Published online May 6, 2022. doi: 10.12998/wjcc.v10.i13.4119
Peer-review started: December 8, 2021
First decision: January 25, 2022
Revised: February 7, 2022
Accepted: March 16, 2022
Article in press: March 16, 2022
Published online: May 6, 2022
Processing time: 142 Days and 23.9 Hours
The clinical role of perioperative respiratory muscle training (RMT), including inspiratory muscle training (IMT) and expiratory muscle training (EMT) in patients undergoing pulmonary surgery remains unclear up to now.
To evaluate whether perioperative RMT is effective in improving postoperative outcomes such as the respiratory muscle strength and physical activity level of patients receiving lung surgery.
The PubMed, EMBASE (via OVID), Web of Science, Cochrane Library and Physiotherapy Evidence Database (PEDro) were systematically searched to obtain eligible randomized controlled trials (RCTs). Primary outcome was postoperative respiratory muscle strength expressed as the maximal inspiratory pressure (MIP) and maximal expiratory pressure (MEP). Secondary outcomes were physical activity, exercise capacity, including the 6-min walking distance and peak oxygen consumption during the cardio-pulmonary exercise test, pulmonary function and the quality of life.
Seven studies involving 240 participants were included in this systematic review and meta-analysis. Among them, four studies focused on IMT and the other three studies focused on RMT, one of which included IMT, EMT and also combined RMT (IMT-EMT-RMT). Three studies applied the intervention postoperative, one study preoperative and the other three studies included both pre- and postoperative training. For primary outcomes, the pooled results indicated that perioperative RMT improved the postoperative MIP (mean = 8.13 cmH2O, 95%CI: 1.31 to 14.95, P = 0.02) and tended to increase MEP (mean = 13.51 cmH2O, 95%CI: -4.47 to 31.48, P = 0.14). For secondary outcomes, perioperative RMT enhanced postoperative physical activity significantly (P = 0.006) and a trend of improved postoperative pulmonary function was observed.
Perioperative RMT enhanced postoperative respiratory muscle strength and physical activity level of patients receiving lung surgery. However, RCTs with large samples are needed to evaluate effects of perioperative RMT on postoperative outcomes in patients undergoing lung surgery.
Core Tip: Our study indicated that perioperative respiratory muscle training (RMT) improved the postoperative maximal inspiratory pressure (P = 0.02) and tended to increase maximal expiratory pressure (P = 0.14). For secondary outcomes, perioperative RMT enhanced postoperative physical activity significantly (P = 0.006) and a trend of improved postoperative pulmonary function was observed. Perioperative RMT enhanced postoperative respiratory muscle strength and physical activity level of patients receiving lung surgery.
- Citation: Yang MX, Wang J, Zhang X, Luo ZR, Yu PM. Perioperative respiratory muscle training improves respiratory muscle strength and physical activity of patients receiving lung surgery: A meta-analysis. World J Clin Cases 2022; 10(13): 4119-4130
- URL: https://www.wjgnet.com/2307-8960/full/v10/i13/4119.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i13.4119
Respiratory muscle strength, representing as maximal inspiratory pressure (MIP) and maximal expiratory pressure (MEP), and physical activity level decreased inevitably after thoracic surgery due to pain and ineffective coughing[1,2]. These will adversely affect postoperative recovery and quality of life[3,4]. The function of respiratory muscles is directly impaired by the surgical incision in the chest wall. Meanwhile, the total chest compliance is reduced due to the injured respiratory muscles after thoracic surgery, especially after lung surgery[4]. The impairment of respiratory muscle strength after pulmonary resection leads to an adverse effect on the expectoration of sputum[4,5].
With the great advance of the enhanced recovery after surgery concept, a number of physiotherapy methods have been widely introduced and applied in clinical practice in order to remove secretions from the lungs and decrease respiratory work load following thoracic surgery. These methods include airway clearance techniques, active cycle of breathing, incentive spirometry, breathing exercises, early mobilization and also respiratory muscle training (RMT). RMT includes both inspiratory muscle training (IMT) and expiratory muscle training (EMT)[6-8]. IMT increases inspiratory muscle strength, relieve inspiratory muscle tension, improve diaphragm function and contributes to lung expansion, thereby helping to maintain the airway patency[9,10]. Meanwhile, it could also inhibit sympathetic nerve function, improve vagus nerve activity and reduce peripheral vascular resistance[9,10]. EMT help create high expiratory flows to remove airway secretions and increases the overall effectiveness of participants’ voluntary cough, which effectively reduces the incidence of pulmonary complications[11,12]. For most of patients receiving lung surgery, including patients who receive the segmentectomy, lobectomy or pneumonectomy with video-assisted thoracic surgery (VATS) or open thoracotomy, the RMT is applicable. However, in some conditions such as combining the tracheotomy, recurrent paralysis, myasthenia gravis or unstable coronary artery disease, the RMT is prohibited. Up to now, a large number of studies have investigated the clinical effects of perioperative RMT in patients undergoing major surgery. Mans et al[13] analyzed eight relevant studies involving 295 participants undergoing upper abdominal or cardiothoracic surgery. They demonstrated that preoperative IMT could substantially improve MIP (mean = 15 cmH2O, 95%CI: 9 to 21 cmH2O, P < 0.001) and reduce postoperative pulmonary complications (PPCs) [relative risk (RR) = 0.48, 95%CI: 0.26 to 0.89, P = 0.02]. However, large differences exist between lung surgery and other types of surgery, including the effect on respiratory muscle function, level of physical activity and risk for PPCs[13-15].
Therefore, we conducted this systematic review and meta-analysis to further investigate the effect of perioperative RMT on postoperative outcomes, especially the respiratory muscle strength and physical activity, in patients following lung surgery, which also helps strengthen the understanding of the value of RMT before and after lung surgery.
We performed this systematic review and meta-analysis according to the preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines [16]. Meanwhile, it has been registered with PROSPERO (ID: CRD42020214940).
The electronic databases of PubMed, EMBASE (via OVID), Web of Science, Cochrane Library and PEDro were systematically searched from inception to March 24, 2021. The following MeSH terms were used for literature search: “respiratory muscle training”, “inspiratory muscle training”, “expiratory muscle training”, “lung resection”, “pulmonary resection”, “lung surgery”, “lobectomy”, “segmentectomy”, “wedge resection”, “pneumonectomy”, “video-assisted thoracoscopic surgery”, “video-assisted thoracic surgery’ and “VATS”. The specific search strategy was: (respiratory muscle training OR inspiratory muscle training OR expiratory muscle training) AND (lung resection OR pulmonary resection OR lung surgery OR pulmonary surgery OR lobectomy OR segmentectomy OR wedge resection OR pneumonectomy OR video-assisted thoracic surgery OR video-assisted thoracoscopic surgery OR VATS). The reference lists of included studies were also reviewed for eligibility.
The following inclusion criteria were applied: (1) randomized controlled trials (RCT) investigating the effects of perioperative RMT, compared with sham RMT or no RMT; (2) participants were adults; (3) articles were published in English; and (4) at least one of the following outcomes was reported.
The exclusion criteria of this study were as follows: (1) meeting abstracts, letters, reviews, non-human trials, protocols, case reports; (2) other perioperative interventions were combined; and (3) training programs were poorly designed and the clinical parameters and training doses of patients were not reported.
Primary outcome was the postoperative respiratory muscle strength representing as the MIP and MEP.
Secondary outcomes were the physical activity, exercise capacity including the 6-min walking distance (6MWD) and peak oxygen consumption (VO2peak) during the cardio-pulmonary exercise test (CPET), pulmonary function such as the forced expiratory volume in one second (FEV1) and forced vital capacity (FVC), and the quality of life representing as the intensity of pain and dyspnoea.
Two authors (YP and YM) screened the records for availability independently. At first, the titles and abstracts were reviewed. Then, the full-texts were further assessed to determine the eligibility when the information in the titles or abstracts was potentially related or insufficient and the availability of relevant data was verified. Any discrepancy was solved by team discussion.
The following data were extracted: author, publication year, country, sample size, type of surgery, specific intervention strategy including the initial training pressure, training time, frequency and duration of training program, treatment strategy of control group, information necessary to calculate the PEDro scale score, primary outcomes and secondary outcomes.
All patients in the included studies received usual care after surgery. Usual care consists of different breathing exercises aiming pulmonary re-expansion and bronchial clearance, early ambulation and mobilization.
The methodological quality of included studies was assessed by two independent investigators (MY and JW) using the PEDro. The high quality was defined as a PEDro score of 6 or higher, the fair quality was defined as a PEDro score of 4 or 5 and a score of 3 or lower indicated poor quality[17,18].
All statistical analysis was performed by RevMan version. The heterogeneity between included studies was quantified by the
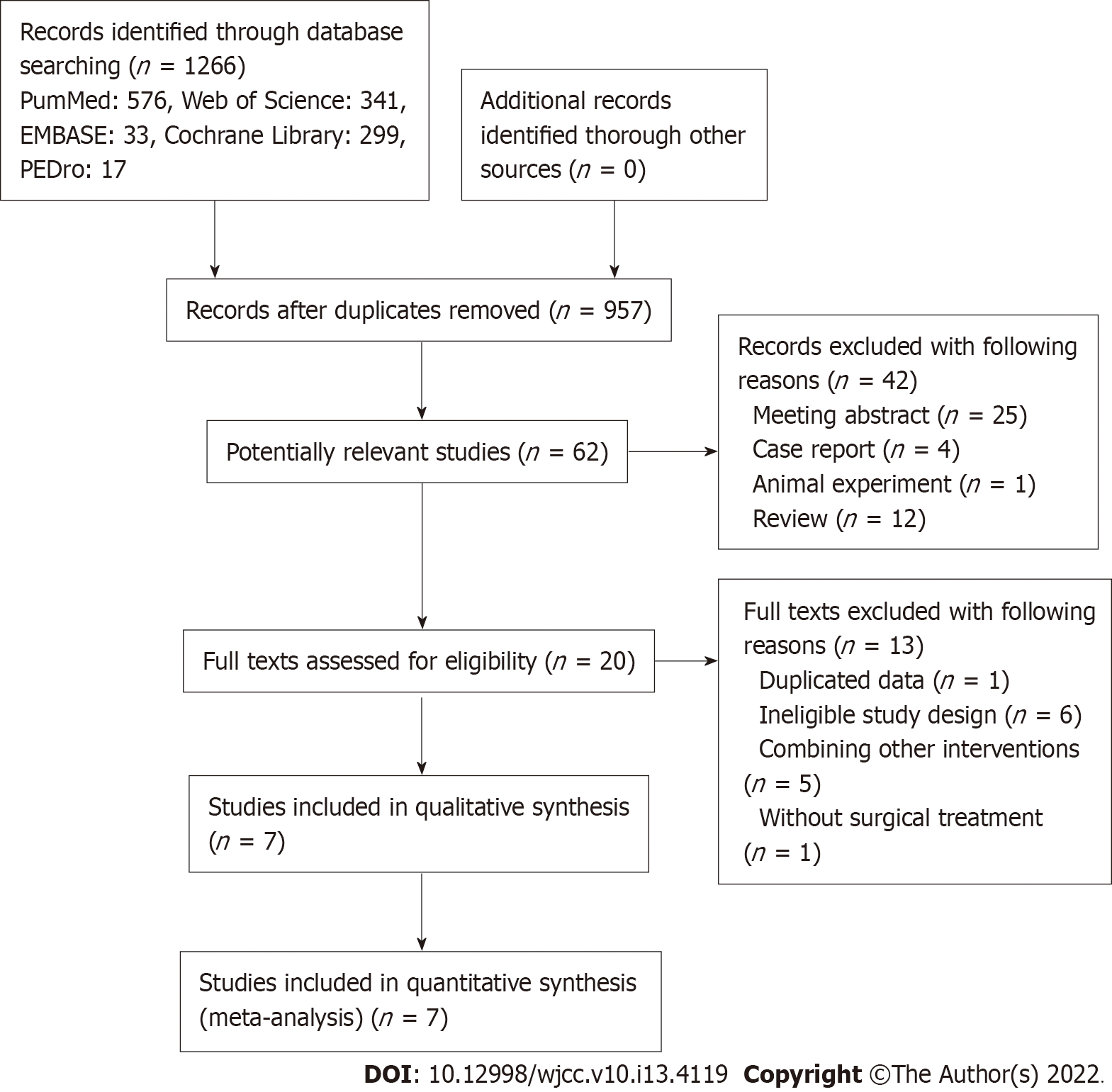
The PRISMA statement flowchart displayed the process of literature search, records selection and reasons for exclusion (Figure 1). At first, 1266 records were searched and 309 duplicated records were removed. After screening the titles and abstracts, 895 irrelevant publications were excluded. Then 62 potentially related publications were screened for eligibility 42 publications were excluded due to the study design. Among remaining 20 publications, 12 records were excluded on the basis of the study not meeting the inclusion criteria and 1 record was excluded because of duplicated data after reviewing the full texts. Finally, only seven articles were included in this meta- analysis after reviewing the full texts of the remaining 20 studies[22-28].
The included seven trials reported data on 240 participants with the sample size ranged from 26 to 68. It should be noted that Brocki et al[23,24] described different outcomes of the same group of patients in two articles. Three studies explored the clinical effect of IMT[22-24], and the other four trials evaluated the clinical effect of RMT, including the IMT and EMT, in patients undergoing lung surgery[25-28]. One study focused on preoperative RMT[28], three studies focused on the postoperative RMT[25-27] and the other three studies contained both pre and postoperative RMT[22-25]. Detailed information is presented in Table 1.
| Ref. | Year | Country | Sample size | Surgery type | Intervention | Control | Initial training pressure (%) | Training time (min/d) | Sessions (n/wk) | Duration (wk) |
| Weiner et al[22] | 1997 | Israel | IMT: 17, Con: 15 | NR | Preoperative and postoperative IMT, incentive spirometry | Usual care | 15 | 60 | 6 | 14 |
| Brocki et al[23] | 2016 | Denmark | IMT: 34, Con: 34 | VATS: 35, thoracotomy: 33 | Preoperative IMT 30% MIP, postoperative: 15% MIP, breathing exercises, early mobilization | Breathing exercises, early mobilization | 30 | 15 | 7 | 2 |
| Brocki et al[24] | 2018 | Denmark | IMT: 34, Con: 34 | VATS: 35, thoracotomy: 33 | Preoperative IMT 30% MIP, postoperative: 15% MIP, breathing exercises, early mobilization | Breathing exercises, early mobilization | 30 | 15 | 7 | 2 |
| Taşkin et al[25] | 2018 | Turkey | RMT: 20, Con: 20 | Thoracotomy | Postoperative RMT, chest physiotherapy, early mobilization | Chest physiotherapy, early mobilization | 15 | Six sessions consisting of 3 sets of 10 breaths | 5 | NR |
| Messaggi-Sartor et al[26] | 2019 | Spain | RMT: 16, Con: 21 | VATS: 3, thoracotomy: 34 | Postoperative RMT, aerobic exercise | Usual care | 30 | 60 | 3 | 8 |
| Kendall et al[27] | 2020 | Portugal | IMT: 13, EMT: 13, IMT + EMT: 18, Con: 19 | Thoracotomy | Postoperative IMT or EMT or IMT + EMT, usual care | Usual care | 25 | 15 | 7 | 8 |
| Laurent et al[28] | 2020 | France | RMT: 14, Con: 12 | VATS or thoracotomy | Preoperative RMT, usual chest physical therapy | Usual chest physical therapy | 30 | 30 | 4 | 3 |
The average score of included RCTs in the PEDro scale was 6.43, ranging from 5 to 7, which indicates high quality (Table 2).
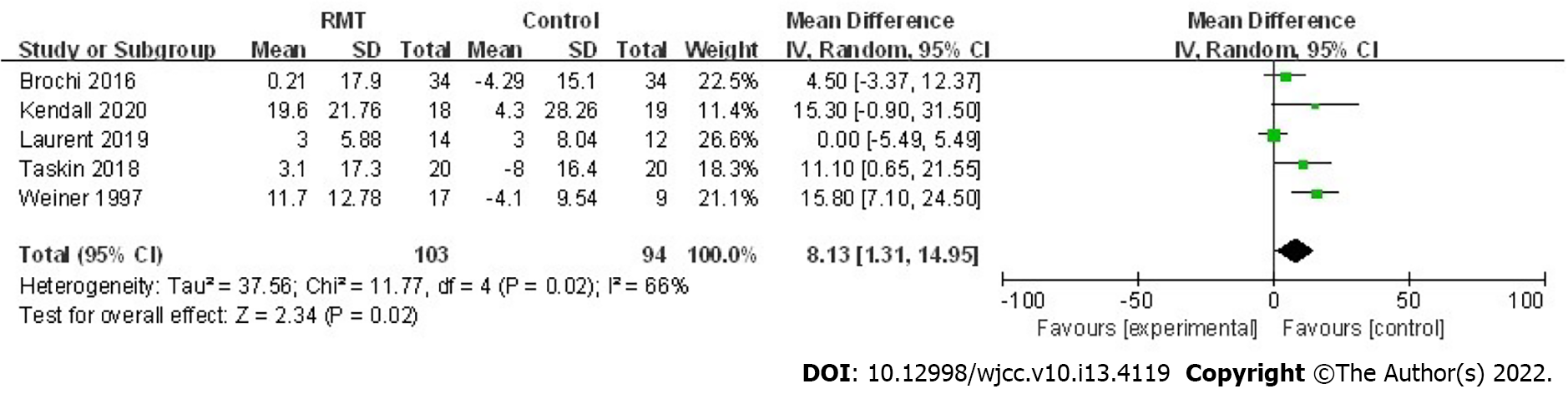
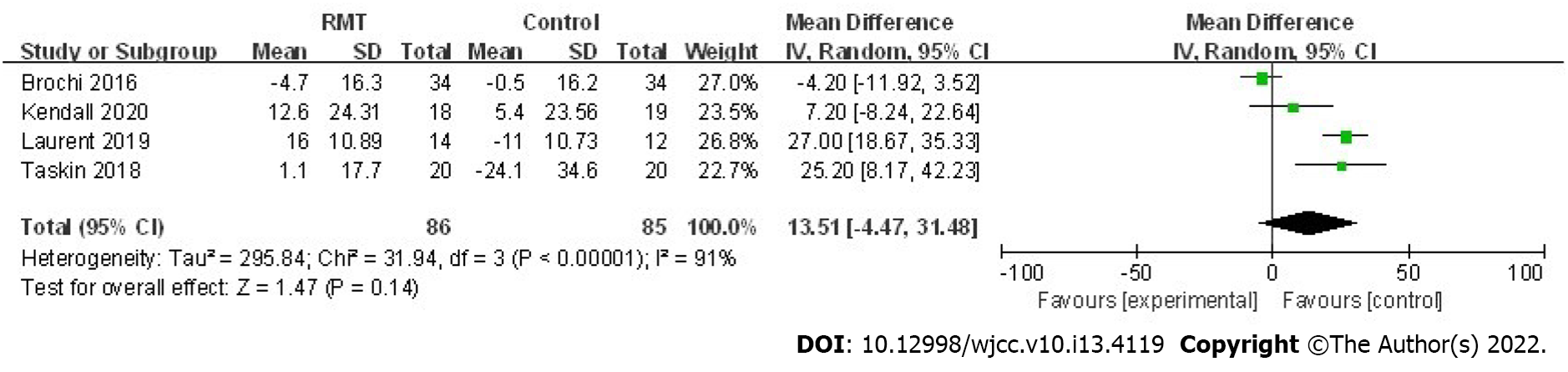
A total of five trials assessed the effect of RMT on the postoperative MIP in 197 patients[22,23,25,27,28]. The pooled results indicated that perioperative RMT improved the postoperative MIP significantly (mean = 8.13 cmH2O, 95%CI: 1.31 to 14.95, P = 0.02; I2 = 66%, Pheterogeneity = 0.02) (Figure 2). Furthermore, perioperative RMT tended to increase the postoperative MEP (mean = 13.51 cmH2O, 95%CI: -4.47 to 31.48, P = 0.14; I2 = 91%, Pheterogeneity < 0.001) after combining four relevant studies including involving 171 patients (Figure 3)[23,25,27,28], although statistical significant differences were not reached.
Subsequently, a subgroup analysis was conducted by stratifying intervention time and training method. For MIP, the results indicated that postoperative RMT significantly increased postoperative MIP (mean = 12.33 cmH2O, 95%CI: 3.55 to 21.11 cmH2O, P = 0.006;
| No. of studies | Mean | 95%CI | P value | Pheterogeneity | ||
| MIP | ||||||
| Intervention time | ||||||
| Preoperative | 1 | 12.33 | -5.49, 5.49 | > 0.999 | - | - |
| Postoperative | 2 | 12.33 | 3.55, 21.11 | 0.006 | 0.0 | 0.67 |
| Training method | ||||||
| IMT | 3 | 9.53 | 3.98, 15.08 | < 0.001 | 44 | 0.17 |
| EMT | 1 | 9.00 | -9.00, 27.00 | 0.33 | - | 0.13 |
| RMT | 3 | 6.97 | -2.81, 16.74 | 0.16 | 64 | - |
| MEP | ||||||
| Intervention time | ||||||
| Preoperative | 1 | 27 | 18.67, 35.33 | < 0.001 | - | - |
| Postoperative | 2 | 15.83 | -1.80, 33.45 | 0.08 | 58 | 0.12 |
| Training method | ||||||
| IMT | 2 | -3.49 | -10.57, 3.60 | 0.33 | 0 | 0.65 |
| EMT | 1 | 1.70 | -14.67 to 18.07 | 0.84 | - | - |
| RMT | 3 | 20.72 | 8.60, 32.84 | < 0.001 | 60 | 0.08 |
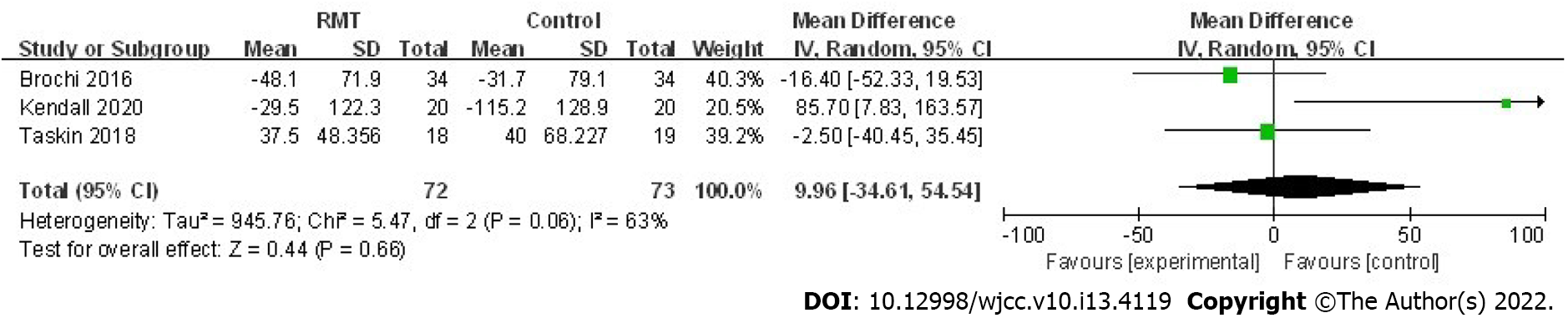
Brocki et al[24] evaluated the effect of IMT on postoperative self-reported physical activity (Physical Activity Scale 2.1 questionnaire[29]) and their results revealed that patients receiving two weeks of postoperative IMT had higher physical activity level than those who received usual care only (sedentary 6% vs 22%, moderate activity 38% vs 12%, low activity 56% vs 66%, respectively; P = 0.006). Furthermore, results of the study conducted by Kendall et al[27] also indicated that perioperative RMT could improve sedentary physical activity (P = 0.009) and total physical activity (P = 0.035). (Table 4) Three trials assessed the effect of RMT on 6MWD[23,25,27] and the pooled results manifested that postoperative 6MWD of patients who received RMT did not increase compared to those who received usual care (mean = 9.96 m, 95%CI: -34.61 to 54.54, P = 0.66; I2 = 63%, Pheterogeneity = 0.06) (Figure 4). Besides, two studies reported the effect of RMT on VO2peak during the CPET[26,28] and pooled results indicated that RMT did not improve VO2peak (mean = 2.44 mL/min/kg, 95%CI: -2.36 to 7.24, P = 0.32; I2 = 96%,
| No. of studies | Mean | 95%CI | P value | Pheterogeneity | ||
| Primary outcomes | ||||||
| Respiratory function | ||||||
| MIP (cmH2O) | 5 | 8.13 | 1.31, 14.95 | 0.02 | 66 | 0.02 |
| MEP (cmH2O) | 4 | 13.51 | -4.47, 31.48 | 0.14 | 91 | < 0.001 |
| Secondary outcomes | ||||||
| Physical activity | 2 | - | - | 0.006/0.035 | - | - |
| Exercise capacity | ||||||
| 6MWD (m) | 3 | 9.96 | -34.61, 54.54 | 0.66 | 63 | 0.06 |
| CPET/VO2peak (mL/min/kg) | 2 | 2.44 | -2.36, 7.24 | 0.32 | 96 | < 0.001 |
| Pulmonary function | ||||||
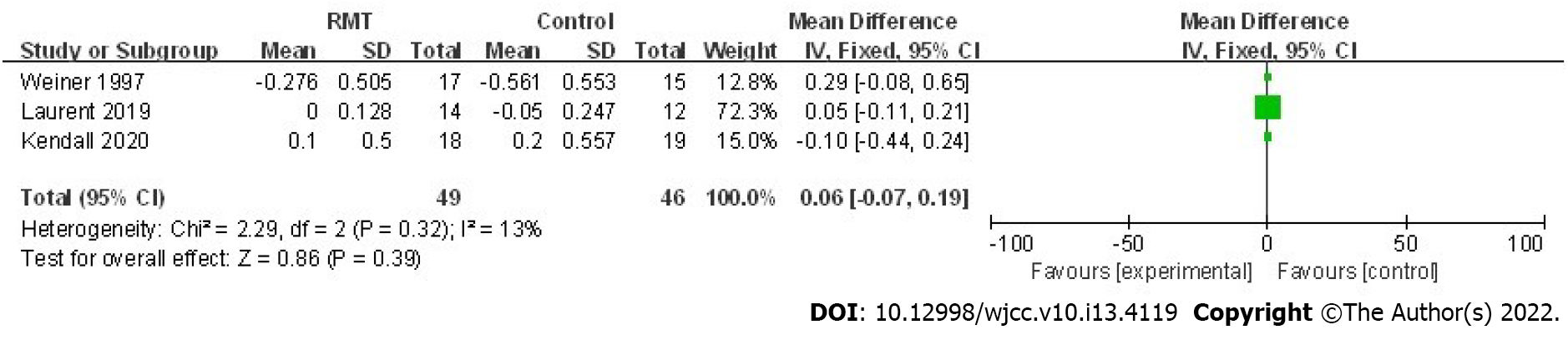
| FEV1 (L) | 3 | 0.06 | -0.07, 0.19 | 0.39 | 13 | 0.32 |
| FVC (L) | 2 | 0.29 | -0.05, 0.64 | 0.10 | 0 | 0.96 |
| Quality of life | ||||||
| Pain (VAS) | 2 | 0.67 | -0.99, 2.32 | 0.43 | 61 | 0.11 |
| Dyspnoea (VAS) | 2 | -0.16 | -0.58, 0.25 | 0.44 | 0 | 0.61 |
| EORTC QLQ-C30 | 1 | - | - | - | - | - |
Regarding the pulmonary function, four trials investigated the effect of RMT on the postoperative FEV1 and FVC[22,23,27,28]. According to the pooled results of our meta-analysis, none of these indexes were increased significantly by the RMT. However, there was a trend that RMT could improve the postoperative FEV1 (mean = 0.06 L, 95%CI: -0.07 to 0.19, P = 0.39; I2 = 13%, Pheterogeneity = 0.32) (Figure 5) and FVC (mean = 0.29, 95%CI: -0.05 to 0.64, P = 0.10; I2 = 0%, Pheterogeneity = 0.96) (Table 4).
Postoperative RMT did not improve the symptoms of pain [visual analog scale (VAS) (mean = 0.67, 95%CI: -0.99 to 2.32, P = 0.43;
To the best of our knowledge, this is the first to comprehensively identify the clinical role of perioperative RMT in patients receiving lung surgery in the form of a meta-analysis after reviewing several relevant studies. To some extent, this is the highest-quality study with the GRADE A to assess the clinical value of RMT in patients undergoing pulmonary resection. Our results demonstrated that perioperative RMT improved respiratory muscle strength and physical activity of patients undergoing lung resection. Furthermore, perioperative RMT might also improve the pulmonary function representing as the FEV1 and FVC. However, the exercise capacity and quality of life were not significantly improved by RMT due to the limitations of small sample size and heterogeneity between included studies, more RCTs with high quality are still needed to verify our finding.
The pooled results indicate that additional perioperative RMT increases the MIP (P = 0.02) of patients receiving lung surgery significantly compared with usual perioperative care alone such as the breathing exercises, chest physiotherapy. For patients receiving major surgery, postoperative reductions in MIP are regarded as the result of altered respiratory mechanics and pain and may be a contributor of PPCs[30-32]. Besides, increased MIP would assist postoperative lung expansion especially in patients who receive lung surgery which, in turn, contributes to the generation of forceful expiratory manoeuvres for secretion clearance[13]. The meta-analysis conducted by Mans et al[13] manifested that preoperative IMT could not only increase MIP (mean = 15 cmH2O, 95%CI: 9 to 21, P < 0.001) but also reduce PPCs (RR = 0.48, 95%CI: 0.26-0.89, P = 0.02) in patients receiving cardiothoracic or upper abdominal surgery, which is consistent with our results and above inferences. Although the pooled results for the effect of perioperative RMT on MEP did not reach the statistical difference, an obvious trend that perioperative RMT may improve MEP was also observed (mean = 13.51 cmH2O, 95%CI: -4.47 to 31.48, P = 0.14). Furthermore, two of included studies reported positive findings that the MEP was increased significantly with the mean changes of 25.20 cmH2O and 27 cmH2O after the postoperative RMT and preoperative RMT, respectively[25,28]. Thus, the authors deem that perioperative RMT may also increase MEP of patients undergoing pulmonary resection.
A postoperative decline of physical activity level is commonly observed in patients undergoing major surgery because of acute pain or (and) temporary decrease of cardiopulmonary function, which may result in adverse postoperative recovery. Brocki et al[24] verified that perioperative IMT was effective to prevent the postoperative decline of physical activity level in high-risk patients following pulmonary resection, which is consistent with the results shown in the research performed by Kendall et al[27].
The 6MWD is widely applied to evaluate the effect of rehabilitation therapy in clinics. The pooled results based on three included trials indicated nonsignificant effect of perioperative RMT on 6MWD in patients receiving lung surgery (P = 0.66). However, 6MWD is often used to assess the exercise endurance and cardiopulmonary function of patients with cardiopulmonary diseases; and actually, improving daily physical activity level is more important for short-term recovery after surgery than increasing exercise endurance, which means physical activity level assessed by sufficient data may be a more meaningful index in evaluating effects of perioperative rehabilitation treatment than single 6MWD. Meanwhile, in the trial conducted by Kendall et al[27], IMT plus EMT was significantly effective in preventing the decline of 6MWD postoperatively, although the other two studies reported negative results[23,25,27]. Thus, more trials investigating the effect of perioperative RMT on 6MWD are still needed.
With the great advances of RMT technologies in recent years, RMT has been widely applied in various types of surgeries including the lung surgery during the perioperative period. RMT is believed to play an essential role in postoperative recovery for patients who receiving pulmonary resection since the lung works as a respiratory organ. However, the clinical value of RMT in lung surgery has not been well recognized, especially in our country. Furthermore, there are many fields worth investigating about the effect of RMT in patients undergoing pulmonary resection. For example, the parameters of initial training pressure, training time, sessions and duration time for different groups of patients should be different. Brocki et al[23] and Laurent et al[28] defined 30% of MIP as the initial training pressure for preoperative RMT and Weiner et al[22], Brocki et al[24], and Taşkin et al[25] defined 15% of MIP as the initial training pressure for postoperative RMT. However, Weiner et al[22] defined 15% of MIP as the initial training pressure and Messaggi-Sartor et al[26] and Kendall et al[27] defined 30% and 25% of MIP as the initial training pressure for postoperative RMT, respectively. Besides, RMT consists of IMT and EMT, it is necessary to compare the differences between the effects of IMT, EMT and IMT-EMT-RMT in different outcomes like Kendall et al[27]. According to the information provided by their trial, IMT alone showed a similar effect on MIP as IMT-EMT-RMT, nevertheless IMT-EMT-RMT was more effective to enhance 6MWD than IMT or EMT alone. Furthermore, the comparison between the effects of preoperative, postoperative and pre plus postoperative RMT is also important, especially in different groups of patients. It is believed that pre plus postoperative RMT is more significant in high-risk patients than in patients with good physical and more effective in enhancing recovery after lung surgery than pre or postoperative RMT alone.
This systematic review and meta-analysis manifested the effects of perioperative RMT on most of postoperative outcomes except for PPCs by combining seven relevant RCTs. This is the first study to comprehensively review clinical value of perioperative RMT in patients undergoing lung surgery, which may provide us some novel suggestions for clinical application of RMT. Besides, we also showed current evidence on the clinical effect of RMT and proposed some valuable directions worth further investigating, which might contribute to the development of RMT in lung surgery.
There are several limitations in this study. First, the sample sizes are relatively small and we were unable to control for some important pretreatment parameters which could affect the outcomes, like the pretreatment pulmonary function indexes. Second, the parameters of RMT are not the same in each included study, such as the initial training pressure ranging from 15% to 30% of MIP and training time ranging from 15 min to 60 min per day. It was too hard to establish a general perioperative RMT protocol in this meta-analysis. Third, although we conducted subgroup analysis stratified by the period (pre or postoperative) and type of RMT (IMT, EMT or IMT + EMT), the results did not well verify the conclusion of our study due to the limited included trials. Four, we contacted all the corresponding authors for original data we needed; however, no response was received. Five, only articles published in English were included in this meta-analysis.
In conclusion, this systematic review and meta-analysis demonstrated that perioperative RMT could enhance the postoperative respiratory muscle strength and physical activity in patients undergoing lung resection. However, more trials with high quality are still needed to verify the effects of perioperative RMT on postoperative outcomes in patients receiving lung surgery.
The clinical values of perioperative respiratory muscle training (RMT), including inspiratory muscle training and expiratory muscle training in patients receiving lung surgery are not clear now.
To evaluate whether perioperative RMT is effective in improving postoperative outcomes such as the respiratory muscle strength and physical activity level in patients receiving lung surgery.
To further identify the clinical role of perioperative RMT in patients undergoing pulmonary surgery.
Several databases were systematically searched to obtain eligible randomized controlled trials (RCTs). Primary outcome was postoperative respiratory muscle strength expressed as the maximal inspiratory pressure (MIP) and maximal expiratory pressure (MEP). Secondary outcomes were physical activity, exercise capacity, including the 6-min walking distance and peak oxygen consumption during the cardio-pulmonary exercise test, pulmonary function and the quality of life.
For primary outcomes, the pooled results indicated that perioperative RMT improved the postoperative MIP (mean = 8.13 cmH2O, P = 0.02) and tended to increase MEP (mean = 13.51 cmH2O, P = 0.14). For secondary outcomes, perioperative RMT enhanced postoperative physical activity significantly (P = 0.006) and a trend of improved postoperative pulmonary function was observed.
Perioperative RMT enhanced postoperative respiratory muscle strength and physical activity level of patients receiving lung surgery.
However, RCTs with large samples are needed to evaluate effects of perioperative RMT on posto
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Rehabilitation
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Batalik L, Czech Republic; Patoulias D, Greece S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Menezes TC, Bassi D, Cavalcanti RC, Barros JESL, Granja KSB, Calles ACDN, Exel AL. Comparisons and correlations of pain intensity and respiratory and peripheral muscle strength in the pre- and postoperative periods of cardiac surgery. Rev Bras Ter Intensiva. 2018;30:479-486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Weissman C. Pulmonary function after cardiac and thoracic surgery. Curr Opin Anaesthesiol. 2000;13:47-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Brunelli A. Risk assessment for pulmonary resection. Semin Thorac Cardiovasc Surg. 2010;22:2-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Kendall F, Abreu P, Pinho P, Oliveira J, Bastos P. The role of physiotherapy in patients undergoing pulmonary surgery for lung cancer. A literature review. Rev Port Pneumol (2006). 2017;23:343-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Martín-Valero R, Jimenez-Cebrian AM, Moral-Munoz JA, de-la-Casa-Almeida M, Rodriguez-Huguet M, Casuso-Holgado MJ. The Efficacy of Therapeutic Respiratory Muscle Training Interventions in People with Bronchiectasis: A Systematic Review and Meta-Analysis. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Templeman L, Roberts F. Effectiveness of expiratory muscle strength training on expiratory strength, pulmonary function and cough in the adult population: a systematic review. Physiotherapy. 2020;106:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Nomori H, Kobayashi R, Fuyuno G, Morinaga S, Yashima H. Preoperative respiratory muscle training. Assessment in thoracic surgery patients with special reference to postoperative pulmonary complications. Chest. 1994;105:1782-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 80] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Drummond G. Surgery and respiratory muscles. Thorax. 1999;54:1140-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 9. | Jaworski A, Goldberg SK, Walkenstein MD, Wilson B, Lippmann ML. Utility of immediate postlobectomy fiberoptic bronchoscopy in preventing atelectasis. Chest. 1988;94:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | O'Donohue WJ Jr. National survey of the usage of lung expansion modalities for the prevention and treatment of postoperative atelectasis following abdominal and thoracic surgery. Chest. 1985;87:76-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 50] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Pitts T, Bolser D, Rosenbek J, Troche M, Okun MS, Sapienza C. Impact of expiratory muscle strength training on voluntary cough and swallow function in Parkinson disease. Chest. 2009;135:1301-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 196] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 12. | Smith Hammond CA, Goldstein LB, Zajac DJ, Gray L, Davenport PW, Bolser DC. Assessment of aspiration risk in stroke patients with quantification of voluntary cough. Neurology. 2001;56:502-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 103] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Mans CM, Reeve JC, Elkins MR. Postoperative outcomes following preoperative inspiratory muscle training in patients undergoing cardiothoracic or upper abdominal surgery: a systematic review and meta analysis. Clin Rehabil. 2015;29:426-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Kendall F, Oliveira J, Peleteiro B, Pinho P, Bastos PT. Inspiratory muscle training is effective to reduce postoperative pulmonary complications and length of hospital stay: a systematic review and meta-analysis. Disabil Rehabil. 2018;40:864-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 15. | Ge X, Wang W, Hou L, Yang K, Fa X. Inspiratory muscle training is associated with decreased postoperative pulmonary complications: Evidence from randomized trials. J Thorac Cardiovasc Surg. 2018;156:1290-1300.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg. 2011;39:91-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 583] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 17. | de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. 2009;55:129-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1009] [Cited by in RCA: 1416] [Article Influence: 88.5] [Reference Citation Analysis (0)] |
| 18. | Moseley AM, Herbert RD, Sherrington C, Maher CG. Evidence for physiotherapy practice: a survey of the Physiotherapy Evidence Database (PEDro). Aust J Physiother. 2002;48:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 605] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 19. | Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21630] [Cited by in RCA: 25789] [Article Influence: 1121.3] [Reference Citation Analysis (0)] |
| 20. | Zintzaras E, Ioannidis JP. HEGESMA: genome search meta-analysis and heterogeneity testing. Bioinformatics. 2005;21:3672-3673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 363] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 21. | Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4895] [Cited by in RCA: 6895] [Article Influence: 344.8] [Reference Citation Analysis (0)] |
| 22. | Weiner P, Man A, Weiner M, Rabner M, Waizman J, Magadle R, Zamir D, Greiff Y. The effect of incentive spirometry and inspiratory muscle training on pulmonary function after lung resection. J Thorac Cardiovasc Surg. 1997;113:552-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 88] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Brocki BC, Andreasen JJ, Langer D, Souza DS, Westerdahl E. Postoperative inspiratory muscle training in addition to breathing exercises and early mobilization improves oxygenation in high-risk patients after lung cancer surgery: a randomized controlled trial. Eur J Cardiothorac Surg. 2016;49:1483-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 24. | Brocki BC, Andreasen JJ, Westerdahl E. Inspiratory Muscle Training in High-Risk Patients Following Lung Resection May Prevent a Postoperative Decline in Physical Activity Level. Integr Cancer Ther. 2018;17:1095-1102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Taşkin H PT, MSc, Telli Atalay O PT, PhD, Yuncu G MD, Taşpinar B PT, Yalman A PT, Şenol H MSc. Postoperative respiratory muscle training in addition to chest physiotherapy after pulmonary resection: A randomized controlled study. Physiother Theory Pract. 2020;36:378-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Messaggi-Sartor M, Marco E, Martínez-Téllez E, Rodriguez-Fuster A, Palomares C, Chiarella S, Muniesa JM, Orozco-Levi M, Barreiro E, Güell MR. Combined aerobic exercise and high-intensity respiratory muscle training in patients surgically treated for non-small cell lung cancer: a pilot randomized clinical trial. Eur J Phys Rehabil Med. 2019;55:113-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | Kendall F, Silva G, Almeida J, Eusébio E, Pinho P, Oliveira J, Bastos PT. Influence of Respiratory Muscle Training on Patients' Recovery after Lung Resection. Int J Sports Med. 2020;41:484-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (2)] |
| 28. | Laurent H, Aubreton S, Galvaing G, Pereira B, Merle P, Richard R, Costes F, Filaire M. Preoperative respiratory muscle endurance training improves ventilatory capacity and prevents pulmonary postoperative complications after lung surgery. Eur J Phys Rehabil Med. 2020;56:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Andersen LG, Groenvold M, Jørgensen T, Aadahl M. Construct validity of a revised Physical Activity Scale and testing by cognitive interviewing. Scand J Public Health. 2010;38:707-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Nomori H, Horio H, Fuyuno G, Kobayashi R, Yashima H. Respiratory muscle strength after lung resection with special reference to age and procedures of thoracotomy. Eur J Cardiothorac Surg. 1996;10:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Canet J, Gallart L, Gomar C, Paluzie G, Vallès J, Castillo J, Sabaté S, Mazo V, Briones Z, Sanchis J; ARISCAT Group. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology. 2010;113:1338-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 632] [Cited by in RCA: 810] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 32. | Moreno AM, Castro RR, Sorares PP, Sant' Anna M, Cravo SL, Nóbrega AC. Longitudinal evaluation the pulmonary function of the pre and postoperative periods in the coronary artery bypass graft surgery of patients treated with a physiotherapy protocol. J Cardiothorac Surg. 2011;6:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |