Published online May 6, 2022. doi: 10.12998/wjcc.v10.i13.4097
Peer-review started: July 26, 2021
First decision: August 19, 2021
Revised: September 1, 2021
Accepted: March 16, 2022
Article in press: March 16, 2022
Published online: May 6, 2022
Processing time: 277 Days and 14 Hours
Recently, nonalcoholic fatty liver disease (NAFLD) has been renamed metabolic-associated fatty liver disease (MAFLD). Based on the definition for MAFLD, a group of non-obese and metabolically healthy individuals with fatty liver are excluded from the newly proposed nomenclature.
To analyze the histologic features in the MAFLD and non-MAFLD subgroups of NAFLD.
Eighty-three patients with biopsy-proven NAFLD were separated into MAFLD and non-MAFLD groups. The diagnosis of MAFLD was established as hepatic steatosis along with obesity/diabetes or evidence of metabolic dysfunction. The histologic features were compared according to different metabolic disorders and liver enzyme levels.
MAFLD individuals had a higher NAFLD activity score (P = 0.002) and higher severity of hepatic steatosis (42.6% Grade 1, 42.6% Grade 2, and 14.8% Grade 3 in MAFLD; 81.8% Grade 1, 13.6% Grade 2, and 4.5% Grade 3 in non-MAFLD; P = 0.007) than the non-MAFLD group. Lobular and portal inflammation, hepatic ballooning, fibrosis grade, and the presence of nonalcoholic steatohepatitis (NASH) and significant fibrosis were comparable between the two groups. The higher the liver enzyme levels, the more severe the grades of hepatic steatosis (75.0% Grade 1 and 25.0% Grade 2 in normal liver function; 56.6% Grade 1, 39.6% Grade 2, and 3.8% Grade 3 in increased liver enzyme levels; 27.8% Grade 1, 27.8% Grade 2, and 44.4% Grade 3 in liver injury; P < 0.001). Patients with liver injury (alanine aminotransferase > 3 × upper limit of normal) presented a higher severity of hepatocellular ballooning (P = 0.021). Moreover, the grade of steatosis correlated significantly with hepatocellular ballooning degree (r = 0.338, P = 0.002) and the presence of NASH (r = 0.466, P < 0.001).
Metabolic dysfunction is associated with hepatic steatosis but no other histologic features in NAFLD. Further research is needed to assess the dynamic histologic characteristics in NAFLD based on the presence or absence of metabolic disorders.
Core Tip: Non-obese and metabolically healthy patients with fatty liver are excluded from the definition of metabolic-associated fatty liver disease (MAFLD), but their clinical course has seldom been demonstrated. We analysed a group of nonalcoholic fatty liver disease (NAFLD) subjects, and found that the MAFLD subgroup had a higher NAFLD activity score and higher severity of hepatic steatosis than the non-MAFLD subgroup. There was no difference in other histologic features, including lobular and portal inflammation, balloon degeneration, and fibrosis, between the MAFLD and non-MAFLD patients. The grade of steatosis correlated positively with the hepatocellular ballooning degree, and the presence of nonalcoholic steatohepatitis. We believe that our study can provide insight into the histologic features of various subsets of fatty liver disease.
- Citation: Dai YN, Xu CF, Pan HY, Huang HJ, Chen MJ, Li YM, Yu CH. Metabolic dysfunction is associated with steatosis but no other histologic features in nonalcoholic fatty liver disease. World J Clin Cases 2022; 10(13): 4097-4109
- URL: https://www.wjgnet.com/2307-8960/full/v10/i13/4097.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i13.4097
Nonalcoholic fatty liver disease (NAFLD), characterized by the presence of steatosis in > 5% of hepatocytes without other causes of liver injury, including excess alcohol consumption, has become a growing social health problem[1]. NAFLD covers a broad spectrum of disease severity[2-5], ranging from simple fatty liver to nonalcoholic steatohepatitis (NASH), and can even lead to cirrhosis and hepatocellular carcinoma. Currently, it is widely accepted that many cases of cryptogenic cirrhosis actually result from NAFLD, in which steatosis vanishes in the late cirrhotic stage.
Metabolic dysfunction is generally defined as obesity, type 2 diabetes mellitus (T2DM), and conditions including excess weight around the waist, hypertension, hyperlipidaemia, prediabetes, and insulin resistance. Currently, it is well recognized that NAFLD originates from an underlying condition of systemic metabolic dysfunction and represents the hepatic manifestation of metabolic syndrome (MS). Actually, it should not be defined as a state of “exclusion”, such as the exclusion of excess alcohol consumption or viral hepatitis. A group of experts have recently suggested that the outdated nomenclature of NAFLD should be renamed metabolic-associated fatty liver disease (MAFLD)[6,7]. Briefly, evidence of hepatic steatosis along with metabolic disorders establishes a diagnosis of MAFLD.
While MAFLD represents the majority of those previously diagnosed with NAFLD in clinical practice, a group of non-obese and metabolically healthy individuals with fatty liver are excluded from MAFLD based on the international expert consensus statement[8]. With regard to this subset of fatty liver, the clinical course and outcomes have seldom been demonstrated. In this study, we aimed to analyze the hepatic histologic characteristics in the MAFLD and non-MAFLD subgroups of NAFLD. In addition, we conducted subgroup analyses according to the presence of obesity, glycemia, and liver enzyme levels to explore histologic features in various subsets of fatty liver disease.
Patients diagnosed with NAFLD, based on the presence of steatosis in more than 5% of hepatocytes with the exclusion of other chronic liver diseases and alcohol consumption, were recruited at Zhejiang Provincial People’s Hospital. They were further divided into MAFLD and non-MAFLD groups. MAFLD was defined as hepatic steatosis along with one of the following three standards, i.e., obesity [body mass index (BMI) ≥ 23 kg/m2], T2DM, or evidence of metabolic dysfunction. The latter was based on the presence of at least two of the following metabolic abnormalities: Waist circumference (WC) ≥ 90 cm in men and ≥ 80 cm in women; blood pressure ≥ 130/85 mmHg or diagnosis of high blood pressure under specific drug treatment; serum triglycerides (TG) ≥ 1.70 mmol/L or specific drug therapy; serum high-density lipoprotein cholesterol (HDL-C) < 1.0 mmol/L for men and < 1.3 mmol/L for women, or under specific drug therapy; fasting glucose between 5.6 to 6.9 mmol/L, or 2-h post-load glucose between 7.8 to 11.0 mmol/L, or HbA1c level between 5.7% to 6.4%, which indicate a condition with prediabetes; homeostasis model assessment for insulin resistance (HOMA-IR) score ≥ 2.5; and high-sensitivity C-reactive protein (hs-CRP) level > 2 mg/L[8]. Non-MAFLD referred to NAFLD participants without obesity, T2DM, or the above metabolic disorders. In particular, “alternative causes” of steatosis, such as medications, celiac disease, severe surgical weight loss, starvation, or total parenteral nutrition, were not allowed for inclusion. However, we did not assess disorders of lipid metabolism, genetic abnormalities, or other rare metabolic disorders as possible causes for non-MAFLD.
The exclusion criteria were as follows: (1) Children or adolescents less than 18 years old; (2) excessive alcohol drinkers (weekly ethanol consumption more than 140 g in men and 70 g in women); (3) documented hepatitis B or C; (4) other chronic liver disease (e.g., autoimmune liver disease, drug-induced liver injury, or hereditary disorders); (5) dysfunction of coagulation; and (6) cirrhosis, malignancy, severe organ dysfunctions such as cardiopulmonary dysfunction, renal inadequacy, or pregnancy.
Asian subjects with a BMI < 23 kg/m2 were defined as having lean NAFLD. The diagnosis of T2DM was in reference to the widely accepted international criteria[9]. In addition, the participants were classified into three groups according to their liver enzyme levels: Normal liver function, increased liver enzyme level, and liver injury[10]. Increased liver enzyme levels were defined as any liver enzyme level above the upper limit of normal (ULN), with an alanine aminotransferase (ALT) level < 3-fold of the ULN. Liver injury conformed to ALT concentrations > 3-fold of the ULN.
All eligible subjects received liver biopsy, and written informed consent was obtained from each participant. The study was in agreement with the Declaration of Helsinki and was approved by the Ethics Committee, People’s Hospital of Hangzhou Medical College.
Anthropometric data were obtained for each subject by a well-trained nurse. We calculated BMI as the weight divided by the square of the height (g/m2). WC refers to the minimum circumference between the umbilicus and the xiphoid process[11]. Systolic and diastolic blood pressures (SBP and DBP) were measured using a sphygmomanometer with the subject in a sitting position.
Blood biochemistry tests evaluating ALT, aspartate transferase (AST), alkaline phosphatase (AKP), gamma-glutamyltransferase (GGT), albumin (ALB), globulin (GLB), cholinesterase (CHE), uric acid (UA), TG, total cholesterol (TC), HDL-C, low-density lipoprotein cholesterol (LDL-C), glucose, complete blood counts, and hs-CRP were performed in our clinical laboratory using automatic analysers.
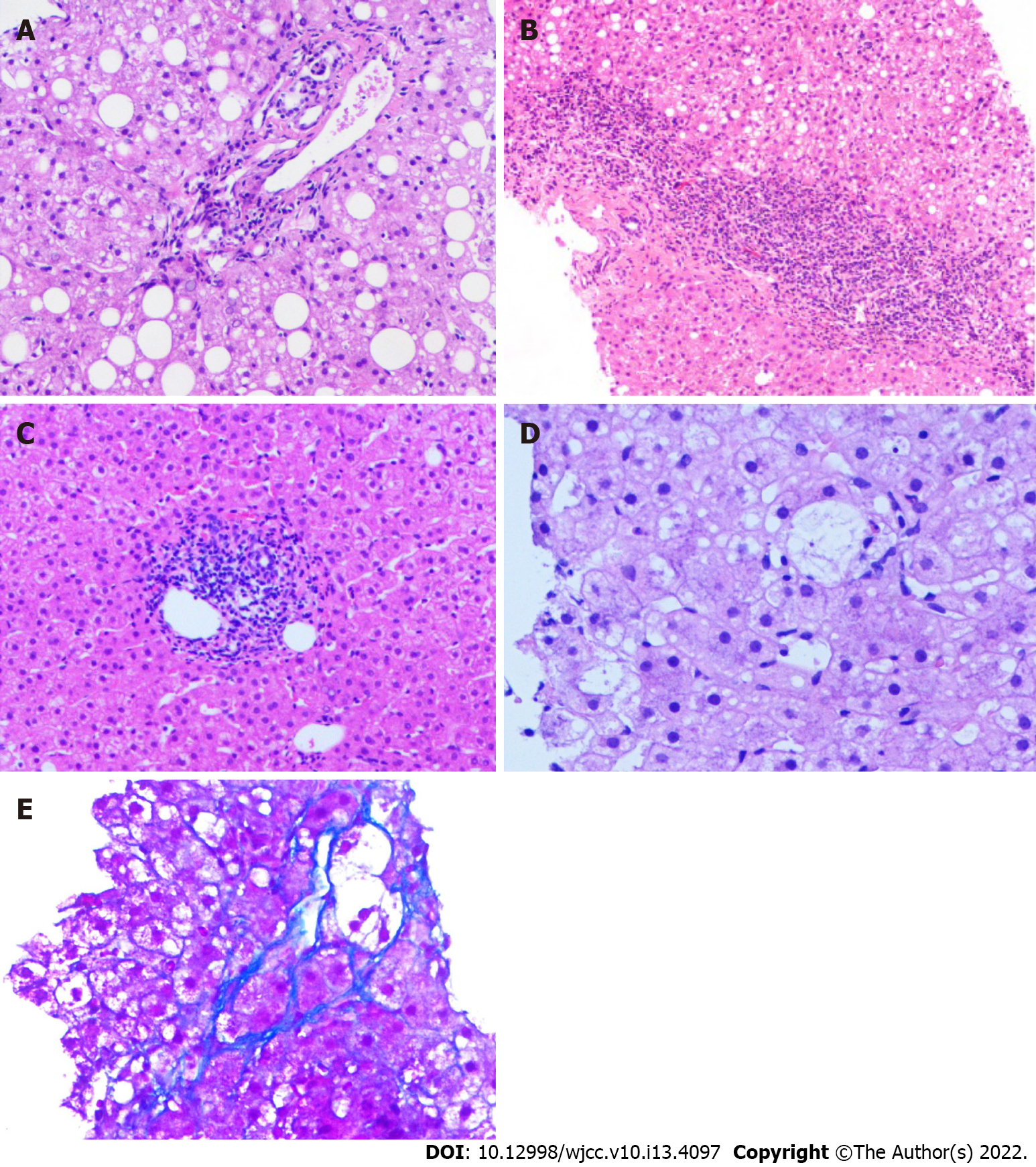
Experienced doctors performed ultrasound-guided percutaneous liver biopsy using the MAX-CORE Disposable Core Biopsy Instrument (Bard Peripheral Vascular, Inc., Mexico), with specimens fixed, paraffin-embedded, and stained with haematoxylin and eosin (H&E) and Masson’s trichrome.
A skilled liver pathologist who was unaware of the participants' clinical data reviewed the slides of liver biopsies. All eligible liver biopsy slides in this study were qualified for grading and staging of the histologic features.
A threshold of 5% macrovesicular steatosis established a diagnosis of NAFLD[12]. Steatosis was graded as the percentage of liver parenchyma replaced by fat: (1) 5%-33%; (2) 34%-66%; or (3) more than 66%[13]. Lobular inflammation was scored on a scale of 0-3: (0) none; (1) mild; (2) moderate; and (3) many. In addition, portal inflammation and hepatocellular ballooning were scored as follows: (0) none; (1) mild inflammation or few balloon cells; and (2) more than mild or prominent ballooning. The degree of fibrosis was divided as “(0) none; (1a) slight perisinusoidal fibrosis, (1b) moderate perisinusoidal fibrosis, (1c) periportal/portal fibrosis; (2) perisinusoidal and periportal/portal fibrosis; (3) bridging fibrosis; and (4) cirrhosis”. Fibrotic stage ≥ 2 was considered significant fibrosis. The NAFLD activity score (NAS) was documented as the summation of the scores for steatosis, lobular inflammation, and ballooning. A NAS of ≥ 5 confirmed the diagnosis of NASH, while a NAS of < 3 was considered non-NASH. If the NAS was between 3 and 4, we diagnosed NASH when pathohistological features, including steatosis, lobular inflammation, and ballooning, existed simultaneously.
All statistical analyses were conducted with SPSS software (version 23) for Windows. Continuous variables are presented as the mean ± SD, and categorical variables are presented as numbers (percentages). The independent t-test or the chi-squared test was used to evaluate differences between groups. One-way analysis of variance (ANOVA) was performed for multiple comparisons. Bivariate correlations between steatosis grade and other histologic parameters were examined using Spearman’s correlation test. A two-sided P value < 0.05 was considered statistically significant.
A total of 83 patients with biopsy-proven NAFLD were included in the study, with 54 males and 29 females. Among them, 61 subjects suffered from MAFLD, while the other 22 had NAFLD without overweight or metabolic dysfunction (defined as the non-MAFLD group). Demographic and laboratory information for the enrolled population is summarized in Table 1.
| MAFLD (n = 61, male 39a) | Non-MAFLD (n = 22, male 15a) | P value | |||
| mean | SD | mean | SD | ||
| Age | 42.23 | 12.62 | 41.23 | 12.08 | 0.743 |
| Height (m) | 1.66 | 0.08 | 1.65 | 0.08 | 0.640 |
| Weight (kg) | 73.65 | 13.10 | 60.31 | 7.48 | < 0.001 |
| BMI | 26.43 | 3.38 | 21.93 | 1.09 | < 0.001 |
| WC (cm) | 89.87 | 8. 53 | 80.91 | 3.93 | < 0.001 |
| SBP (mmHg) | 126.11 | 14.50 | 118.36 | 10.12 | 0.009 |
| DBP (mmHg) | 77.30 | 8.68 | 71.82 | 8.72 | 0.016 |
| HR (per min) | 77.51 | 9.10 | 76.05 | 8.25 | 0.492 |
| WBC (× 109/L) | 6.22 | 1.29 | 5.76 | 1.25 | 0.153 |
| Neu% | 58.75 | 8.33 | 57.42 | 9.77 | 0.573 |
| HGB (g/L) | 146.20 | 16.66 | 149.23 | 15.57 | 0.447 |
| PLT (× 109/L) | 213.95 | 70.66 | 201.18 | 59.33 | 0.416 |
| hs-CRP (mg/L) | 2.39 | 2.78 | 1.92 | 1.08 | 0.284 |
| ALB (g/L) | 45.14 | 4.42 | 43.41 | 4.32 | 0.117 |
| GLB (g/L) | 28.62 | 4.37 | 29.06 | 3.76 | 0.656 |
| UA (μmol/L) | 372.82 | 103.65 | 341.05 | 73.45 | 0.128 |
| TG (mmol/L) | 2.54 | 1.63 | 1.32 | 0.53 | <0.001 |
| TC (mmol/L) | 4.89 | 1.26 | 4.68 | 0.91 | 0.441 |
| LDLC (mmol/L) | 2.84 | 0.93 | 2.67 | 0.57 | 0.334 |
| HDLC (mmol/L) | 1.03 | 0.23 | 1.15 | 0.17 | 0.018 |
| ALT (U/L) | 85.43 | 60.31 | 65.36 | 38.65 | 0.081 |
| AST (U/L) | 53.28 | 31.67 | 46.32 | 26.15 | 0.318 |
| GGT (U/L) | 84.36 | 52.04 | 60.45 | 50.48 | 0.067 |
| ALP (U/L) | 100.34 | 29.11 | 94.95 | 25.03 | 0.412 |
| ChE (U/L) | 9756.44 | 1990.84 | 8791.95 | 1987.90 | 0.059 |
| GLU (mmol/L) | 5.56 | 1.01 | 4.99 | 0.63 | 0.003 |
MAFLD patients had significantly higher body weight (73.65 ± 13.10 vs 60.31 ± 7.48 kg, P < 0.001), BMI (26.43 ± 3.38 vs 21.93 ± 1.09 kg/m2, P < 0.001), and WC (89.87 ± 8.53 vs 80.91 ± 3.93 cm, P < 0.001) than the non-MAFLD group. Moreover, the blood pressures in the MAFLD group were higher than those in the non-MAFLD group (P = 0.009 for SBP and P = 0.016 for DBP). In addition, subjects with MAFLD had lower HDL-C (P = 0.018) and elevated TG (P < 0.001) and glucose (P = 0.003) than the non-MAFLD participants. The liver functions in the two groups were not significantly different.
Grade 1 steatosis was found in 44 (53.0%), and grades 2-3 steatosis was found in 39 individuals (47.0%). Lobular inflammation was found in 79 (95.2%) and absent in 4 (4.8%) patients. Portal inflammation was found in 73 (95.2%) and absent in 10 (4.8%) patients. Sixteen patients (19.3%) had hepatocellular ballooning, while 67 (80.7%) did not. Fibrosis of any degree was present in 59 (71.1%) and absent in 24 patients (28.9%). Among the 83 biopsy-proven NAFLD patients, 19 (22.9%) had NASH, and 15 (18.1%) had significant fibrosis. Figure 1 demonstrates the representative histological images to provide an overview of the pathological changes.
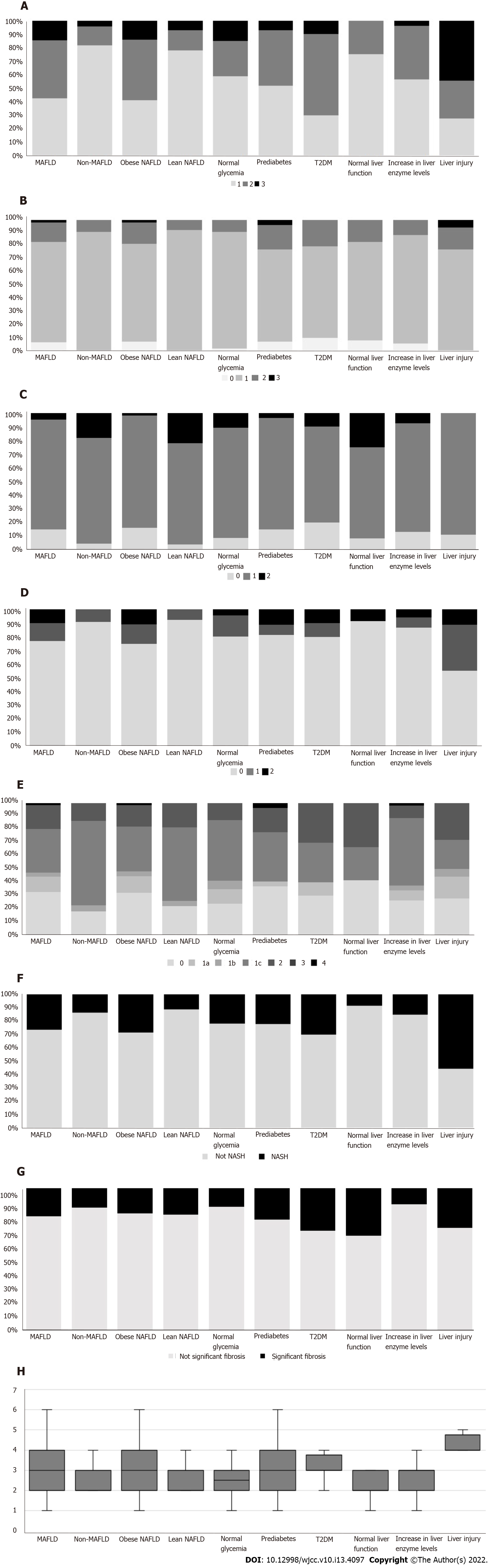
The comparison of histologic characteristics between the MAFLD and non-MAFLD subgroups is shown in Figure 2. There was a significant difference in steatosis degree. Compared with non-MAFLD subjects, MAFLD subjects had a higher severity of hepatic steatosis (42.6% Grade 1, 42.6% Grade 2, and 14.8% Grade 3 in MAFLD; 81.8% Grade 1, 13.6% Grade 2, and 4.5% Grade 3 in non-MAFLD; P = 0.007, Figure 2A). The MAFLD group also had a higher NAS than the non-MAFLD group (3.11 ± 1.29 vs 2.41 ± 0.67, P = 0.002, Figure 2H). However, lobular and portal inflammation, hepatic ballooning, fibrosis grade, and the presence of NASH and significant fibrosis were comparable between the two groups (P = 0.461, 0.091, 0.251, 0.151, 0.228, and 0.749, respectively; Figure 2B-G).
There were 56 obese NAFLD patients and 27 lean NAFLD patients based on a BMI threshold of 23 kg/m2. Among the lean NAFLD patients, five were defined as having MAFLD, one of whom had T2DM, three of whom had hypertriglyceridemia and low HDL-C, and the other had low HDL-C and elevated postprandial glucose levels.
Similarly, obese NAFLD patients had a higher severity of hepatic steatosis (41.1% Grade 1, 44.6% Grade 2, and 14.3% Grade 3 in obese NAFLD; 77.8% Grade 1, 14.8% Grade 2, and 7.4% Grade 3 in lean NAFLD; P = 0.007, Figure 2A) and higher NAS (3.16 ± 1.32 vs 2.44 ± 0.70, P = 0.002, Figure 2H) than lean NAFLD patients. However, obese NAFLD patients had milder portal inflammation than their lean counterparts (16.1% Grade 0, 82.1% Grade 1, and 1.8% Grade 2 in obese NAFLD; 3.7% Grade 0, 74.1% Grade 1, and 22.2% Grade 2 in lean NAFLD; P = 0.003, Figure 2C). As presented in Figure 2, lobular inflammation, ballooning, fibrosis grade, and the presence of NASH and significant fibrosis were comparable between the two groups (P = 0.247, 0.116, 0.250, 0.098, and 1.000, respectively).
Ten patients suffered from T2DM; 27 had prediabetes, while the other 46 had normal blood sugar. There were no significant differences among individuals based on glycemia in terms of any histologic characteristic (steatosis: P = 0.260; lobular inflammation: P = 0.400; portal inflammation: P = 0.676; balloon degeneration: P = 0.717; fibrosis: P = 0.563; NAS: P = 0.141; NASH: P = 0.849; significant fibrosis: P = 0.357) (Figure 2).
Based on the markers of liver injury, 12 patients presented with normal liver function, 53 had elevated liver enzyme levels, and 18 had liver injury (ALT > 3 × ULN). As shown in Figure 2A, the higher the liver enzyme levels, the more severe the grades of hepatic steatosis (75.0% Grade 1 and 25.0% Grade 2 in normal liver function; 56.6% Grade 1, 39.6% Grade 2, and 3.8% Grade 3 in increased liver enzyme levels; 27.8% Grade 1, 27.8% Grade 2, and 44.4% Grade 3 in liver injury; P < 0.001). Moreover, the liver injury group presented with much graver hepatocellular ballooning (91.7% Grade 0 and 8.3% Grade 2 in normal liver function; 86.8% Grade 0, 7.5% Grade 1, and 5.7% Grade 2 in increased liver enzyme levels; 55.6% Grade 0, 33.3% Grade 1, and 11.1% Grade 2 in liver injury; P = 0.021; Figure 2D). There was one case of NASH in the normal liver function group (8.33%), eight patients with NASH in the group with increased liver enzyme levels (15.09%), and ten patients with NASH in the liver injury group (55.56%, P = 0.001; Figure 2F). The NASs in the three groups were 2.50 ± 1.00, 2.70 ± 1.01 (P = 0.545 vs the normal liver function group), and 3.89 ± 1.37 (P = 0.003 vs the normal liver function group, P = 0.002 vs the group with increased liver enzymes), respectively (Figure 2H). No significant differences in fibrosis grades (P = 0.223) or the presence of significant fibrosis (P = 0.097) were seen among the three groups (Figure 2E and G). In addition, there was no significant difference in terms of lobular (P = 0.496) or portal inflammation (P = 0.190) in the comparison of the three groups according to liver function (Figure 2B and C).
Interestingly, steatosis grade correlated significantly with hepatocellular ballooning degree (r = 0.338, P = 0.002) and the presence of NASH (r = 0.466, P < 0.001). In contrast, Spearman’s analysis did not find any correlation between steatosis grade and other hepatic histologic features, including inflammation or fibrosis (Table 2).
| Histologic feature | Correlation (r) | P value |
| Lobular inflammation | 0.122 | 0.272 |
| Portal inflammation | 0.005 | 0.968 |
| Balloon degeneration | 0.338 | 0.002 |
| Liver fibrosis | 0.060 | 0.588 |
| Significant liver fibrosis | 0.151 | 0.172 |
| NASH | 0.466 | < 0.001 |
Generally, NAFLD accompanies obesity and its comorbidities[14]. Nevertheless, it can also occur in individuals within a BMI cut-off of 25 kg/m2 in Caucasians and 23 kg/m2 in Asians, the so-called “lean” NAFLD[15]. There is growing evidence that lean patients with metabolic disorders display superior ectopic fat accumulation, with higher risks of fatty liver and cardiovascular disease[16-18]. A recent study identified that non-obese NAFLD patients with MS presented a comparable degree of liver histologic severity to their obese counterparts without MS[19]. While weight gain and insulin resistance are well-known predictors of long-term outcomes of NAFLD[20,21], other metabolic disorders also play a crucial role in NAFLD pathogenesis. Herein, a nomenclature of MAFLD was proposed based on the presence of fatty liver and metabolic dysfunction. According to the definition of MAFLD, a group of lean and metabolically healthy individuals are not included. In this study, we focused on the comparison of histologic characteristics between the non-MAFLD and MAFLD subgroups of NAFLD, with the purpose of demonstrating the histologic performance of lean NAFLD without metabolic disorders.
The results of this study indicated that MAFLD individuals had a higher NAS than non-MAFLD individuals. Specifically, the difference in NAS originated from the severity of steatosis other than inflammation or balloon degeneration. While the grades of inflammation and balloon degeneration were similar between the two groups, there was also no difference in the presence of NASH or significant fibrosis. Given the substantial heterogeneity of MAFLD, sub-classifications might present with different histologic features and lead to different clinical outcomes[22]. Therefore, subgroup analyses were conducted according to the presence of elements of metabolic syndrome. Similar results were observed in obese and lean NAFLD individuals, except for the difference in degrees of portal inflammation. However, diabetes was not associated with any hepatic histologic features.
It has been proven that NASH rather than simple fatty liver is related to a worse prognosis. Hepatic steatosis with minimal or no inflammation appears to follow a comparatively benign clinical course[23-25]. Nevertheless, a longitudinal study showed that only fibrosis stage instead of other histologic features of NASH was related to end-stage liver diseases and all-cause mortality, and long-term prognosis did not depend upon a diagnosis of NASH but hepatic fibrosis[12]. Moreover, fibrosis develops not only in those with steatohepatitis but also in those with steatosis alone[26]. As shown in this study, steatosis grade had a positive correlation with ballooning degree and the presence of NASH. While steatosis and other histologic alterations represent a continuous process, the current histologic assessment cannot reflect the dynamic changes of liver histology or represent future disease progression. That is, the more severe steatosis grade in the MAFLD group might possibly result in advanced fibrosis in the future, consequently leading to a poorer outcome. However, this hypothesis needs to be verified in a longitudinal, large-cohort study.
Recently, NAFLD has been reconsidered as the correct name for fatty liver owing to metabolic factors, and experts have appealed to revise its nomenclature based on the following reasons. First, NAFLD is a condition of “exclusion”, while metabolic liver disease coexists with other causes of liver injury, such as chronic viral hepatitis, alcohol consumption, and autoimmune liver diseases. Currently, fatty liver disease has a dichotomous division into simple fatty liver and NASH, which remains a great matter of debate due to its limit of capturing the complete spectrum of disease course[2]. As mentioned above, fibrosis has been considered the major determinant of adverse outcomes[12,25]. Consequently, perhaps NAFLD should be regarded similarly to other chronic liver diseases, paying particular attention to the degrees of activity and fibrosis. With a wide spectrum of disease severities of NAFLD, ranging from simple fatty liver, NASH, cirrhosis, and even hepatocarcinoma, more precise subphenotypes of the disease and appropriate patient stratification should be proposed according to the heterogeneous pathogenesis. Given the currently recognized pathogenesis of fatty liver, MAFLD, which focuses on the presence of obesity and metabolic disorders along with hepatic steatosis, is recommended to be a more suitable definition. In the meantime, although the “overwhelming majority” of the previously called NAFLD patients will meet the criteria for MAFLD, we should also make great efforts to map the landscapes of those individuals with NAFLD not presenting obesity, T2DM, or evidence of metabolic dysfunction. A study revealed that NAFLD patients who cannot be diagnosed with MAFLD may have severe hepatic steatosis, significant liver injury, and fibrosis[27]. Another study indicated that the MAFLD criteria seem to be less accurate in identifying a higher cardiometabolic risk in obese children[28]. Therefore, emphasis on MAFLD alone might lead to underestimation of the progression of fatty liver disease and cardiometabolic risk.
According to the results of this study, metabolic dysfunction is associated with only steatosis but no other histologic features in NAFLD. Thus, whether the renaming of NAFLD to MAFLD is rational still requires further studies on the dynamic histologic changes and long-term clinical outcomes between the MAFLD and non-MAFLD subgroups.
However, liver enzymes, particularly ALT, as markers of liver injury have repeatedly failed to identify a large number of patients with hepatic injury. Previous studies have indicated that advanced inflammation or fibrosis is present in many hepatitis B patients with persistently normal ALT levels[29,30]. Although this study found that higher liver enzymes were associated with more severe histologic performance (higher grades of steatosis, balloon degeneration, higher NAS, and larger proportions of NASH), there were still a number of cases with NASH and significant fibrosis with normal or mildly elevated liver function. Therefore, NAFLD with normal liver function can still have significant disease activity and might progress to hepatic decompensation.
This study had several limitations. The major reason was the small size of the study population, especially for the non-MAFLD group, because fatty liver without metabolic dysregulations is relatively rare. Second, due to the cross-sectional nature of this research, we were unable to follow up on the course of the disease. The current histologic features cannot reflect the possible different risks for future disease progression between MAFLD and NAFLD without metabolic dysfunctions. Third, we did not analyze any biomolecules involved in the pathogenesis and progression of MAFLD and NAFLD. Finally, the definition of MAFLD actually includes those patients that also have concomitant conditions, such as alcohol consumption and chronic viral hepatitis. Therefore, further investigation and characterization of this group of MAFLD patients are urgently needed.
In conclusion, MAFLD presents with more severe hepatic steatosis and a higher NAS than the non-MAFLD subgroup of NAFLD. However, there are no differences in other hepatic histologic characteristics, including inflammation and fibrosis, between the two groups. Further longitudinal large-cohort studies are needed to discover the dynamic histologic features and prognosis in NAFLD based on the presence or absence of metabolic disorders.
Non-obese and metabolically healthy patients with nonalcoholic fatty liver disease (NAFLD) are excluded from the definition of metabolic-associated fatty liver disease (MAFLD), but their clinical course has been seldom demonstrated.
To study the hepatic histologic characteristics in various subsets of NAFLD based on different metabolic disorders and liver enzyme levels.
To compare the histologic features in various subsets of NAFLD.
A total of 83 patients with biopsy-proven NAFLD were divided into MAFLD and non-MAFLD groups. MAFLD was defined as hepatic steatosis along with obesity/diabetes or evidence of metabolic dysfunction. The histologic features were compared in different subgroups.
The MAFLD subgroup had a higher NAFLD activity score and higher severity of hepatic steatosis than the non-MAFLD subgroup of NAFLD. There were no differences for other histologic features including lobular and portal inflammation, balloon degeneration, and fibrosis between MAFLD and non-MAFLD. The higher the liver enzyme levels, the more severe the grades of hepatic steatosis. Patients with liver injury had a higher severity of hepatocellular ballooning. The grade of steatosis correlated positively with hepatocellular ballooning degree, and presence of nonalcoholic steatohepatitis.
Metabolic dysfunction is related to hepatic steatosis in NAFLD, but other histologic features including inflammation and fibrosis are similar in the MAFLD and non-MAFLD subgroups.
Dynamic histologic characteristics should be assessed in more NAFLD patients based on the presence or absence of metabolic disorders.
We thank Dr. Yu LL for the assistance in histopathological assessment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Di Sessa A, Italy; Protopapas AA, Greece; Ulaşoglu C, Turkey; Yang M, United States S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Yan JP
| 1. | Sarin SK, Kumar M, Eslam M, George J, Al Mahtab M, Akbar SMF, Jia J, Tian Q, Aggarwal R, Muljono DH, Omata M, Ooka Y, Han KH, Lee HW, Jafri W, Butt AS, Chong CH, Lim SG, Pwu RF, Chen DS. Liver diseases in the Asia-Pacific region: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol. 2020;5:167-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 390] [Cited by in RCA: 371] [Article Influence: 74.2] [Reference Citation Analysis (0)] |
| 2. | Brunt EM, Kleiner DE, Wilson LA, Belt P, Neuschwander-Tetri BA; NASH Clinical Research Network (CRN). Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53:810-820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 997] [Cited by in RCA: 952] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 3. | Wong VW, Chan WK, Chitturi S, Chawla Y, Dan YY, Duseja A, Fan J, Goh KL, Hamaguchi M, Hashimoto E, Kim SU, Lesmana LA, Lin YC, Liu CJ, Ni YH, Sollano J, Wong SK, Wong GL, Chan HL, Farrell G. Asia-Pacific Working Party on Non-alcoholic Fatty Liver Disease guidelines 2017-Part 1: Definition, risk factors and assessment. J Gastroenterol Hepatol. 2018;33:70-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 358] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 4. | Leoni S, Tovoli F, Napoli L, Serio I, Ferri S, Bolondi L. Current guidelines for the management of non-alcoholic fatty liver disease: A systematic review with comparative analysis. World J Gastroenterol. 24:3361-3373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 412] [Cited by in RCA: 393] [Article Influence: 56.1] [Reference Citation Analysis (7)] |
| 5. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia. 2016;59:1121-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 505] [Article Influence: 56.1] [Reference Citation Analysis (2)] |
| 6. | Eslam M, Sanyal AJ, George J; International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999-2014.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2367] [Cited by in RCA: 2207] [Article Influence: 441.4] [Reference Citation Analysis (1)] |
| 7. | Fouad Y, Waked I, Bollipo S, Gomaa A, Ajlouni Y, Attia D. What's in a name? Liver Int. 2020;40:1254-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 211] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 8. | Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2883] [Cited by in RCA: 2828] [Article Influence: 565.6] [Reference Citation Analysis (1)] |
| 9. | Jia W, Weng J, Zhu D, Ji L, Lu J, Zhou Z, Zou D, Guo L, Ji Q, Chen L, Dou J, Guo X, Kuang H, Li L, Li Q, Li X, Liu J, Ran X, Shi L, Song G, Xiao X, Yang L, Zhao Z; Chinese Diabetes Society. Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab Res Rev. 2019;35:e3158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 485] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 10. | Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, Zhang XJ, Cai J, Lin L, Ouyang S, Wang X, Yang C, Cheng X, Liu W, Li H, Xie J, Wu B, Luo H, Xiao F, Chen J, Tao L, Cheng G, She ZG, Zhou J, Wang H, Lin J, Luo P, Fu S, Ye P, Xiao B, Mao W, Liu L, Yan Y, Chen G, Huang X, Zhang BH, Yuan Y. Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China. Hepatology. 2020;72:389-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 309] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 11. | Woo J, Ho SC, Yu AL, Sham A. Is waist circumference a useful measure in predicting health outcomes in the elderly? Int J Obes Relat Metab Disord. 2002;26:1349-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A, Haflidadottir S, Bendtsen F. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149:389-97.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2304] [Cited by in RCA: 2230] [Article Influence: 223.0] [Reference Citation Analysis (0)] |
| 13. | Tiniakos DG. Nonalcoholic fatty liver disease/nonalcoholic steatohepatitis: histological diagnostic criteria and scoring systems. Eur J Gastroenterol Hepatol. 2010;22:643-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | ZELMAN S. The liver in obesity. AMA Arch Intern Med. 1952;90:141-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Younes R, Bugianesi E. NASH in Lean Individuals. Semin Liver Dis. 2019;39:86-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 165] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 16. | Stefan N, Schick F, Häring HU. Causes, Characteristics, and Consequences of Metabolically Unhealthy Normal Weight in Humans. Cell Metab. 2017;26:292-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 389] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 17. | Eshraghian A, Nikeghbalian S, Geramizadeh B, Kazemi K, Shamsaeefar A, Malek-Hosseini SA. Characterization of biopsy proven non-alcoholic fatty liver disease in healthy non-obese and lean population of living liver donors: The impact of uric acid. Clin Res Hepatol Gastroenterol. 2020;44:572-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Kawaguchi T, Torimura T. Is metabolic syndrome responsible for the progression from NAFLD to NASH in non-obese patients? J Gastroenterol. 2020;55:363-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Kim D, Kim W, Joo SK, Han J, Kim JH, Harrison SA, Younossi ZM, Ahmed A. Association between body size-metabolic phenotype and nonalcoholic steatohepatitis and significant fibrosis. J Gastroenterol. 2020;55:330-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J Hepatol. 2017;67:862-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 848] [Cited by in RCA: 801] [Article Influence: 100.1] [Reference Citation Analysis (2)] |
| 21. | Li H, Guo M, An Z, Meng J, Jiang J, Song J, Wu W. Prevalence and Risk Factors of Metabolic Associated Fatty Liver Disease in Xinxiang, China. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 22. | Idalsoaga F, Kulkarni AV, Mousa OY, Arrese M, Arab JP. Non-alcoholic Fatty Liver Disease and Alcohol-Related Liver Disease: Two Intertwined Entities. Front Med (Lausanne). 2020;7:448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 23. | Dam-Larsen S, Becker U, Franzmann MB, Larsen K, Christoffersen P, Bendtsen F. Final results of a long-term, clinical follow-up in fatty liver patients. Scand J Gastroenterol. 2009;44:1236-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 24. | Kleiner DE, Brunt EM, Wilson LA, Behling C, Guy C, Contos M, Cummings O, Yeh M, Gill R, Chalasani N, Neuschwander-Tetri BA, Diehl AM, Dasarathy S, Terrault N, Kowdley K, Loomba R, Belt P, Tonascia J, Lavine JE, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network. Association of Histologic Disease Activity With Progression of Nonalcoholic Fatty Liver Disease. JAMA Netw Open. 2019;2:e1912565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 278] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 25. | Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, Castellanos M, Aller-de la Fuente R, Metwally M, Eslam M, Gonzalez-Fabian L, Alvarez-Quiñones Sanz M, Conde-Martin AF, De Boer B, McLeod D, Hung Chan AW, Chalasani N, George J, Adams LA, Romero-Gomez M. Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients With Advanced Nonalcoholic Fatty Liver Disease: A Multi-National Cohort Study. Gastroenterology. 2018;155:443-457.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 601] [Article Influence: 85.9] [Reference Citation Analysis (0)] |
| 26. | Siddiqui MS, Harrison SA, Abdelmalek MF, Anstee QM, Bedossa P, Castera L, Dimick-Santos L, Friedman SL, Greene K, Kleiner DE, Megnien S, Neuschwander-Tetri BA, Ratziu V, Schabel E, Miller V, Sanyal AJ; Liver Forum Case Definitions Working Group. Case definitions for inclusion and analysis of endpoints in clinical trials for nonalcoholic steatohepatitis through the lens of regulatory science. Hepatology. 2018;67:2001-2012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 133] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 27. | Huang J, Kumar R, Wang M, Zhu Y, Lin S. MAFLD criteria overlooks a number of patients with severe steatosis: Is it clinically relevant? J Hepatol. 2020;73:1265-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 28. | Di Sessa A, Guarino S, Umano GR, Arenella M, Alfiero S, Quaranta G, Miraglia Del Giudice E, Marzuillo P. MAFLD in Obese Children: A Challenging Definition. Children (Basel). 2021;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Huang H, Sun Z, Pan H, Chen M, Tong Y, Zhang J, Chen D, Su X, Li L. Serum metabolomic signatures discriminate early liver inflammation and fibrosis stages in patients with chronic hepatitis B. Sci Rep. 2016;6:30853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Wang H, Xue L, Yan R, Zhou Y, Wang MS, Cheng MJ, Hai-Jun Huang. Comparison of histologic characteristics of Chinese chronic hepatitis B patients with persistently normal or mildly elevated ALT. PLoS One. 2013;8:e80585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |