Published online May 6, 2022. doi: 10.12998/wjcc.v10.i13.4226
Peer-review started: September 23, 2021
First decision: January 10, 2022
Revised: January 21, 2022
Accepted: March 16, 2022
Article in press: March 16, 2022
Published online: May 6, 2022
Processing time: 218 Days and 18.9 Hours
Thrombotic pulmonary embolism (TPE) is one of the most critical diseases in obstetrics but is rarely reported in caesarean section (CS) because TPE patients in CS have a high risk of death and are difficult to diagnose. This case report of TPE during CS was recorded by transthoracic echocardiography (TTE) and can provide a reference for the differential diagnosis of critical illnesses in CS.
A 37-year-old pregnant woman with rheumatic heart disease (RHD), gravida 5 and para 1 (G5P1), presented for emergency CS at 33 wk and 3 d of gestation under general anesthesia because of acute heart failure, pulmonary hypertension and arrhythmia. After placental removal during CS, TTE revealed a nascent thrombus in the inferior vena cava (IVC) that elongated, detached and fragmented leading to acute thromboembolic events and acute TPE. This report presents the whole process and details of TPE during CS and successful rescue without any sequelae in the patient. This case gives us new ideas for the diagnosis of death or cardiovascular accidents during CS in pregnant women with heart disease and the detailed presentation of the rapid development of TPE may also elucidate new ideas for treatment. This case also highlighted the importance of prophylactic anticoagulation in the management of heart disease during pregnancy.
Pregnancy with heart failure could trigger inferior vena cava (IVC)-origin TPE during CS. Detection and timely treatment can avoid serious consequences.
Core Tip: Thrombotic pulmonary embolism (TPE) is one of the most critical diseases in obstetrics but is rarely reported in caesarean section (CS), especially thrombus originating from the inferior vena cava. This case report describes a 37-year-old pregnant woman with rheumatic heart disease who developed TPE during CS and recorded the whole process by transthoracic echocardiography. This case provided experience for the differential diagnosis of amniotic fluid embolism in CS. This case also reminds us of the importance of pregnancy management and prophylactic anticoagulants in pregnant women with heart disease, and if not, an intensive care team is needed to ensure patient safety.
- Citation: Jiang L, Liang WX, Yan Y, Wang SP, Dai L, Chen DJ. Thrombotic pulmonary embolism of inferior vena cava during caesarean section: A case report and review of the literature. World J Clin Cases 2022; 10(13): 4226-4235
- URL: https://www.wjgnet.com/2307-8960/full/v10/i13/4226.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i13.4226
Thrombotic pulmonary embolism (TPE) occurs when a blood clot wedges into an artery in the lungs[1]. It remains a major cause of cardiovascular and respiratory collapse and maternal mortality during caesarean section (CS)[2-4]. Most reported cases of TPE during pregnancy or CS are considered to be caused by thrombosis originating from the deep veins of the lower limbs or the pelvic veins[5]. In this case report, we present a pregnant patient with rheumatic heart disease (RHD) who developed a nascent thrombus in the inferior vena cava (IVC) during CS. The thrombus elongated to the heart and detached off to the pulmonary artery where it fragmented and resulted in an acute incomplete bilateral pulmonary embolism. It is valuable for us to understand the occurrence, development and pathogenesis of TPE and no previous cases have been reported. Due to the timely diagnosis and treatment during CS, this case did not cause serious consequences, but her pregnancy management deserves our reflection. Moreover, this case also suggests that intraoperative transthoracic echocardiography (TTE) has very important clinical value in critically pregnant patients for monitoring safety when undergoing CS.
A 37-year-old pregnant woman at 33 wk and 3 d of gestation had been diagnosed with RHD 4 mo ago. She was admitted to the intensive care unit (ICU) of our hospital complaining of orthopnea, anhelation and lower extremity edema.
The patient was first diagnosed with RHD at 12 wk of gestation. She developed cardiac enlargement with moderate pulmonary hypertension (PH) at 28 wk of gestation followed by atrial fibrillation (AF) at 29 wk of gestation. At 33 wk of gestation, she experienced severe heart failure which had worsened in the last 24 h.
The patient was gravida 5, 1-0-0-0-1, with 3 spontaneous abortions because of embryo hepatocytes. The outcome of her first pregnancy more than 10 years earlier was a natural birth without any discomfort.
The patient denied a history of thrombosis, smoking, tuberculosis, and alcohol or drug use. No family members had similar diseases.
The patient’s heart rate was 145 beats/min, blood pressure was 113/82 mmHg, respiratory rate was 25 breaths/min, and oxygen saturation by pulse oximetry (SaO2) was 100% on room air. The patient’s current weight was 82 kg, BMI (before pregnancy) was 25.3 kg/m2, a total weight gain was 15 kg during pregnancy. There were no obvious abnormalities in the fetus.
The patient’s N-terminal pro brain-type natriuretic peptide was 1880 pg/mL, activated partial thromboplastin time (APTT) was 27.0 s, fibrinogen was 4.45 g/L, and D-dimer was 660 ng/mL. The cardiogram showed AF. The urine analysis and arterial blood gas was normal.
The TTE showed RHD with severe mitral regurgitation (MR), mild mitral stenosis, moderate aortic regurgitation (AR), severe tricuspid regurgitation (TR), heart dilatation, left ventricular ejection fraction (LVEF) of 52% and a pulmonary artery systolic pressure (PASP) of 75 mmHg. The patient’s right heart (RH) chamber was enlarged and the left ventricle was hypokinetic. The routine TTE monitoring in pregnancy are shown in Table 1.
| Gestation (W) | LAD (mm) | LVDD (mm) | RA (mm) | LVEF (%) | LVFS (%) | MVA (cm2) | MR (cm2) | AR (cm2) | TR (cm2) | PASP (mmHg) | Cardiac rhythm |
| 28 + | 63 | 58 | 56 × 41 | 58 | 31 | 2.3 | 23.3 | 2.7 | 5.1 | 63 | Normal |
| 29 + | 64 | 61 | 54 × 40 | 67 | 38 | 2.3 | 17.6 | 3.6 | 3.5 | 46 | AF |
| 30 + | 65 | 59 | 51 × 43 | 66 | 37 | 2.3 | 18.4 | 3.6 | 2.1 | 37 | AF |
| 33 + | 65 | 54 | 60 × 48 | 52 | 27 | 2.3 | 26.7 | 4.0 | 14.5 | 75 | AF |
| Preoperative day 1 | 63 | 54 | 59 × 51 | 52 | 27 | 2.3 | 24.6 | 4.2 | 14.3 | 53 | AF |
| Postoperative day 1 | 62 | 52 | 58 × 46 | 45 | 22 | 2.3 | 19.5 | 7.3 | 8.1 | 54 | AF |
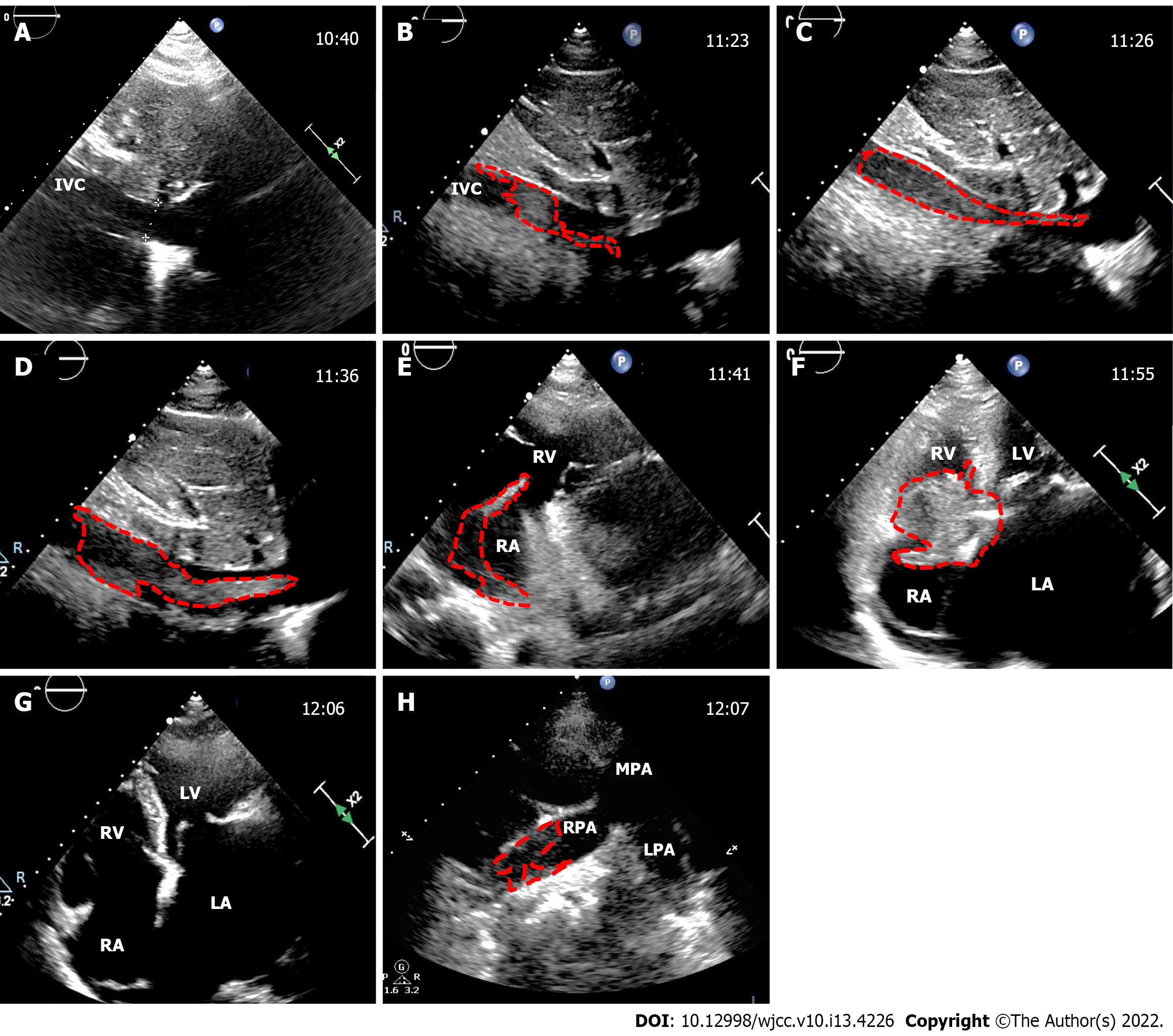
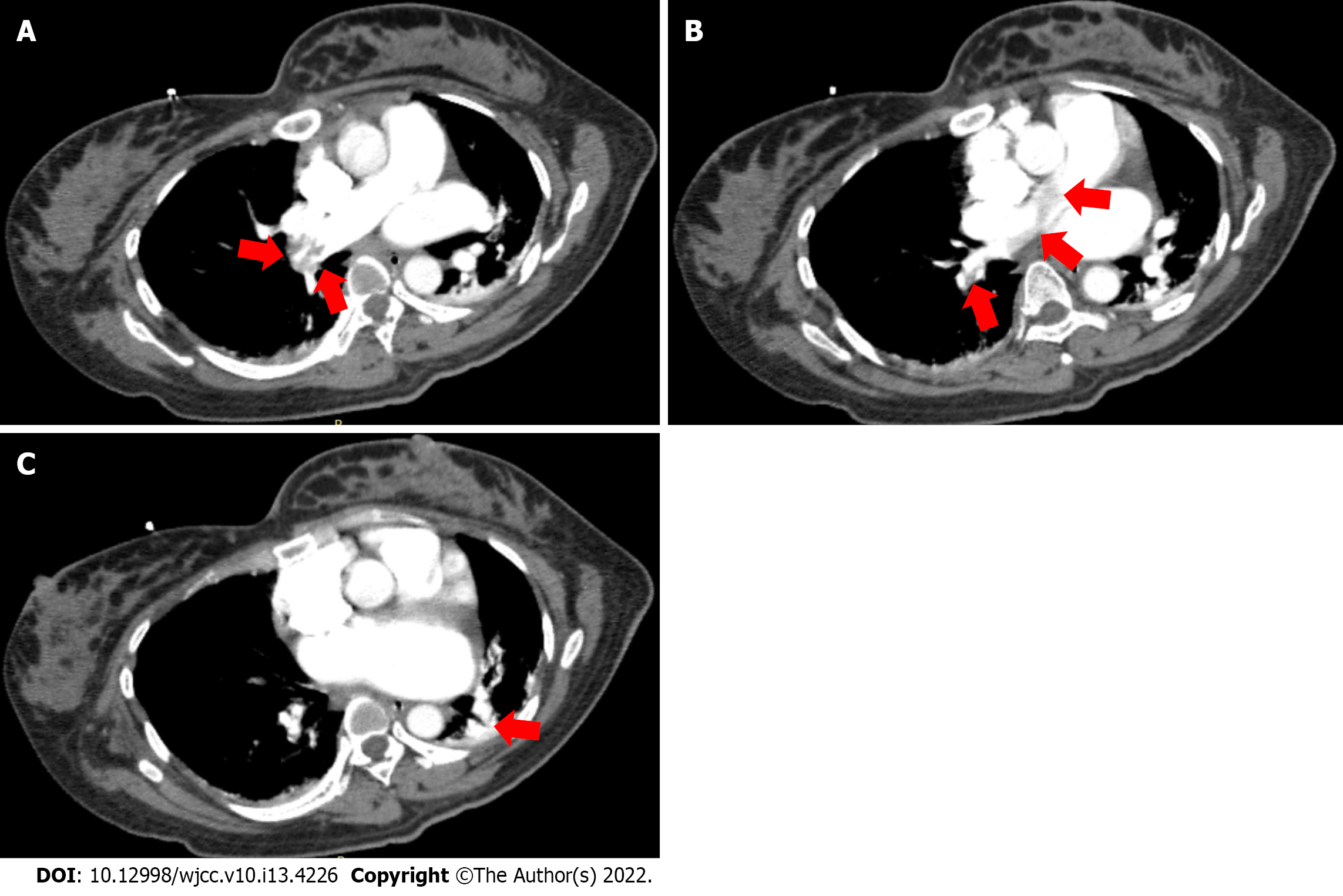
During the CS, the TTE revealed a nascent thrombus that fluttered in a serpentine manner in the IVC after placental removal. The thrombus grew rapidly. From 81 mm at discovery to 155 mm in 18 min. The average growth rate was 4.1 mm/min. At 32 min after discovery, the thrombus detached to the tricuspid valve. At 43 min after discovery, the thrombus detached off to the pulmonary artery, where it fragmented and resulted in acute incomplete bilateral pulmonary embolism, and contemporaneously with the PASP increasing sharply to 93 mmHg, SaO2 decreased to 96%. Moreover, small thrombosis attachment to the mitral valve and left atrial appendage (LAA) were detected. The detailed thrombotic changes are shown in Figure 1. Postoperative computed tomography angiography showed that the right main pulmonary artery, the middle and lower branch of the right pulmonary artery and its branches, and the left lower branch of the pulmonary artery were embolized (Figure 2). Ultrasonic examination revealed no deep vein thrombus of the lower extremities during the perioperative period.
When TTE discovered the IVC thrombus, we urgently organized the multidisciplinary team (MDT) discussion for rescue plan. The team included the department of obstetrics, anesthesiology, vascular surgery, cardiology, cardiac surgery, ICU, ultrasound medicine, etc. The obstetrician’s advice was that the patient with heart disease already has two children and that the uterus could be removed in this emergency to avoid the risk of massive bleeding. The anesthesiology and cardiology’s advice were that once the fetus had been removed, thrombolytic and anticoagulant therapy should be performed immediately and the circulatory system could be stabilized through various drugs to ensure the safety of the maternal life. Vascular surgery suggested that ultrasound-guided vascular interventional thrombolytic therapy could be performed if a TPE occurred. Cardiac surgery suggested emergency thoracotomy for thrombus removal when those treatments failed. The ICU recommended extracorporeal membrane oxygenation at all times to ensure maternal safety.
The patient was diagnosed with a TPE.
At 12 wk of gestation, the patient had no PH or clinical symptoms. After health education, the physician required a regular follow-up to prenatal examination once a month in the second trimester, but the patient did not comply.
At 28 wk of gestation, the patient was referred to the critical maternal care center of our hospital for cardiac enlargement and moderate PH and developed AF at 29 wk. The patient did not show significant discomfort. Through diuretic and cardiotonic property treatments, cardiac indicators decreased, accompanied by mild PH. Considering that the patient had no history of thrombosis and related family history, prophylactic anticoagulation was not performed during the 2 wk of treatment and was discharged to home. The patient was advised to return for examinations 3 d later but the patient did not comply.
At 33 wk of gestation, she experienced severe heart failure and was admitted to the ICU at our hospital. The patient was treated for cardio tonicity, diuresis and the reduction in PASP but the signs and symptoms of heart failure were not alleviated after 2 d. After an MDT discussion, emergency CS was performed under general anesthesia, and TTE was used for intraoperative monitoring for patient safety.
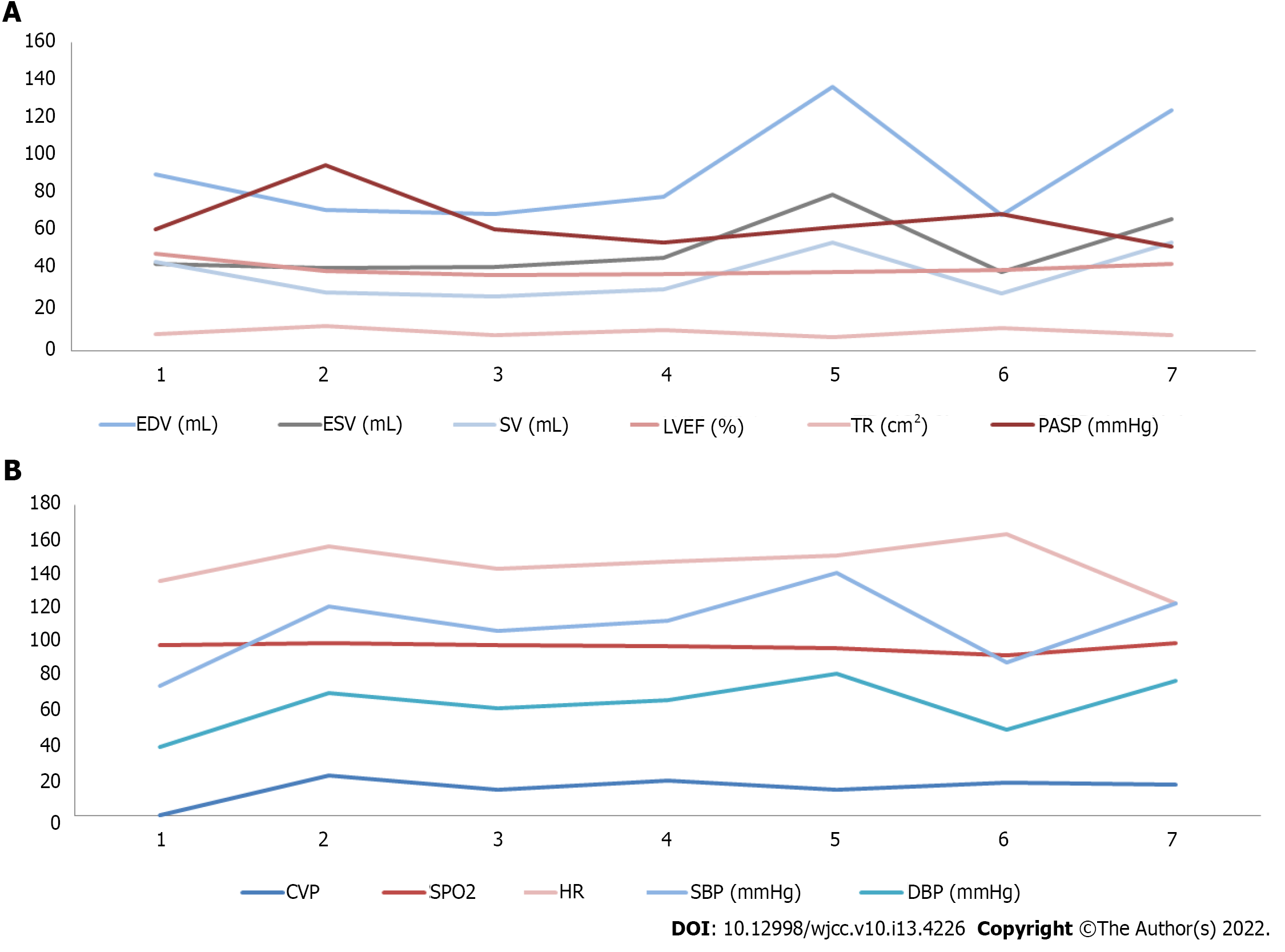
During the CS, we discovered the IVC thrombus and made an MDT discussion and informed consent with the patient’s family. Low molecular weight heparin (LMWH) at 140 mL intravenous injection was performed immediately. After hysterectomy, 60 mL and 50 mL LMWH were performed separately and the SaO2 increased to 98%, PASP gradually decreased to 71 mmHg and the patient’s condition stabilized. The patient’s cardiac volume and dynamics indexes during the perioperative period of CS is shown in Figure 3.
After the operation, the patient was treated with LMWH 30 mL q12 h for 3 d, and the patient’s SaO2 stabilized at 100%. The patient’s condition remained stable. One week later she was discharged home with warfarin 2.5 mg qd anticoagulant therapy. Details and timeline of thrombosis development and anticoagulation process are shown in Table 2.
| Check times | Patient states | End of the thrombus reaches | Thrombus size (long × thick, mm) | Tr (cm2) | Pasp (mmHg) | SaO2 (%) | Act (s) | Lmwh dose (mL) |
| 10:40 am | 20 min before CS | - | - | 8.5 | 45 | 99 | - | - |
| 11:23 am | After removed fetus | IVC | 81 × 11 | 10.8 | 56 | 96 | - | - |
| 11:26 am | Suturing the uterus | IVC entrance | 86 × 12 | 13.7 | 56 | 96 | - | - |
| 11:36 am | After uterine suture | RA | 100 × 13 | 10.7 | 54 | 98 | - | - |
| 11:41 am | Ligation uterine arteries | Tricuspid valve | 155 × 13 | 6.8 | 64 | 99 | - | - |
| 11:55 am | Suturing the skin | Fall off to RH | 55 × 28 | 5.0 | 69 | 98 | 114 | 140, iv |
| 12:06 am | Informed consent | Fall off to LPA, RPA | Undeterminable | 12.2 | 93 | 96 | 380 | - |
| 12:10 am | Hysterectomy | Located at LPA, RPA | Undeterminable | 11.0 | 90 | 97 | 429 | 60, iv |
| 1:50 pm | Closed abdomen | MV and LAA appeared thrombus | 17 × 15 in LAA, others undeterminable | 11.6 | 71 | 98 | 384 | 50, iv |
| Postoperative day 1 | In ICU | Located at LPA, RPA, LAA | 17 × 15 in LAA, Others undeterminable | 8.1 | 54 | 100 | - | 30/q12h, HI |
After treatment of the patient, the heart failure gradually improved and hemodynamics returned to normal. The patient was followed up for 6 mo and showed no discomfort, such as, shortness of breath or dyspnea during this period.
Heart disease increases the risk of complications during pregnancy, especially during the perinatal period and CS[6]. Pregnancy in women with heart disease will not only lead to the deterioration of heart disease but also increase the mortality during CS, especially in patients with PH, whose perinatal mortality is 5% to 36%[7-9], and the cause of death is often complex and varied.
The pregnant woman in this case had RHD and a thrombus occurred in the IVC rather than in the LAA or deep veins of the lower limbs. The possible reasons are as follows: (1) There may have been injury to the endothelium of the IVC because the patient had long-term right heart overload and a considerable amount of TR in the third trimester of pregnancy. and the patient’s IVC had widened with a collapsible index of less than 50%. Therefore, damage to the endothelium of the IVC, which is one of the conditions for the development of a thrombus can be easily caused[10]; (2) PH increases the resistance of the IVC blood flow to the heart. The patient in this case developed severe PH which resulted in an unstable blood flow state. PH also caused right heart failure and severe TR. These factors cause red blood cells to accumulate in the IVC which is another condition for the development of thrombi; (3) the blood is hypercoagulable during pregnancy; (4) CS itself is a risk factor for venous thrombosis[11]; and (5) AF and neither standardized anticoagulation therapy nor anti-heart failure treatment were administered during pregnancy. Therefore, those factors might have triggered thrombosis during CS[12,13].
We reviewed that the patient’s pregnancy was poorly managed, the patient lacked awareness about her disease and physicians failed to follow up with the patient in time resulting in the failure of strict management of the pregnant patient with heart disease as required. In addition, physicians’ insufficient understanding of prophylactic anticoagulation during pregnancy was also a reason. The patient was at multiple risk of thromboses, such as pregnancy, CS, RHD, PH and AF; prophylactic anticoagulation should be considered to reduce the risk of perinatal maternal death[14,15].
The patient reported in this case was fortunate to avoid serious adverse events. From this case, we also learned that comprehensive anti-risk management is important if we encounter patients who fail to receive standardized treatment during pregnancy. Intraoperative TTE can be used to monitor the heart for perioperative management.
Some case reports indicate that intraoperative TPE occurs after removal of the placenta during CS[16,17]. In this case, the placenta had just been removed when we detected the thrombus and TPE occurred 43 min after removal of the placenta. In addition, the sharp increase in PASP during the suction of amniotic fluid may be an adverse factor. We speculate that the time of thrombus development may have been after amniotic fluid absorption. Therefore, TTE can be used for key monitoring at these two stages during CS for the early detection of anomalies. In this case, the development and changes in the thrombus moved rapidly but without serious consequences. We consider that the morphology and timely dissolution of the thrombus by total heparinization treatment stopped it from occluding the main pulmonary artery and prevented thrombus enlargement in other areas such as the LAA and mitral valve. Therefore, the patient was able to avoid cardiovascular and respiratory collapse.
TPE patients present with wheezing, dyspnea, chest pain or gasping. If not treated correctly, disseminated intravascular coagulation (DIC) can occur[18]. Therefore, it is necessary to distinguish amniotic fluid embolism (AFE). Venous air embolism (VAE) during CS also needs to be differential with TPE. TPE, AFE and VAE are the most common critical diseases in CS. They have the same clinical features including PH, heart failure, hypoxia, hypotension and DIC. However, their pathological mechanisms are completely different.
AFE is an exclusive diagnosis that excludes complications of CS with similar clinical presentations, such as acute pulmonary embolism, acute myocardial infarction, perinatal cardiomyopathy, anaphylactic shock, and anesthesia accidents. Intraoperative TTE can be used to diagnose the differential of these diseases. The patients with AFE have no specificity image by TTE. The main manifestations image are cardiac enlargement, PH, cardiac dysfunction and cardiac arrest[19-22]. However, cardiac abnormalities in patients with acute TPE usually manifest as an increase in the right heart volume, a decrease in the left heart volume and right heart dysfunction[23]. These differences can be distinguished from AFE, but if the patient has a pre-existing heart disease, the cardiac changes in acute pulmonary embolism are atypical and can be identified by finding an embolus as reported in this case.
VAE is extremely rare, air pulmonary embolism (APE) is the most common type[24]. The disease progression and clinical symptoms of APE and TPE were not significantly different. Their differentiation is the acoustic characteristics of the embolus[25]. In APE, a large amount of air entering the right heart can mix with the blood to form foamy blood, which can lead to circulatory failure and even death[26]. The TTE acoustic characteristics of foamy blood show strong echoes of bubbles of different sizes in the blood. Bubbles can also attach to the endocardium and chordae tendineae and color Doppler displays show that laminar flow was replaced by turbulence in the heart cavity[27]. It is important to observe granular bubbles in the right heart by TTE before developing APE.
Intraoperative echocardiographic monitoring has received increasing attention[28,29]. Intraoperative echocardiographic monitoring is an advantage to obtain cardiac structural images and data than other methods. In addition, it has no effect on the surgical process but it must be performed by doctors with specialized training. Its main applications include: (1) Routine surgical monitoring by transesophageal echocardiography (TEE), which is mainly used for real-time monitoring of changes in patients’ cardiac function and blood volume, especially elderly patients or patients whose volume needs strict control[30]; (2) intraoperative monitoring of cardiovascular surgery by TEE can improve the success rate of surgery and reduce the occurrence of surgical complications. For example, in valve replacement, plastic surgery, left atrial auricular occlusion, atrial septal puncture, etc. Intraoperative TEE can accurately provide images and data for surgeons[31-33]; and (3) intraoperative monitoring of non-cardiovascular surgery by TTE. Compared with TEE, TTE is safer to operate and has larger echo windows in which more information is available. TTE is mainly used to monitor potential cardiovascular problems during surgery. However, preoperative evaluation and intraoperative risk factor analysis should be performed in advance according to each patient’s condition to formulate the main monitoring content and prevent the occurrence of unknown risks[29,34].
Pregnancy with heart failure could trigger TPE during CS if the patient is not strictly managed and administered prophylactic anticoagulation. In addition to the deep veins of the lower limbs and pelvic veins, thrombi may form in the IVC. The speed of thrombus aggregation in CS is very rapid, and timely treatment can greatly reduce the occurrence of serious complications. Intraoperative TTE can be used to detect thrombosis timely and distinguish TPE from AFE and APE.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Obstetrics and gynecology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ito S, Japan; Socea B, Romania S-Editor: Chen YL L-Editor: Filipodia P-Editor: Chen YL
| 1. | Ortel TL, Neumann I, Ageno W, Beyth R, Clark NP, Cuker A, Hutten BA, Jaff MR, Manja V, Schulman S, Thurston C, Vedantham S, Verhamme P, Witt DM, D Florez I, Izcovich A, Nieuwlaat R, Ross S, J Schünemann H, Wiercioch W, Zhang Y. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020;4:4693-4738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 831] [Article Influence: 166.2] [Reference Citation Analysis (0)] |
| 2. | Shirazi M, Sahebdel B, Torkzaban M, Feizabad E, Ghaemi M. Maternal mortality following thromboembolism; incidences and prophylaxis strategies. Thromb J. 2020;18:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Lai J, Venu I, Malinowski AK, Gandhi S, McLeod A, Nisenbaum R, Zaffar N, Shehata N. Thromboembolism following cesarean section: a retrospective study. Hematology. 2018;23:351-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Bates SM, Rajasekhar A, Middeldorp S, McLintock C, Rodger MA, James AH, Vazquez SR, Greer IA, Riva JJ, Bhatt M, Schwab N, Barrett D, LaHaye A, Rochwerg B. American Society of Hematology 2018 guidelines for management of venous thromboembolism: venous thromboembolism in the context of pregnancy. Blood Adv. 2018;2:3317-3359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 349] [Article Influence: 58.2] [Reference Citation Analysis (0)] |
| 5. | Sousa Gomes M, Guimarães M, Montenegro N. Thrombolysis in pregnancy: a literature review. J Matern Fetal Neonatal Med. 2019;32:2418-2428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Sharma G, Ying W, Silversides CK. The Importance of Cardiovascular Risk Assessment and Pregnancy Heart Team in the Management of Cardiovascular Disease in Pregnancy. Cardiol Clin. 2021;39:7-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Zhou Q, Peng P, Liu X, Liu J, Gao J, Chen W. Evaluation of maternal and fetal outcomes in pregnancy complicated with pulmonary arterial hypertension. Ann Palliat Med. 2021;10:1404-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Low TT, Guron N, Ducas R, Yamamura K, Charla P, Granton J, Silversides CK. Pulmonary arterial hypertension in pregnancy-a systematic review of outcomes in the modern era. Pulm Circ. 2021;11:20458940211013671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 9. | Yang JZ, Fernandes TM, Kim NH, Poch DS, Kerr KM, Lombardi S, Melber D, Kelly T, Papamatheakis DG. Pregnancy and pulmonary arterial hypertension: a case series and literature review. Am J Obstet Gynecol MFM. 2021;3:100358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Dargaud Y, Rugeri L, Fleury C, Battie C, Gaucherand P, Huissoud C, Rudigoz RC, Desmurs-Clavel H, Ninet J, Trzeciak MC. Personalized thromboprophylaxis using a risk score for the management of pregnancies with high risk of thrombosis: a prospective clinical study. J Thromb Haemost. 2017;15:897-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Cao W, Ni X, Wang Q, Li J, Li Y, Chen T, Wang X. Early diagnosis and precision treatment of right ovarian vein and inferior vena cava thrombosis following caesarean section: A case report. Exp Ther Med. 2020;19:2923-2926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Trahan MJ, Bastrash MP, Mardigyan V, Klam S. Management of New-Onset Atrial Fibrillation in Pregnancy: When Should Early Delivery Be Considered? J Obstet Gynaecol Can. 2020;42:1012-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Mehta LS, Warnes CA, Bradley E, Burton T, Economy K, Mehran R, Safdar B, Sharma G, Wood M, Valente AM, Volgman AS; American Heart Association Council on Clinical Cardiology; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; and Stroke Council. Cardiovascular Considerations in Caring for Pregnant Patients: A Scientific Statement From the American Heart Association. Circulation. 2020;141:e884-e903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 239] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 14. | Salciccioli KB, Cotts TB. Pregnancy in Women with Adult Congenital Heart Disease. Cardiol Clin. 2021;39:55-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Ferrara F, Zhou X, Gargani L, Wierzbowska-Drabik K, Vriz O, Fadel BM, Stanziola AA, Kasprzak J, Vannan M, Bossone E. Echocardiography in Pulmonary Arterial Hypertension. Curr Cardiol Rep. 2019;21:22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Oda Y, Fujita M, Motohisa C, Nakata S, Shimada M, Komatsu R. Pulmonary embolism caused by ovarian vein thrombosis during cesarean section: a case report. JA Clin Rep. 2018;4:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Anderson DJ, Liu H, Kumar D, Patel M, Kim S. Placenta Percreta Complications. Cureus. 2021;13:e18842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Hepburn-Brown M, Darvall J, Hammerschlag G. Acute pulmonary embolism: a concise review of diagnosis and management. Intern Med J. 2019;49:15-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 19. | Hassan R, Ferland A, Hawkins TL, Miller RJH. McConnell’s Sign in a Patient with Amniotic Fluid Embolism and Severe Right Ventricular Dysfunction. CASE (Phila). 2021;5:354-357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Lemoine S, Jost D, Barre-Drouard C, Tourtier JP. Re: Tawfik M.M., et al "Circulatory collapse in a parturient undergoing cesarean delivery: a diagnostic dilemma.". Int J Obstet Anesth. 2018;33:97-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Cahan T, De Castro H, Kalter A, Simchen MJ. Amniotic fluid embolism - implementation of international diagnosis criteria and subsequent pregnancy recurrence risk. J Perinat Med. 2021;49:546-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Pilecky D, Sollfrank R, Wiesinger T, Balogh E, Elsner D. Echocardiographic diagnosis of amniotic fluid embolism with paradoxical embolism. Eur Heart J Cardiovasc Imaging. 2021;22:e150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Pacheco LD, Clark SL, Klassen M, Hankins GDV. Amniotic fluid embolism: principles of early clinical management. Am J Obstet Gynecol. 2020;222:48-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 24. | Brull SJ, Prielipp RC. Vascular air embolism: A silent hazard to patient safety. J Crit Care. 2017;42:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 25. | Al-Afif S, Elkayekh H, Omer M, Heissler HE, Scheinichen D, Palmaers T, Nakamura M, Hermann EJ, Samii M, Krauss JK. Analysis of risk factors for venous air embolism in the semisitting position and its impact on outcome in a consecutive series of 740 patients. J Neurosurg. 2021;1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Guo JL, Wang HB, Wang H, Le Y, He J, Zheng XQ, Zhang ZH, Duan GR. Transesophageal echocardiography detection of air embolism during endoscopic surgery and validity of hyperbaric oxygen therapy: Case report. Medicine (Baltimore). 2021;100:e26304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Rahman ZU, Murtaza G, Pourmorteza M, El Minaoui WK, Sethi P, Mamdouhi P, Paul T. Cardiac Arrest as a Consequence of Air Embolism: A Case Report and Literature Review. Case Rep Med. 2016;2016:8236845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Nagre AS. Focus-assessed transthoracic echocardiography: Implications in perioperative and intensive care. Ann Card Anaesth. 2019;22:302-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 29. | Vojnar B, Zoremba M. [Focused Transthoracic Echocardiography in the Perioperative Setting]. Anasthesiol Intensivmed Notfallmed Schmerzther. 2019;54:90-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Fayad A, Shillcutt SK. Perioperative transesophageal echocardiography for non-cardiac surgery. Can J Anaesth. 2018;65:381-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Elsherbiny M, Abdelwahab Y, Nagy K, Mannaa A, Hassabelnaby Y. Role of Intraoperative Transesophageal Echocardiography in Cardiac Surgery: an Observational Study. Open Access Maced J Med Sci. 2019;7:2480-2483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Prempeh ABA, Scherman J, Swanevelder JL. Transesophageal echocardiography in minimally invasive cardiac surgery. Curr Opin Anaesthesiol. 2020;33:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Subramaniam K, Ibarra A, Boisen ML. Echocardiographic Guidance of AMPLATZER Amulet Left Atrial Appendage Occlusion Device Placement. Semin Cardiothorac Vasc Anesth. 2019;23:248-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Saranteas T, Manikis D, Papadimos T, Mavrogenis AF, Kostopanagiotou G, Panou F. Intraoperative TTE inferior vena cava monitoring in elderly orthopaedic patients with cardiac disease and spinal-induced hypotension. J Clin Monit Comput. 2017;31:919-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |