Published online May 6, 2022. doi: 10.12998/wjcc.v10.i13.4207
Peer-review started: September 9, 2021
First decision: December 9, 2021
Revised: December 17, 2021
Accepted: March 16, 2022
Article in press: March 16, 2022
Published online: May 6, 2022
Processing time: 232 Days and 7.5 Hours
Congenital cataract, facial dysmorphism, and neuropathy (CCFDN) syndrome is an extremely rare multiorgan disorder. Characteristics include congenital cataracts, facial deformation, extremity deformities, and demyelinating neuro
We report the anesthetic management of a 13-year-old girl with CCFDN syndrome scheduled for posterior neuromuscular scoliosis correction surgery. The patient suffered from extensive progressive neuromuscular scoliosis with a Cobb angle of 83°. Her limitations included neuropathy and a scoliotic curve. This condition negatively impacted her quality of life. This case reflects the potential anesthetic complications for posterior scoliosis correction and CCFDN syndrome. The challenge for our anesthetic team was the limited amount of data about anesthetic management of this condition. In total, one case report without any data about endotracheal intubation of patients with this condition was available. Endotracheal intubation in our case was uncomplicated. Another focus of our case was the prevention of possible complications associated with this syndrome, including rhabdomyolysis and seizures. Rhabdomyolysis can be triggered by some types of anesthetic agents like suxamethonium or volatile anesthetics, especially in patients with certain types of myopathies.
Adequate understanding of the anesthetic management of CCFDN syndrome can reduce perioperative complications and improve patient outcome after surgery.
Core Tip: We report on a rare case of anesthetic management of a patient with congenital cataract, facial dysmorphism, and neuropathy (CCFDN) syndrome for posterior neuromuscular scoliosis correction. Additionally, this case report is unique as it for the first time presents successful endotracheal intubation in a patient with CCFDN syndrome associated with facial dysmorphism.
- Citation: Hudec J, Kosinova M, Prokopova T, Filipovic M, Repko M, Stourac P. Anesthesia of a patient with congenital cataract, facial dysmorphism, and neuropathy syndrome for posterior scoliosis: A case report . World J Clin Cases 2022; 10(13): 4207-4213
- URL: https://www.wjgnet.com/2307-8960/full/v10/i13/4207.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i13.4207
Congenital cataract, facial dysmorphism, and neuropathy (CCFDN) syndrome is an extremely rare multiorgan disorder. It is an autosomal recessive disease with unknown prevalence, described in Roma ethnicity[1]. The diagnosis is based on clinical examination. Typical abnormalities include ophthalmic problems like bilateral congenital cataracts, nystagmus, microphthalmia or microcorneae, facial dysmorphism with micrognathia, scoliosis or extremity deformities, hypo/demyelinating symmetric peripheral neuropathy, and developmental delay[2-6].
CCFDN syndrome is associated with increased risk during anesthesia. Rhabdomyolysis, pulmonary oedema, inspiratory stridor after anesthesia, or epileptic seizures are described in the literature. Although there is no literature about difficult airways in these patients, difficult airway management (DAM) should be expected due to facial dysmorphism and irregular anatomy. There is no convincing data about an association with malignant hyperthermia[3,8].
Only one case study was published regarding the anesthetic management of a patient with CCFDN syndrome. That case involved a 9-year-old boy from the Czech Republic who underwent surgery at Birmingham, UK and repeatedly at a younger age in Czech that was not published. In that case, a laryngeal mask was used for airway management[8].
We report on the first orthopedic case of a 13-year-old girl with CCFDN syndrome for posterior correction and fusion for neuromuscular scoliosis in which the first successful endotracheal intubation and anesthesia by total intravenous anesthesia (TIVA) in a major orthopedic surgery on a patient suffering from CCFDN syndrome is documented.
Our patient is a 13-year-old Roma girl (ASA physical status III) prepared for T2–L2 posterior scoliosis correction and fusion.
The patient suffered from mild mental retardation; however, communication was unhindered. She walked without support for a distance of approximately 20 m, but had limitation in movement of the upper extremities due to peripheral neuropathy. Facial dysmorphism was presented by prominent nasal philtrum and upper incisors.
The progressive curve of scoliosis limited the possibility of rehabilitation, therefore brace treatment was not prescribed for our patient. The Cobb angle was 83°.
Previous general anesthesia for cataract operation at the University Hospital in Prague was uncomplicated. We could not obtain more information about the course of anesthesia used.
The patient was diagnosed with a patent foramen ovale and pulmonary valve stenosis, both hemodynamically insignificant without contraindication to the procedure in the prone position.
The patient had no significant family history. Rhabdomyolysis, seizure, or CCFDN syndrome was not present in her family relatives.
The patient's body weight was 45 kg and her height was 136 cm; however, the scoliosis curve reduced her actual height. Her Mallampati score was 1. The cardiovascular system was not affected and valve disease was insignificant. Her ASA score was III.
On admission, laboratory test results were within normal limits. There was no abnormality in blood count, biochemical tests, or coagulation.
Pulmonary functions were dominantly limited by restrictive lung disease (forced vital capacity, 54%; forced expiratory volume in 1 s, 62%).
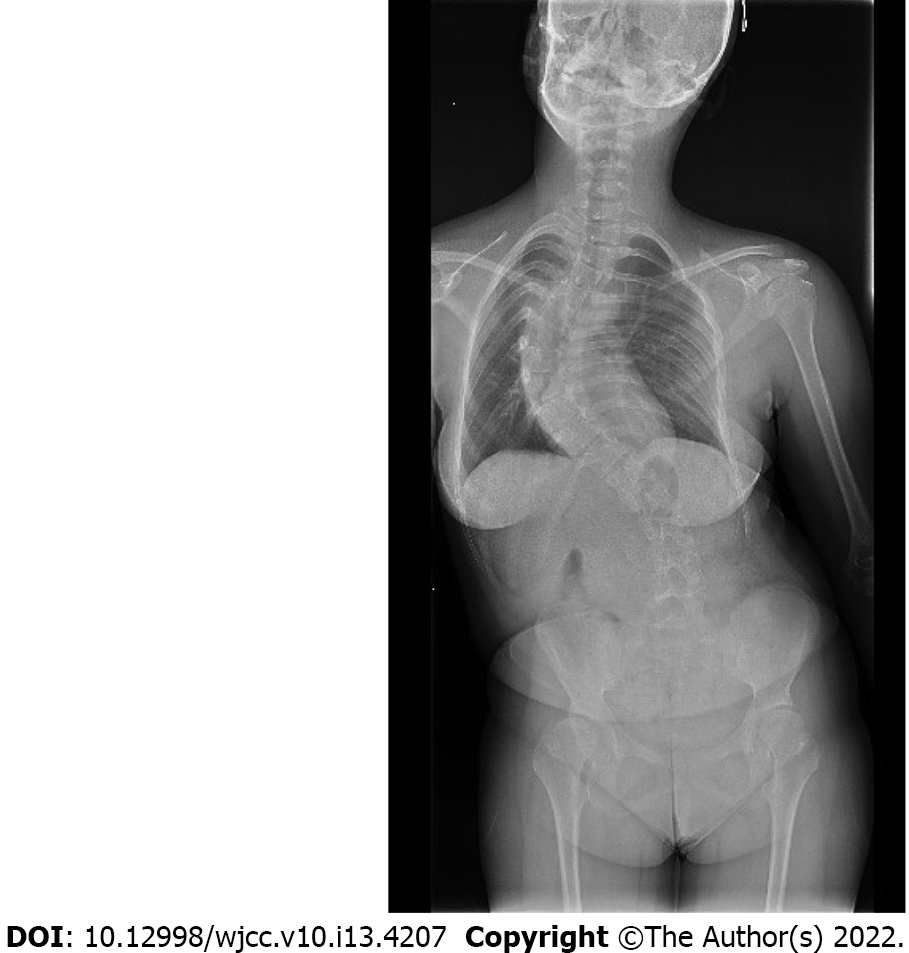
Chest X-ray was limited by chest scoliosis and revealed no abnormal lung and heart pathology. Echocardiography found hemodynamically insignificant valve disease. X-ray demonstrated a significant and progressive scoliosis curve with a Cobb angle 83° (Figure 1).
The final diagnosis was extensive progressive neuromuscular scoliosis in a patient with CCFDN syndrome indicated for surgical correction and fusion.
Total intravenous anesthesia was the preferred anesthesia technique due to the possibility of motor evoked potential (MEP) monitoring and to minimize the higher risk of rhabdomyolysis described in these patients that is associated with other types of anesthesia. We prepared two infusion pumps, first with 1% propofol and a second with remifentanil (4 mg/20 mL).
Along with standard vital sign monitoring, we monitored the bispectral index (BIS) to prevent propofol overdosing as the patient presented with abnormal body proportions due to the disfigurement of disease. We cannulated a peripheral IV line G20 before anesthesia induction through which we performed the total IV anesthesia. For induction, we administered a bolus of 120 mg propofol followed by the continuous infusion of 3 mg/kg IV after 2 min pre-oxygenation. We gave a single bolus dose of 0.6 mg/kg rocuronium for intubation relaxation. For analgesia, a remifentanil infusion was used at a dose of 0.1 µg/kg/min. The patient’s airway was evaluated as having a Cormack-Lehane score of 1 during direct laryngoscopy with a Macintosh blade size of 3. The airway was secured with a cuffed 6.5 mm endotracheal tube on the first attempt. No complications were experienced during either pre-intubation mask ventilation or intubation.
Post intubation, we targeted the dose of propofol and remifentanil to a BIS value between 40-60. Before MEP monitoring, the patient had no residual neuromuscular blockade (TOF ratio more than 90%). We cannulated a second peripheral IV line G18 and an arterial line G20 for invasive blood pressure monitoring. A dose of 700 mg tranexamic acid was given IV before the start of the operation for expected blood loss. The patient was pronated for surgery. Special gel pads were placed to position the patient to prevent iatrogenic trauma since patients with CCFDN syndrome suffer from osteoporosis and pose a higher risk of iatrogenic trauma. The body temperature was maintained between 36.5-34.7 °C with warming blankets applied on the upper and lower extremities. Total blood loss was 1600 mL (36 mL/kg). We administered blood derivatives based on the thromboelastometry results and complete blood count. Perioperative fluid management, considering evaporation from the large wound, was performed according to pulse pressure variation. The patient received 754 mL of red blood cells, 200 mL of purified plasma and 4000 mg of fibrinogen, 3500 mL of balanced crystalloids, and 500 mL of 4% gelatine. We administered paracetamol 700 mg IV and piritramide 7.5 mg SC prior to the end of the procedure. No fluctuation in MEP occurred during the duration of the procedure.
The propofol infusion was suspended approximately 30 min before the skin suture with continual BIS monitoring. Remifentanil continued until positioning to the supine position. We extubated the patient 4 min after positioning. Perioperative rhabdomyolysis or seizures did not develop in our patient.
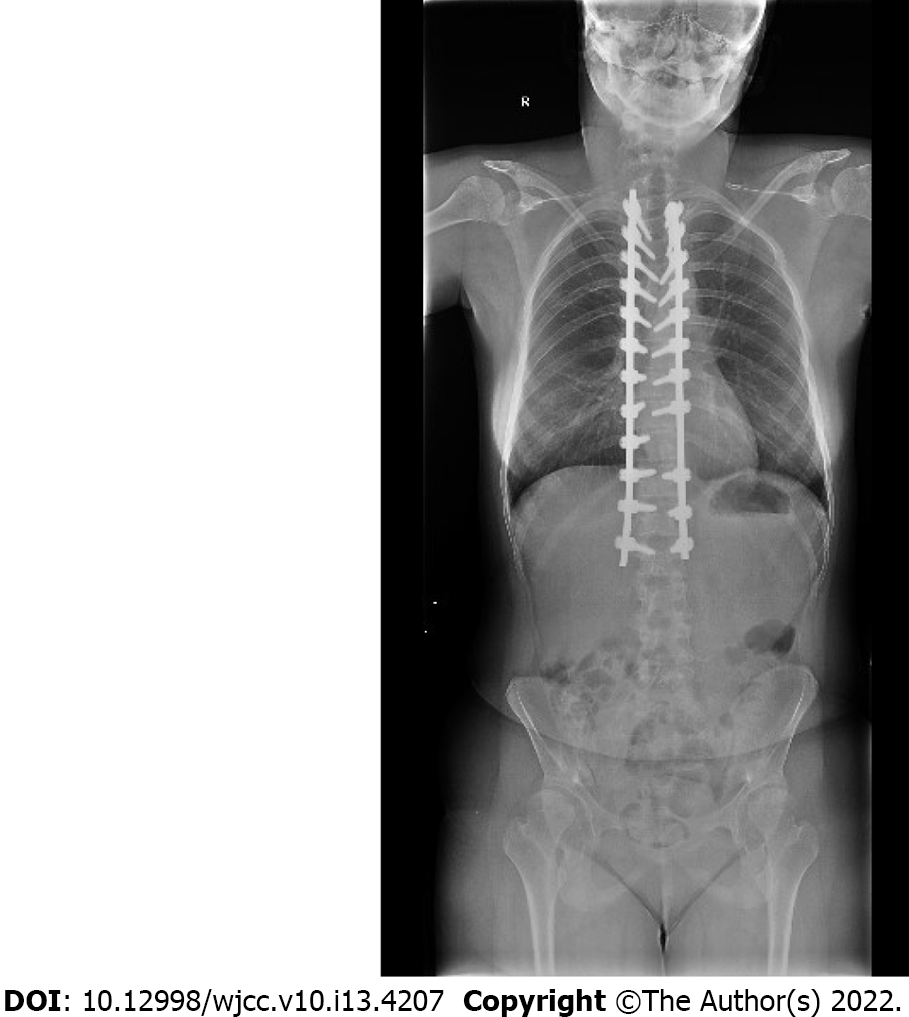
Postoperative care in the intensive care unit was without cardiorespiratory complications. The patient’s rehabilitation was uneventful, the surgical wound healed "per primam intentionem". The spine instrumentation was without dislocation on the control X-ray. The patient recovered without new neurological deficit and was discharged home on the eleventh day post surgery.
The 6-mo outcome was favorable. The patient arrived with compensated scoliosis and a nearly immeasurable curve. The spine instrumentation was sufficient (Figure 2). There was no new neurological deficit present. The patient visits a physiotherapist regularly.
CCFDN syndrome is a rare multisystem disorder. The most typical abnormalities include ophthalmic problems, facial dysmorphism, scoliosis, extremity deformities, and hypo/demyelinating symmetric peripheral neuropathy (Table 1). This syndrome has similar clinical manifestations to Marinesco-Sjögren syndrome. However, molecular testing revealed that these syndromes are genetically different. The definitive diagnosis is confirmed by genetic testing. There is a mutation in the CTDP1 gene on chromosome 18q23 with a homozygous mutation 863+389C>T. Today we know that the best prevention of the repetition of the disease in affected families is molecular testing of all Roma children with congenital cataracts[1].
| Clinical sign | Possible anesthetic complication |
| Cataract | Iatrogenic eye damage |
| Microcorneae | Iatrogenic eye damage |
| Microphthalmia | Iatrogenic eye damage |
| Malar prominence | Difficult airway management |
| Micrognathia | Difficult airway management |
| Prominent upper incisors | Difficult airway management |
| Prominent nasal philtrum | Difficult airway management |
| Cervical spine abnormality | Difficult airway management |
| Development delay | Limited cooperation for invasive procedures |
| Peripheral neuropathy | Rhabdomyolysis |
| Scoliosis | Restrictive lung disease |
| Osteoporosis | Iatrogenic injury |
Anesthesiologists usually manage these patients for skeletal corrections or ophthalmic operations of the cataract and rarely for gonad abnormality correction[8]. Only one case report mentions anesthetic management of these patients for orchidopexy of the patient with CCFDN syndrome where a laryngeal mask was used for airway management[8]. We report the first case of successful, uncomplicated endotracheal intubation of a patient with CCFDN syndrome without need of using any special equipment for DAM. Anesthesiologists should always anticipate possible difficult airway management in patients with facial dysmorphism[3]. A clear plan for airway management is necessary, including preparation of anticipated equipment in accordance with local or international guidelines for difficult airway management. In this case, we managed to secure the airway with our first-choice device - a size 6.5 mm cuffed endotracheal tube.
Scoliosis is one of the most frequent deformities in patients with neuromuscular disease. The effect of scoliosis is complex with multiorgan involvement, greater than just the motor system of the patients affecting daily care, walking, and sitting. Other systems have secondary involvement, dominantly the cardiovascular and respiratory systems depending on the curve's severity and progression[9-13]. Perioperative care during posterior scoliosis procedures is unique due to the patient's prone positioning, evoked potential monitoring[9], higher blood loss, body temperature loss, and increased evaporation from the large wound[10]. Positioning, especially in patients with CCFDN syndrome, is complicated from peripheral neuropathy with osteoporosis. There is a higher risk of iatrogenic injury with these patients, therefore the manipulation and positioning must be done with extreme care[11]. TIVA is the preferred method for procedures with MEP monitoring[12]. Non-depolarising muscle relaxants should be used only during intubation. Another dose of relaxant is contraindicated to enable MEP monitoring. Neuromuscular blockade monitoring is strictly recommended when non-depolarising muscle relaxants are used. The blockade can be prolonged in patients with a neuromuscular disease like CCFDN syndrome, therefore rocuronium is preferred as the antidote sugammadex can be used to suspend muscle relaxation to avoid prolonged neuromuscular blockade[13,14]. Evoked potentials can be altered, and the amplitudes can be lower in CCFDN syndrome with progressive peripheral neuropathy. The adequate depth of anesthesia excludes the adverse effect of deep anesthesia on MEP reproducibility[15]. We decided to avoid volatile anesthetics as the risk of rhabdomyolysis and malignant hyperthermia is elevated with those agents. The association with malignant hyperthermia is unlikely from the different gene locations of CCFDN syndrome and malignant hyperthermia[8]. Rhabdomyolysis presents an acute crisis associated with exposure to suxamethonium or volatile anesthetics and it is known as “anesthesia-induced rhabdomyolysis.” Rhabdomyolysis may present as sudden bradycardia with peaked T waves on ECG or cardiac arrest. Hyperkalemia and raised creatine kinase levels are present, especially in patients with neuromuscular disease[1]. Myoglobinuria monitoring is used to detect rhabdomyolysis postoperatively. Controlled awakening, especially in patients with a neuromuscular disorder, can reduce the length of mechanical ventilation required for anesthesia. Managing the depth of anesthesia according to BIS value can shorten the awakening from general anesthesia[8]. The dose of propofol and remifentanil was managed by targeting administration to BIS values from 40 to 60. The patient was extubated 4 min after postoperative supine positioning. Regional analgesia in patients with CCFDN syndrome is described in the literature[8]; however, the mental status of the patient is of grave concern during regional anesthesia of a developmentally delayed patient. Regional blockade is preferably combined with general anesthesia, especially in children with development delay[16]. There is no specific access or contraindications for regional anesthesia in patients with CCFDN syndrome although there can be anatomical abnormalities due to peripheral neuropathy and contractions. Ultrasound-guided regional anesthesia is the method of choice. In this case, the large extent of the procedure from T2 to L2 and the risk of local anesthetic toxicity prevented the use of regional analgesia techniques. Postoperative care depends on the patient's comorbidities and the type of surgery. There is a higher risk of postoperative complications as prolonged neuromuscular blockade or respiratory insufficiency are potential complications. Intensive care of the patient is indicated after prolonged or high-risk surgeries. These patients will profit from early mobilization and rehabilitation[14].
Anesthesia for posterior scoliosis correction can be challenging for anesthesia and the orthopedic team. In this case, the anesthesiologist should know specifics for posterior scoliosis correction and CCFDN syndrome. TIVA and reported perioperative management with safety measures respecting the specifics of both the patient and procedure helped reduce risks described in the CCFDN syndrome and provided early recovery of our patient from the procedure.
A rare case of anesthetic management of a patient with CCFDN syndrome undergoing general anesthesia for posterior neuromuscular scoliosis correction has been described. Understanding this syndrome eliminates perioperative complications and enables excellent postoperative patient outcome.
The publication of the first successful endotracheal intubation and uncomplicated perioperative period in major surgery in a patient with CCFDN syndrome is presented. Although airway management was uncomplicated in the end, DAM should be expected in these patients. It is recommended that DAM should be anticipated and advanced equipment for DAM should be available.
The figures were used with permission of the Department of Radiology and Nuclear Medicine, University Hospital Brno. This case report was presented as a poster at the World Congress of Anaesthesiologists in September 2021.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: European Society of Anaesthesiology and Intensive Care, 189008; Czech Society of Anaesthesiology and Intensive Care Medicine.
Specialty type: Anesthesiology
Country/Territory of origin: Czech Republic
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Higa K, Japan S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Lassuthova P, Sišková D, Haberlová J, Sakmaryová I, Filouš A, Seeman P. Congenital cataract, facial dysmorphism and demyelinating neuropathy (CCFDN) in 10 Czech Gypsy children--frequent and underestimated cause of disability among Czech Gypsies. Orphanet J Rare Dis. 2014;9:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Gray RM. Anesthesia-induced rhabdomyolysis or malignant hyperthermia: is defining the crisis important? PaediatrAnaesth. 2017;27:490-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Kalaydjieva L. Congenital cataracts-facial dysmorphism-neuropathy. Orphanet J Rare Dis. 2006;1:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Merlini L, Gooding R, Lochmüller H, Müller-Felber W, Walter MC, Angelicheva D, Talim B, Hallmayer J, Kalaydjieva L. Genetic identity of Marinesco-Sjögren/myoglobinuria and CCFDN syndromes. Neurology. 2002;58:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Walter MC, Bernert G, Zimmermann U, Müllner-Eidenböck A, Moser E, Kalaydjieva L, Lochmüller H, Müller-Felber W. Long-term follow-up in patients with CCFDN syndrome. Neurology. 2014;83:1337-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Müllner-Eidenböck A, Moser E, Klebermass N, Amon M, Walter MC, Lochmüller H, Gooding R, Kalaydjieva L. Ocular features of the congenital cataracts facial dysmorphism neuropathy syndrome. Ophthalmology. 2004;111:1415-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Angelicheva D, Turnev I, Dye D, Chandler D, Thomas PK, Kalaydjieva L. Congenital cataracts facial dysmorphism neuropathy (CCFDN) syndrome: a novel developmental disorder in Gypsies maps to 18qter. Eur J Hum Genet. 1999;7:560-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Masters OW, Bergmans E, Thies KC. Anaesthesia and orphan disease: A child with Congenital Cataract Facial Dysmorphism neuropathy (CCFDN) syndrome: a case report. Eur J Anaesthesiol. 2017;34:178-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Punjasawadwong Y, Phongchiewboon A, Bunchungmongkol N. Bispectral index for improving anaesthetic delivery and postoperative recovery. Cochrane Database Syst Rev 2014: CD003843. [RCA] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Toll BJ, Samdani AF, Janjua MB, Gandhi S, Pahys JM, Hwang SW. Perioperative complications and risk factors in neuromuscular scoliosis surgery. J NeurosurgPediatr. 2018;22:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Grover M, Bachrach LK. Osteoporosis in Children with Chronic Illnesses: Diagnosis, Monitoring, and Treatment. CurrOsteoporos Rep. 2017;15:271-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Wang AC, Than KD, Etame AB, La Marca F, Park P. Impact of anesthesia on transcranial electric motor evoked potential monitoring during spine surgery: a review of the literature. Neurosurg Focus. 2009;27:E7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Katz JA, Murphy GS. Anesthetic consideration for neuromuscular diseases. CurrOpinAnaesthesiol. 2017;30:435-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Racca F, Mongini T, Wolfler A, Vianello A, Cutrera R, Del Sorbo L, Capello EC, Gregoretti C, Massa R, De Luca D, Conti G, Tegazzin V, Toscano A, Ranieri VM. Recommendations for anesthesia and perioperative management of patients with neuromuscular disorders. Minerva Anestesiol. 2013;79:419-433. [PubMed] |
| 15. | Pastorelli F, Di Silvestre M, Vommaro F, Maredi E, Morigi A, Bacchin MR, Bonarelli S, Plasmati R, Michelucci R, Greggi T. Intraoperative monitoring of somatosensory (SSEPs) and transcranial electric motor-evoked potentials (tce-MEPs) during surgical correction of neuromuscular scoliosis in patients with central or peripheral nervous system diseases. Eur Spine J. 2015;24 Suppl 7:931-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |