rather than retrograde transapical access because it is less invasive and is associated with a lower risk of bleeding. However, there are still advantages in using transapical access, including easier wire probing (especially PVL defects located at the medial aspect and when there is little room for device manipulation) and a shorter procedure time. In the present case, TEE provided precise guidance of transseptal puncture. In contrast to the fixed curve introducer, the Agilis NxT steerable introducer tip can be adjusted to various angles by rotating the knob on the handle. Therefore, we use the Agilis NxT steerable introducer to overcome difficulties when dealing with medial PVL defects. Several occluder devices to close PVL are currently available[4]. Among these devices, Amplatzer Vascular Plug (AVP) III and II are the most commonly used due to the ovoid shape of the lobe of AVP III which can fit the crescentic shape of PVL and the low profile of AVP II.
In our case, a virtual marker indicating the PVL location was shown in real-time on the fluoroscopic screen regardless of the projection angles using EchoNavigator transesophageal echocardiographic-fluoroscopic fusion imaging software. This substantially reduced the time required to orient the steerable introducer and pass the wire through the PVL defect. Fusion imaging using EchoNavigator software is very helpful if the bioprosthetic valve is radiolucent. Other clinical implications of EchoNavigator software include transcatheter edge-to-edge repair of the mitral valve, and transcatheter closure of the left atrial appendage. The procedure can be facilitated by making virtual markers on the site of clipping on the mitral valve and the orifice of the left atrial appendage.
In addition, we sequentially deployed the second AVP II without losing the orientation of the Agilis NxT steerable introducer, and we could pass the wire easily through the defect despite the presence of the first AVP II. Mitral PVL defects are usually crescentic, and if we had used Amplatzer Vascular Plug III we may have been able to close the defect more efficiently. We used real-time three-dimensional TEE imaging (left atrial side view) during the intervention which provided clear imaging without the need for additional radiation exposure or contrast media. Using a combination of fluoroscopy and TEE imaging avoided the need for contrast media, thereby minimizing the risk of further deterioration of renal function.
Transcatheter closure of mitral PVL is feasible and should be the first choice of therapy in experienced centers. Using EchoNavigator virtual marker and Agilis NxT steerable introducer enabled us to finish the challenging intervention more efficiently and safely. Clinical improvements were obvious in our case. Long-term follow-up of these patients is warranted.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Garg A, India; Gulel O, Turkey; Guo L, China; Santomauro M, Italy; Umapathi KK, United States S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Rallidis LS, Moyssakis IE, Ikonomidis I, Nihoyannopoulos P. Natural history of early aortic paraprosthetic regurgitation: a five-year follow-up. Am Heart J. 1999;138:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 162] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 2. | Ionescu A, Fraser AG, Butchart EG. Prevalence and clinical significance of incidental paraprosthetic valvar regurgitation: a prospective study using transoesophageal echocardiography. Heart. 2003;89:1316-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 221] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 3. | Eleid MF, Cabalka AK, Malouf JF, Sanon S, Hagler DJ, Rihal CS. Techniques and Outcomes for the Treatment of Paravalvular Leak. Circ Cardiovasc Interv. 2015;8:e001945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 4. | Gafoor S, Franke J, Bertog S, Lam S, Vaskelyte L, Hofmann I, Sievert H, Matic P. A Quick Guide to Paravalvular Leak Closure. Interv Cardiol. 2015;10:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Sorajja P, Cabalka AK, Hagler DJ, Rihal CS. The learning curve in percutaneous repair of paravalvular prosthetic regurgitation: an analysis of 200 cases. JACC Cardiovasc Interv. 2014;7:521-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Ruiz CE, Jelnin V, Kronzon I, Dudiy Y, Del Valle-Fernandez R, Einhorn BN, Chiam PT, Martinez C, Eiros R, Roubin G, Cohen HA. Clinical outcomes in patients undergoing percutaneous closure of periprosthetic paravalvular leaks. J Am Coll Cardiol. 2011;58:2210-2217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 230] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 7. | Zhang Y, Pan X, Qu X, Wang C, Huang E, Ma L, Wu W, Fang W. Comparison of transcatheter and surgical treatment of paravalvular leak: Results from a 5-year follow-up study. Catheter Cardiovasc Interv. 2019;94:E88-E95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |