Published online May 6, 2022. doi: 10.12998/wjcc.v10.i13.4220
Peer-review started: September 12, 2021
First decision: February 14, 2022
Revised: February 23, 2022
Accepted: March 15, 2022
Article in press: March 15, 2022
Published online: May 6, 2022
Processing time: 229 Days and 14.7 Hours
Primary trigeminal neuralgia can achieve satisfactory results through clinical treatment and intervention. The pathogenesis of neuralgia caused by varicella-zoster virus infection of the trigeminal nerve is more complex, and it is still difficult to relieve the pain in some patients simply by drug treatment or surgical intervention.
A 66-year-old woman was hospitalized with herpetic neuralgia after herpes zoster ophthalmicus (varicella-zoster virus infects the ophthalmic branch of the trigeminal nerve). On admission, the patient showed spontaneous, electric shock-like and acupuncture-like severe pain in the left frontal parietal region, and pain could be induced by touching the herpes area. The numerical rating scale (NRS) was 9. There was no significant pain relief after pulsed radiofrequency and thermocoagulation of the ophthalmic branch of the trigeminal nerve. Combined with patient-controlled intravenous analgesia (PCIA) with esketamine, neuralgia was significantly improved. The patient had no spontaneous pain or allodynia at discharge, and the NRS score decreased to 2 points. The results of follow-up 2 mo after discharge showed that the NRS score was ≤ 3, and the Pittsburgh Sleep Quality Index score was 5 points. There were no adverse reactions.
Trigeminal extracranial thermocoagulation combined with esketamine PCIA may be a feasible method for the treatment of refractory herpetic neuralgia after herpes zoster ophthalmicus.
Core Tip: We applied thermocoagulation combined with esketamine intravenous controlled analgesia for the first time in the treatment of refractory herpetic neuralgia after herpes zoster ophthalmicus.
- Citation: Tao JC, Huang B, Luo G, Zhang ZQ, Xin BY, Yao M. Trigeminal extracranial thermocoagulation along with patient-controlled analgesia with esketamine for refractory postherpetic neuralgia after herpes zoster ophthalmicus: A case report. World J Clin Cases 2022; 10(13): 4220-4225
- URL: https://www.wjgnet.com/2307-8960/full/v10/i13/4220.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i13.4220
Varicella-zoster virus infection can cause varicella and latency in the sensory ganglia, and its reactivation causes herpes zoster in the corresponding innervated area. Older adults and those with impaired immune function are prone to the disease[1]. The most commonly involved site is the thoracic region, followed by the cranial (especially trigeminal), lumbar, cervical and sacral regions. The first branch of the trigeminal nerve (ophthalmic branch) is the most frequently involved branch of the trigeminal nerve, also known as herpes zoster ophthalmicus (HZO), which accounts for 10%-20% of all cases of herpes zoster[2]. This high incidence may be related to long-term ultraviolet exposure[3]. HZO can lead to keratitis and even blindness[4]. In addition, patients may develop postherpetic neuralgia (PHN), so early intervention is important.
Treatments for herpes zoster mainly includes oral analgesics, nerve block and radiofrequency. Radiofrequency in the trigeminal ganglion is a minimally invasive, quickly effective and safe method for the treatment of HZO[5-7]. However, there are still some patients with HZO who cannot be effectively relieved after the above treatment in the clinic. This seriously affects the daily lives of patients. Therefore, it is urgent to explore a new treatment to relieve HZO neuralgia. Ketamine is an N-methyl-D-aspartate receptor (NMDAR) that has been shown to relieve a variety of neuropathic pain, such as complex regional pain syndrome[8], PHN[9], cancer pain[10] and trigeminal neuralgia[11]. We used ketamine for patient-controlled intravenous analgesia (PCIA) for the first time in the treatment of ocular herpes zoster neuralgia, in a new treatment approach for refractory HZO.
A 66-year-old woman, weight 46 kg and height 155 cm, was hospitalized with left frontal parietal herpes for 20 d with pain for 3 d.
At the beginning of the disease, the main manifestation was herpes in the left frontal parietal region without pain. Herpes gradually improved after antiviral treatment [valaciclovir capsules (0.3 g bid) was taken orally and acyclovir cream was applied externally for one week]. Seventeen days later, pain appeared in the herpes area, which was characterized by paroxysmal, acupuncture-like and electric shock-like pain. The frequency of pain attacks was approximately 1 per hour, and each attack lasted from 30 s to 2 min. The numerical rating scale (NRS) was 9. There was no significant pain relief after oral painkillers, resulting in serious impacts to the patient’s daily life.
The patient had a history of diabetes for more than 10 years and was taking metformin sustained release tablets (0.5 g qd) for hypoglycemic treatment. The patient underwent subtotal gastrectomy because of gastric cancer in three years ago.
There was no significant family history and physical examination was normal.
There was no significant family history and physical examination was normal.
All laboratory investigations and imaging investigations were normal including the liver function.
All laboratory investigations and imaging investigations were normal including the liver function.
Postherpetic neuralgia after herpes zoster ophthalmicus; Diabetes.
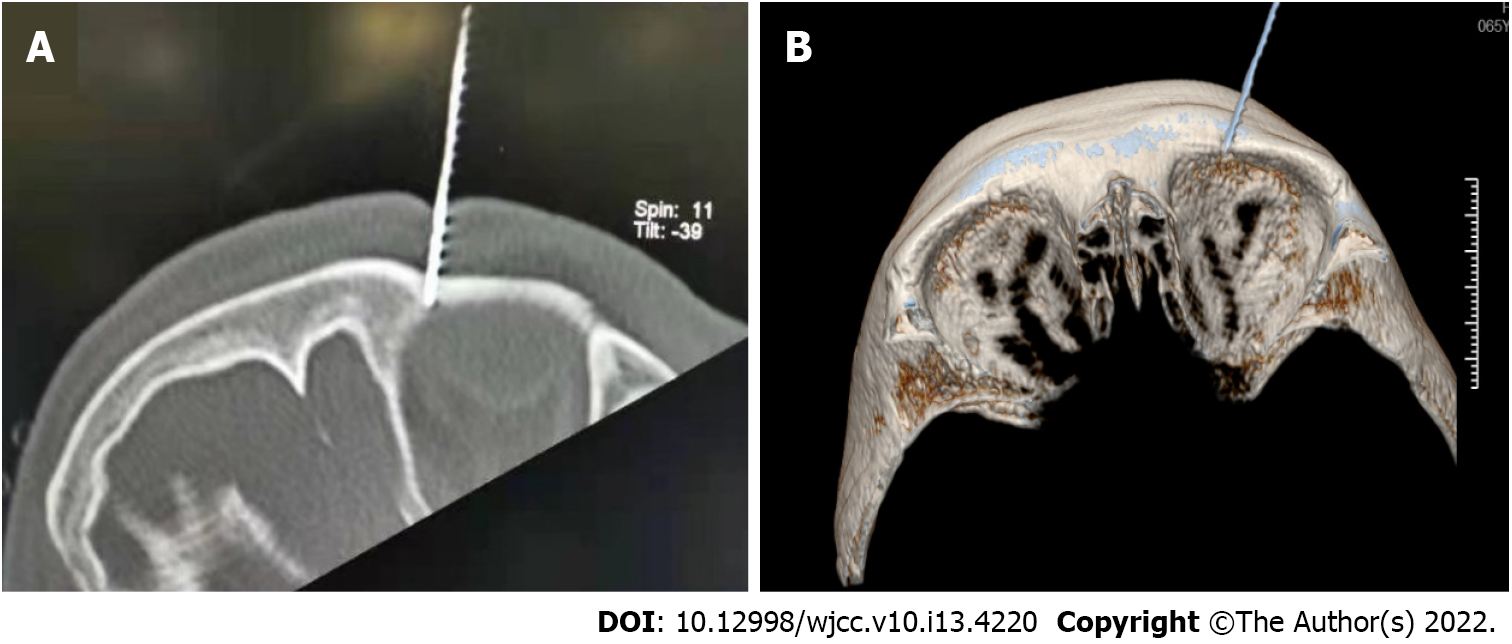
At the beginning of pain (on the 17 d after HZO), the patient was treated with gabapentin capsules (0.3 g bid) and paracetamol oxycodone tablets (5 mg tid) to control the pain, but the pain persisted. After computed tomography (CT)-guided trigeminal nerve pulse radiofrequency procedure, the pain was relieved for 2 h after the procedure, and severe pain later appeared again. A single subcutaneous injection of morphine hydrochloride (5 mg) temporarily relieved pain, but there was still repeated touch-induced pain and spontaneous pain. After obtaining the patient's consent, percutaneous radiofrequency thermocoagulation was performed under the guidance of CT to completely destroy the ophthalmic branch of the trigeminal nerve (Figure 1A and B). After the procedure, the sensation of the innervation area of the ophthalmic branch of the left trigeminal nerve decreased (manifested as numbness), and the touch-induced pain disappeared, but there was no relief of paroxysmal spontaneous pain. On the second day, the patient was given esketamine for PCIA for continuous analgesia. The PCIA formula was esketamine hydrochloride injection (550 mg) combined with midazolam injection (5 mg) diluted in 275 mL (that is, the concentration of esketamine was 2 mg/mL). Parameter settings were as follows: maintenance dose 8 mg/h, additional dose 10 mg, and additional interval time 30 min. The parameters were adjusted according to the pain and tolerance of the patient. The patient was given oxygen inhalation, and vital signs were monitored.
The pain was significantly relieved after two courses of PCIA, and the NRS score was reduced to 2 points, compared with that before treatment, the difference was statistically significant (P < 0.05). There was no spontaneous pain and allodynia. During the use of PCIA, the patient developed lethargy, nausea, hypotension and abnormal liver enzymes. After positive treatment, the above adverse reactions were improved. The patient was discharged after no adverse reactions were observed.
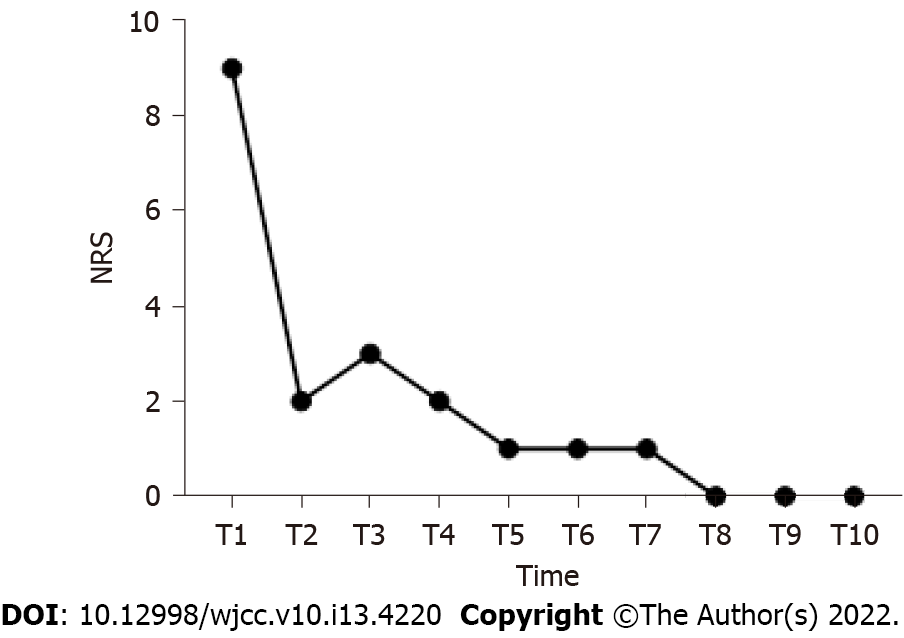
After discharge, the patient was followed up by telephone or outpatient services every week. During the 2-month follow-up, the pain was relieved continuously, and no spontaneous pain or allodynia was reported. The NRS score was ≤ 3, and the Pittsburgh Sleep Quality Index score was 5, which was significantly different from that before treatment (P < 0.05). No adverse events were observed. Figure 2 shows the changing trend of NRS score at admission and during follow-up after discharge.
This case shows that trigeminal thermocoagulation combined with esketamine PCIA can effectively relieve refractory herpetic neuralgia after HZO. In addition, the pain relief lasted at least 2 mo. Primary trigeminal neuralgia can achieve satisfactory curative effects through drug and surgical intervention[12,13]. However, neuralgia caused by varicella-zoster virus infection of the trigeminal nerve is not satisfactorily managed through drugs and surgical treatment, and these methods are ineffective in some patients. This may be related to the mechanism of central sensitization after herpes zoster virus infection. Untimely treatment may lead to keratitis and even permanent visual impairment. Besides, 7% of patients suffer from post herpetic neuralgia after HZO which may last from 30 days to 6 mo[14]. Antiviral treatment, steroids and anti-inflammatory drugs will prevent the complications.
Ketamine is an NMDAR antagonist. NMDARs are excitatory glutamate receptors in the spinal cord and are involved in the transmission of pain signals. Persistent pain receptor stimulation leads to activation and upregulation of synaptic NMDARs in the spinal dorsal horn, which leads to enhanced and amplified transmission of pain signals to the brain (central sensitization). In addition, ketamine can enhance the descending inhibition of the central site and anti-inflammatory effects[15]. It can relieve all kinds of neuropathic pain[16,17]. Esketamine is the dextral form of ketamine and has a higher affinity for NMDARs[10]. Therefore, the dose needed to produce analgesia is lower. Ketamine has been used in the treatment of PHN[9,18]. In this case, PCIA with esketamine was used for the treatment of refractory herpetic neuralgia after HZO for the first time, and the pain was significantly relieved. Previous studies have shown that the duration of relief from neuropathy is related to the total infusion dose and infusion duration of ketamine[19]. Furthermore, intravenous ketamine infusion in the treatment of refractory pain is within the guidelines of the American Society of Anesthesiology and Pain[20]. Therefore, in this case chose the PCIA mode. The PCIA mode can accurately control the infusion speed and infusion dose and achieve continuous analgesia. In addition, when an outbreak of pain occurs, patients can automatically control the additional dose to relieve the pain symptoms in time. The peak effect of ketamine is after 15 to 20 min and hence the lockout time was set to 30 min. The combined use of midazolam can reduce the side effects of unpleasant pseudomental disorders to some extent. There were no psychedelic, paranoid or other mental side effects in this case.
On the second day after treatment with esketamine PCIA, the patient showed drowsiness and nausea, and her blood pressure dropped to 82/53 mmHg. After lowering the dose and rehydration, the patient's drowsiness improved, and blood pressure returned to normal. On the 3rd day, the patient’s liver enzyme index was significantly higher than that before treatment. The liver enzyme decreased after liver protection treatment and returned to normal at discharge. Dizziness and lethargy are common central nervous system symptoms of ketamine. Ketamine is mainly metabolized by the liver, and heavy use of ketamine will damage liver function. Therefore, when using ketamine, we should pay attention to the changes in patients' consciousness, monitor vital signs and review liver function regularly. No myocardial inhibition, cystitis or other adverse reactions were found in this case.
This case was followed up for 2 mo after treatment. Long-term follow-up, large sample size and prospective studies are needed to verify the long-term efficacy of ketamine in the treatment of intractable herpes zoster.
In conclusion, trigeminal extracranial thermocoagulation combined with esketamine PCIA may be a safe and feasible method for the treatment of refractory herpetic neuralgia after herpes zoster ophthalmicus. This may be a new treatment method for refractory ocular herpes zoster neuralgia.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neurosciences
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: DeSousa K, India S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Shiraki K, Toyama N, Shiraki A, Yajima M; Miyazaki Dermatologist Society. Age-dependent trigeminal and female-specific lumbosacral increase in herpes zoster distribution in the elderly. J Dermatol Sci. 2018;90:166-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Liesegang TJ. Herpes zoster ophthalmicus natural history, risk factors, clinical presentation, and morbidity. Ophthalmology. 2008;115:S3-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 274] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 3. | Zak-Prelich M, Borkowski JL, Alexander F, Norval M. The role of solar ultraviolet irradiation in zoster. Epidemiol Infect. 2002;129:593-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Li JY. Herpes zoster ophthalmicus: acute keratitis. Curr Opin Ophthalmol. 2018;29:328-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Zhang H, Ni H, Liu S, Xie K. Supraorbital Nerve Radiofrequency for Severe Neuralgia Caused by Herpes Zoster Ophthalmicus. Pain Res Manag. 2020;2020:3191782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Liu DY, Chen JS, Fang ZZ, Liu SY, Wan L. Pulsed Radiofrequency of the Trigeminal Ganglion for Treating Postherpetic Neuralgia of the Ophthalmic Branch. Pain Res Manag. 2021;2021:6638392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Wan C, Dong DS, Song T. High-Voltage, Long-Duration Pulsed Radiofrequency on Gasserian Ganglion Improves Acute/Subacute Zoster-Related Trigeminal Neuralgia: A Randomized, Double-Blinded, Controlled Trial. Pain Physician. 2019;22:361-368. [PubMed] |
| 8. | Sigtermans MJ, van Hilten JJ, Bauer MCR, Arbous SM, Marinus J, Sarton EY, Dahan A. Ketamine produces effective and long-term pain relief in patients with Complex Regional Pain Syndrome Type 1. Pain. 2009;145:304-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 283] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 9. | Kim YH, Lee PB, Oh TK. Is magnesium sulfate effective for pain in chronic postherpetic neuralgia patients comparing with ketamine infusion therapy? J Clin Anesth. 2015;27:296-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Culp C, Kim HK, Abdi S. Ketamine Use for Cancer and Chronic Pain Management. Front Pharmacol. 2020;11:599721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 11. | Rabben T, Skjelbred P, Oye I. Prolonged analgesic effect of ketamine, an N-methyl-D-aspartate receptor inhibitor, in patients with chronic pain. J Pharmacol Exp Ther. 1999;289:1060-1066. [PubMed] |
| 12. | Sterman-Neto H, Fukuda CY, Duarte KP, Aparecida da Silva V, Rodrigues ALL, Galhardoni R, de Siqueira SRDT, de Siqueira JTT, Teixeira MJ, Ciampi de Andrade D. Balloon compression vs radiofrequency for primary trigeminal neuralgia: a randomized, controlled trial. Pain. 2021;162:919-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Ren H, Zhao C, Wang X, Shen Y, Meng L, Luo F. The Efficacy and Safety of the Application of Pulsed Radiofrequency, Combined With Low-Temperature Continuous Radiofrequency, to the Gasserian Ganglion for the Treatment of Primary Trigeminal Neuralgia: Study Protocol for a Prospective, Open-Label, Parall. Pain Physician. 2021;24:89-97. [PubMed] |
| 14. | Schmader K. Herpes Zoster. Ann Intern Med. 2018;169:ITC19-ITC31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | Niesters M, Martini C, Dahan A. Ketamine for chronic pain: risks and benefits. Br J Clin Pharmacol. 2014;77:357-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 295] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 16. | Marchetti F, Coutaux A, Bellanger A, Magneux C, Bourgeois P, Mion G. Efficacy and safety of oral ketamine for the relief of intractable chronic pain: A retrospective 5-year study of 51 patients. Eur J Pain. 2015;19:984-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Pickering G, Pereira B, Morel V, Corriger A, Giron F, Marcaillou F, Bidar-Beauvallot A, Chandeze E, Lambert C, Bernard L, Delage N. Ketamine and Magnesium for Refractory Neuropathic Pain: A Randomized, Double-blind, Crossover Trial. Anesthesiology. 2020;133:154-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 18. | Sawynok J, Zinger C. Topical amitriptyline and ketamine for post-herpetic neuralgia and other forms of neuropathic pain. Expert Opin Pharmacother. 2016;17:601-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Maher DP, Chen L, Mao J. Intravenous Ketamine Infusions for Neuropathic Pain Management: A Promising Therapy in Need of Optimization. Anesth Analg. 2017;124:661-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 122] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 20. | Schwenk ES, Viscusi ER, Buvanendran A, Hurley RW, Wasan AD, Narouze S, Bhatia A, Davis FN, Hooten WM, Cohen SP. Consensus Guidelines on the Use of Intravenous Ketamine Infusions for Acute Pain Management From the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43:456-466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 146] [Article Influence: 20.9] [Reference Citation Analysis (0)] |