Published online May 6, 2022. doi: 10.12998/wjcc.v10.i13.4084
Peer-review started: April 24, 2021
First decision: June 13, 2021
Revised: June 25, 2021
Accepted: April 2, 2022
Article in press: April 2, 2022
Published online: May 6, 2022
Processing time: 370 Days and 19.2 Hours
Colorectal cancer (CRC) is often associated with elevated platelet count (> 400 × 109/L), known as thrombocytosis. The role of CD40 ligand (CD40L), a member of the tumor necrosis factor family, is controversial in CRC. Circulating CD40L is higher in CRC, but its relationship with disease staging and local and distant metastasis is not clear. Although most of the circulating CD40L is produced by platelets, no previous study investigated its relationship with CRC-related thrombocytosis.
To investigate the role of CD40L to predict the outcome of CRC and its relation to thrombocytosis.
A total of 106 CRC patients and 50 age and sex-matched control subjects were enrolled for the study. Anamnestic data including comorbidities and histopathological data were collected. Laboratory measurements were performed at the time of CRC diagnosis and 1.5 mo and at least 6 mo after the surgical removal of the tumor. Plasma CD40L and thrombopoietin were measured via enzyme-linked immunosorbent assay, while plasma interleukin-6 was measured via electrochemiluminescence immunoassay. Patient follow-ups were terminated on January 31, 2021.
Plasma CD40L of CRC patients was tendentiously higher, while platelet count (P = 0.0479), interleukin-6 (P = 0.0002), and thrombopoietin (P = 0.0024) levels were significantly higher as opposed to the control subjects. Twelve of the 106 CRC patients (11.3%) had thrombocytosis. Significantly higher CD40L was found in the presence of distant metastases (P = 0.0055) and/or thrombocytosis (P = 0.0294). A connection was found between CD40L and platelet count (P = 0.0045), interleukin-6 (P = 0.0130), and thrombocytosis (P = 0.0155). CD40L was constant with the course of CRC, and all baseline differences persisted throughout the whole study. Both pre- and postoperative elevated platelet count, CD40L, and interleukin-6 level were associated with poor overall and disease-specific survival of patients. The negative effect of CD40L and interleukin-6 on patient survival remained even after the stratification by thrombocytosis.
CD40L levels of CRC patients do not change with the course of the disease. The CD40L level is strongly correlated with platelet count, interleukin-6, thrombocytosis, and the presence of distant metastases.
Core Tip: This observational study investigated whether plasma CD40 ligand (CD40L) is related to colorectal cancer (CRC)-associated thrombocytosis and disease severity. Baseline CD40L was significantly higher in patients with distant metastasis and thrombocytosis. An association between CD40L, platelet count, and the interleukin-6 level was found. CD40L was constant with the course of the disease, and all its baseline differences persisted throughout the study. Both pre- and postoperative high CD40L levels negatively affected overall, disease-specific, and thrombocytosis-eliminated survival. A possible connection between elevated CD40L levels and increased general inflammation caused by CRC was also suggested.
- Citation: Herold Z, Herold M, Herczeg G, Fodor A, Szasz AM, Dank M, Somogyi A. High plasma CD40 ligand level is associated with more advanced stages and worse prognosis in colorectal cancer. World J Clin Cases 2022; 10(13): 4084-4096
- URL: https://www.wjgnet.com/2307-8960/full/v10/i13/4084.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i13.4084
According to the GLOBOCAN 2018 data, colorectal cancer (CRC) is the third most common cancer type with over 1.8 million new cases and causing more than 860000 deaths annually[1]. Elevated platelet count (thrombocytosis) has been described previously as a poor prognostic sign in CRC[2]. Thrombocytosis may arise in CRC due to the following: (1) Secondary thrombocytosis caused by the bleeding of the tumor; and (2) paraneoplastic thrombocytosis defined as a metabolic change caused by the tumor itself[2,3]. In the latter, the elevated platelet count can be attributed to the overproduction of cytokines by the tumor, which induces hepatic thrombopoietin production and ultimately increases bone marrow activity[3,4].
CD40 ligand (CD40L, synonym: CD154, gp39, or T-B activating molecule) is a type I transmembrane glycoprotein belonging to the tumor necrosis factor family of cytokines[5,6]. CD40L activates CD40, a transmembrane protein receptor found on antigen-presenting cells[6]. The majority of circulating CD40L is assumed to originate from platelets. A membrane-bound CD40L form is also known[6]. CD40L has been described to have antitumor effects via the inhibition of tumor cell proliferation and pro-apoptotic features through the activation of apoptotic pathways[6-9]. It has been found previously that CRC patients have significantly higher soluble CD40L levels than those of healthy control subjects. Its possible use as a promising biomarker in CRC was proposed[10-12]. The connection between CD40L levels and lymph node involvement or distant metastasis is controversial. One study reported increased CD40L levels in patients with worse disease conditions, while others have found the opposite. Furthermore, neoadjuvant chemoradiotherapy has decreased CD40L levels[10-12].
The role of CD40L in the course of CRC and its relationship with CRC-related thrombocytosis has not been investigated previously. Therefore, a prospective observational study was carried out. Paraneoplastic thrombocytosis was investigated through the measurement of plasma interleukin-6 and thrombopoietin levels. In addition to the latter, the further aim of this study was to try to clarify the discrepancy between plasma CD40L levels and higher tumor stages. The effect of plasma CD40L on survival was also analyzed.
The study was conducted in concordance with the World Medical Association’s Declaration of Helsinki. The study was approved by the Committee of Science and Research Ethics, Hungarian Medical Research Council (ETT TUKEB 8951-3/2015/EKU) and by the institutional ethical committees of Semmelweis University (SE TUKEB 21-12/1994, approval date of latest modification: February 10, 2017) and Szent Imre University Teaching Hospital (SZIK IKEB 5/2017). Handling of patient data was in accordance with the General Data Protection Regulation issued by the European Union.
A prospective, real-life observational cohort study was carried out, and a total of 106 CRC patients were enrolled for the study between 2017 and 2019. Fifty age and sex-matched control subjects were selected from a pool of 166 volunteers. Prior to any study-specific procedures, study subjects signed written informed consents. CRC patients attended the Department of Internal Medicine and Hematology, Semmelweis University, Budapest and the Department of General Surgery, Szent Imre University Teaching Hospital, Budapest. Control subjects attended the Metabolic Outpatient Clinic of the Department of Internal Medicine and Hematology, Semmelweis University, Budapest. Study exclusion criteria included age < 18 years, an Eastern Cooperative Oncology Group performance status > 2, previous malignancy, and systemic autoimmune, inflammatory bowel, inadequately controlled thyroid, hematologic, chronic kidney, or any mental diseases. The usage of erythropoiesis-stimulating agents or recent blood transfusion was also prohibited. In addition to the above, control subjects with any metabolic disease, except type 2 diabetes mellitus, which was present in several of the CRC patients, were excluded from the study.
Body weight, height, and anamnestic data including comorbidities and recent medications were collected. Fasting blood samples were drawn: (1) At the time of CRC diagnosis; (2) at least 6 wk after tumor removal surgery; and (3) at least 6 mo after tumor removal surgery. Several chemotherapeutic agents are known to affect platelet count[13,14]. Therefore, the third measurements were timed so that patients had not received any oncological treatment for at least 6 wk prior to blood sampling. Follow-ups of patients were terminated on January 31, 2021. Routine laboratory measurements were performed at the central laboratories of Semmelweis University and Szent Imre University Teaching Hospital. Complete blood count, liver enzyme, plasma glucose, and creatinine levels were determined. The Chronic Kidney Disease-Epidemiology Collaboration equations were used to calculate the estimated glomerular filtration rate[15].
In addition to routine laboratory measurements, plasma CD40L, interleukin-6, and thrombopoietin levels were measured using the CD40L Human Enzyme-linked Immunosorbent Assay kit (ELISA kit, abcam®, Catalog Number ab99991, Cambridge, MA, United States), ELECSYS® Interleukin-6 electrochemiluminescence immunoassay (ECLIA, Roche Diagnostics GmbH, Mannheim, Germany), and the Human Thrombopoietin Quantikine® ELISA kits (catalog number: DTP00B, R&D Systems, Minneapolis, MN, United States), respectively. As per the manufacturer’s description, thrombopoietin level was obtained from platelet-poor plasma.
The tumor-staging was given by histopathological examination of surgical specimens and imaging studies; the American Joint Committee on Cancer grouping was used[16]. The side of CRC was described[17] as right-sided if the tumor was originating from the cecum, ascending colon, and proximal two-thirds of the transverse colon, while left-sided was described if the tumor originated from the distal one-third of the transverse colon, descending colon, sigmoid colon, and rectum. Chemo
Statistical analysis was performed with R version 4.0.4[18]. Matching of control subjects was done via propensity score matching (R-package Matching[19]). Wilcoxon-Mann-Whitney U-test, Fisher’s exact test, Kruskal-Wallis test with P value corrected pairwise Wilcoxon-Mann-Whitney U-tests as post-hoc, and Spearman’s rank correlation were used. To detect the changes of CD40L in time, linear mixed-effects models were used (R-package nlme)[20]. Overall and CRC-related disease-specific survival of patients were analyzed with Cox regression and cause-specific competing risk survival models (R-package survival)[21], respectively. P < 0.05 was considered as statistically significant, and P values were corrected with the Holm method[22] for multiple comparisons problem. Results were expressed as mean ± SD and as the number of observations (percentage) for continuous and count data, respectively.
A total of 106 CRC patients and 50 age and sex-matched voluntary control subjects were enrolled for the study. Baseline laboratory measurements and anamnestic data were summarized in Table 1, and histopathological data of CRC patients were summarized in Table 2. The two cohorts were well balanced as no significant difference was detected in either of the anamnestic data. On the contrary, most of the parameters of complete blood count within the CRC group were out of normal range (P < 0.05), and 12 of the 106 CRC patients (11.3%) showed signs of thrombocytosis (platelet count > 400 × 109/L). The plasma interleukin-6 (P = 0.0002) and thrombopoietin (P = 0.0024) levels of CRC patients were significantly higher than those of the control subjects. CD40L did not differ between the two cohorts, but it should be highlighted that CD40L of control subjects was tendentiously lower (crude P value: 0.2946; Figure 1).
| Parameter | CRC patients (n = 106) | Controls (n = 50) | P value |
| Age (yr) | 68.55 ± 8.64 | 63.91 ± 10.12 | P = 0.2068 |
| Sex [Male:Female, n (%)] | 71 (67.0):35 (33.0) | 35 (70.0):15 (30.0) | P = 1.0000 |
| Body mass index (kg/m2) | 27.37 ± 4.03 | 29.26 ± 5.07 | P = 0.5995 |
| White blood cell count (109/L) | 8.76 ± 4.56 | 7.41 ± 1.99 | P = 0.7308 |
| Neutrophil count (109/L) | 6.05 ± 3.60 | 4.45 ± 1.64 | P = 0.0205 |
| Eosinophil count (109/L) | 0.28 ± 0.86 | 0.20 ± 0.15 | P = 1.0000 |
| Basophil count (109/L) | 0.06 ± 0.05 | 0.06 ± 0.03 | P = 1.0000 |
| Monocyte count (109/L) | 0.65 ± 0.48 | 0.48 ± 0.12 | P = 0.2532 |
| Lymphocyte count (109/L) | 1.76 ± 1.07 | 2.22 ± 0.72 | P = 0.0002 |
| Red blood cell count (1012/L) | 4.48 ± 0.57 | 4.93 ± 0.51 | P = 0.0002 |
| Hemoglobin (g/L) | 123.67 ± 21.37 | 147.04 ± 12.70 | P < 0.0001 |
| Hematocrit (L/L) | 0.38 ± 0.06 | 0.44 ± 0.04 | P < 0.0001 |
| Mean corpuscular volume (fL) | 84.69 ± 8.29 | 89.22 ± 4.06 | P = 0.0856 |
| Mean corpuscular hemoglobin (pg) | 27.30 ± 3.50 | 29.93 ± 1.77 | P = 0.0001 |
| Mean corpuscular hemoglobin concentration (g/L) | 322.94 ± 17.84 | 335.38 ± 9.44 | P < 0.0001 |
| Red blood cell distribution width (%) | 14.85 ± 3.70 | 13.13 ± 0.82 | P = 0.1911 |
| Platelet count (109/L) | 315.58 ± 124.55 | 259.96 ± 73.98 | P = 0.0479 |
| Aspartate transaminase (U/L) | 25.95 ± 20.22 | 26.52 ± 6.94 | P = 0.0155 |
| Alanine transaminase (U/L) | 22.14 ± 12.74 | 28.62 ± 12.32 | P = 0.0047 |
| Gamma-glutamyl transferase (U/L) | 75.07 ± 130.37 | 37.84 ± 31.74 | P = 1.0000 |
| Plasma glucose (mmol/L) | 5.71 ± 1.23 | 5.84 ± 1.94 | P = 1.0000 |
| Creatinine (µmol/L) | 78.26 ± 20.06 | 74.82 ± 15.28 | P = 1.0000 |
| Estimated glomerular filtration rate [mL/(min × 1.73 m2)] | 81.39 ± 17.00 | 87.21 ± 12.42 | P = 0.7308 |
| Interleukin-6 (pg/mL) | 13.79 ± 28.41 | 3.23 ± 1.69 | P = 0.0005 |
| CD40 ligand (pg/mL) | 273.92 ± 309.03 | 191.84 ± 191.82 | P = 1.0000 |
| Thrombopoietin (pg/mL) | 43.59 ± 30.76 | 26.41 ± 24.15 | P = 0.0029 |
| Known comorbidities, n (%) | |||
| Type 2 diabetes mellitus | 25 (23.6) | 16 (32.0) | P = 1.0000 |
| Hypertension | 68 (64.2) | 26 (52.0) | P = 0.8157 |
| Major cardiovascular event(s) prior CRC | 21 (19.8) | 6 (12.0) | P = 1.0000 |
| Platelet aggregation inhibition, n (%) | 23 (21.7) | 18 (36.0) | P = 0.5517 |
| Parameter | Number of observations |
| AJCC staging[16], n (%) | |
| Stage I | 27 (25.5) |
| Stage II | 26 (24.5) |
| Stage III | 24 (22.6) |
| Stage IV | 29 (27.4) |
| Regional lymph node metastasis, n (%) | |
| N0 | 57 (53.8) |
| N1+ | 49 (46.2) |
| Development of distant metastasis after the tumor removal surgery, n (%) | 14 (13.2) |
| Side of CRC, n (%) | |
| Left-sided | 75 (70.8) |
| Right-sided | 31 (29.2) |
| Chemotherapy, n (%) | |
| None | 51 (48.1) |
| Adjuvant | 21 (19.8) |
| First-line | 11 (10.4) |
| Second-line | 13 (12.3) |
| Third or later-line | 10 (9.4) |
| Usage of biological therapy, n (%) | 22 (20.8) |
To test whether other factors, such as age, sex, body mass index, histopathological data, or the presence of comorbidities, affect plasma CD40L levels, further subgrouping within the individual cohorts and correlation analysis was performed. CD40L level of control subjects was affected by the presence of diabetes (without diabetes: 240.41 ± 207.37 pg/mL; with diabetes: 110.09 ± 112.06 pg/mL; P = 0.0313), while no further parameters had any effect on the CD40L level of control subjects. In contrast, the presence of diabetes had no effect on CD40L levels of CRC patients (P = 0.7377). The presence of regional lymph node metastasis alone was not associated with a higher CD40L level (P = 0.7165), but the CD40L level was significantly higher in the presence of distant metastasis (M0: 228.27 ± 293.30 pg/mL; M1: 395.11 ± 322.00 pg/mL; P = 0.0055; Figure 2A) and with thrombocytosis (without thrombocytosis: 248.15 ± 299.20 pg/mL; with thrombocytosis: 475.77 ± 323.43 pg/mL; P = 0.0294).
Furthermore, a negative correlation was found between CD40L and mean corpuscular volume (Spearman’s ρ = -0.36, P = 0.0048), marginal association with hematocrit (Spearman’s ρ = -0.28, P = 0.0898), mean corpuscular hemoglobin (Spearman’s ρ = -0.29, P = 0.0801), and red blood cell distribution width (Spearman’s ρ = +0.29, P = 0.0805). Higher platelet count was associated with more advanced stages of CRC (P = 0.0079; Figure 2B), similar to those of CD40L levels. Right-sided tumors (left sided: 300.97 ± 114.81 × 109/L; right sided 350.90 ± 141.27 × 109/L; P = 0.0121) and the presence of distant metastasis (M0: 289.16 ± 107.69 × 109/L; M1: 385.72 ± 140.28 × 109/L; P = 0.0006) were also associated with increased platelet count. Moreover, higher interleukin-6 levels were observed in patients with a higher stage range (P = 0.0025; Figure 2C), with the presence of positive regional lymph nodes (N0: 7.03 ± 7.53 pg/mL; N1+: 15.82 ± 24.67 pg/mL; P = 0.0400) and with distant metastasis (M0: 7.03 ± 8.66 pg/mL; M1: 21.88 ± 29.33 pg/mL; P = 0.0005). Plasma thrombopoietin levels were basically equal in all stages except in Stage II, where decreased thrombopoietin levels were observed compared to the other stages (P = 0.0210; Figure 2D).
To further assess the effect of thrombocytosis on CD40L, general linear models were created. Thrombopoietin did not have any effect on CD40L. Higher platelet count or the presence of thrombocytosis and higher plasma interleukin-6 levels were strongly correlated with higher CD40L levels (Table 3). It should be emphasized that both the individual and combined effect of these parameters only slightly explained the increase in CD40L. The explanatory power of the models, based on adjusted R2, was at a maximum of 8.1%.
| Parameter | Individual effect P value | Multiple effect P value | Multiple effect P value |
| Interleukin-6 (pg/mL) | 0.0130 | 0.1720 | 0.0454 |
| Thrombopoietin (pg/mL) | 0.1620 | 0.2393 | 0.1785 |
| Platelet count (109/L) | 0.0045 | 0.0043 | - |
| Presence of thrombocytosis | 0.0155 | - | 0.0138 |
CRC patients were recalled for follow-ups, and 61 of the original 106 patients (call-back rate 57.4%) had at least one repeated measurement of CD40L and other laboratory parameters. Distant metastasis developed in an additional 14 CRC patients following the surgical removal of the primary tumor. Thirty CRC patients had all three sets of measurements (Figure 3). The mean durations between baseline and 6 wk after surgery and between baseline and > 6 mo after surgery were 2.07 ± 1.76 mo and 10.38 ± 3.73 mo, respectively. To determine whether CD40L or the parameters related to thrombocytosis change with respect to the course of CRC, linear mixed-effects models were constructed. A total of 197 measurements were used, where not only the paired but all the baseline and further repeated measurements from all the 106 study participants were used. Based on these estimations, a significant 1.5%-2.7% average decrease in platelet count can be expected per month (P < 0.0001; Figure 3B), while no significant changes were observed in the plasma CD40L (P = 0.6813; Figure 3A), interleukin-6 (P = 0.4497), and thrombopoietin (P = 0.2867) levels of CRC patients.
The effect of regional metastatic lymph node or distant metastasis and thrombocytosis on plasma CD40L levels within the course of the disease was also assessed. After the surgical resection of the primary tumor, the CD40L level of CRC patients with distant metastasis (P = 0.6964) or thrombocytosis (P = 0.7829) did not change over time. The same increased level could be observed throughout the observation time (M1: P = 0.0326; thrombocytosis: P = 0.0008), as described at the baseline. The strong association between CD40L level and platelet count (P = 0.0002) and interleukin-6 level (P = 0.0012), observed at baseline measurements, also persisted throughout the whole time of the study.
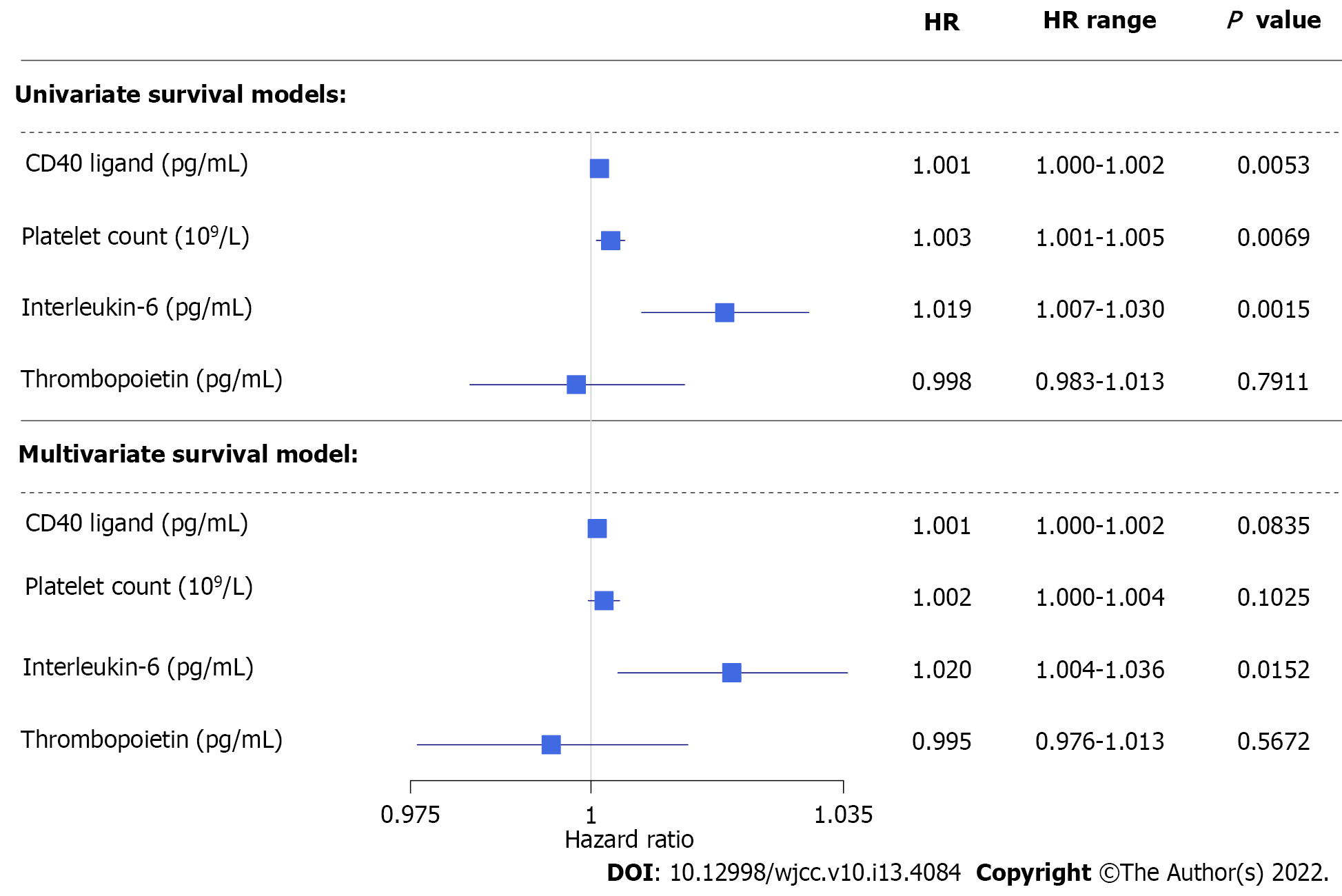
Overall and CRC-related disease-specific survival of patients was calculated. Patient follow-ups were terminated on January 31, 2021. Thirty of the 106 patients (28.3%) died during the study. The three different causes of death were postoperative complications, infection, and CRC-related in 4 cases, 1 case, and 25 cases, respectively. Both pre- and postoperative data had been analyzed; 106 and 61 cases were used for the calculations, respectively. Patients with higher preoperative plasma CD40L level [hazard ratio (HR): 1.001, 95% confidence interval (CI): 1.000-1.002, P = 0.0159], plasma interleukin-6 level (HR: 1.020, 95%CI: 1.010-1.030, P = 0.0001), and platelet count (HR: 1.003, 95%CI: 1.001-1.005, P = 0.0052) had significantly shorter overall survival, while preoperative plasma thrombopoietin level (P = 0.5550) did not affect the overall survival of patients in univariate models. In a multivariate setting, interleukin-6 had the most prominent effect on overall survival (HR: 1.024, 95%CI: 1.010-1.039, P = 0.0012), while CD40L (95%CI: 0.9995-1.0020) and platelet count (95%CI: 0.9996-1.0040) had a marginal effect. Thrombopoietin did not affect the overall survival of patients. The same was observed for preoperative (Figure 4) and postoperative (Figure 5) disease-specific survival.
Using stratified survival models we could assume different preoperative baseline hazards for patients with or without thrombocytosis (platelet count > 400 × 109/L). In a univariate model, higher preoperative plasma CD40L level indicated poor disease-specific prognosis of CRC patients (HR: 1.001, 95%CI: 1.000-1.002, P = 0.0332). However, only a marginal effect was found in multivariate models (HR: 1.001, 95%CI: 0.9998-1.002, P = 0.1196). Neither platelet count (univariate: P = 0.3310; multivariate: P = 0.6237), nor thrombopoietin (univariate: P = 0.9440; multivariate: P = 0.5387) level affected patient survival if stratification by thrombocytosis had been applied. The strong effect of the inflammatory cytokine, interleukin-6 on survival could be demonstrated even by the elimination of the effect of thrombocytosis (univariate: P = 0.0016; multivariate: P = 0.0103).
The role of CD40L in neoplastic diseases is controversial[6]. Cellular model studies have revealed that it can significantly contribute to the immunological activity against cancer, while other studies have reported the complete opposite, i.e. that CD40L contributes to the progression and growth of the tumor[23,24]. The most promising results and antitumor effects have been observed in melanomas and hematological malignancies[6], including enhanced protection of dendritic cells against apoptosis-inducing factors of tumor cells, enhanced maturation and antigen production of B cells, increased T cell-dependent immune response and the CD40L activation-dependent apoptosis of cancer cells[6]. Moreover, CD40- and CD40L-based drugs have been developed recently, and active clinical trials are currently investigating their efficacy[25].
The CRC cell lines HCT116, Colo320, and Caco-2 have been shown to be positive for CD40, while SW480, HT29, Colo741, and LS174T do not express CD40[7,9,24]. The treatment of CD40-positive cell lines with interferon-γ can further increase the expression of CD40[7]. Analysis of resected tumor specimens has shown that approximately every third CRC is moderately or strongly CD40-postive[24]. While in another study CD40- and CD40L-positivity has been observed in 79% and 56% of CRC patients, respectively[8]. Ex vivo treatment of CD40-positive CRC cell lines with recombinant soluble human CD40L can inhibit tumor growth, induce apoptosis[7], inhibit CRC cell proliferation, stall CRC cells at the G0/G1 state, influence cell adhesion and metastasis, and increase aryl hydrocarbon receptor expression[8]. While the T cell membrane bound CD40L can induce enhanced apoptosis of in vitro CRC cells with CD40-positivity[24], less signaling strength has been observed in the case of the soluble form of CD40L. Soluble CD40L can induce apoptosis only following specific pharmacological interventions[24,26].
Previous clinical studies revealed that high CD40 expression and higher soluble CD40L concentration are associated with CRC[9,10], and these elevations are the most prominent with the presence of lymph node metastasis[7,10,27], venous invasion[11], and higher TNM stages[7,10,27]. In vitro stimulation of CD3+, CD4+, and CD8+ T cells of CRC patients resulted in a significantly increased, approximately four-times higher, CD40L expression compared to those of healthy control subjects[28].
In contrast to the results above, Tada et al[11] and Lima et al[12] observed lower soluble CD40L levels within those CRC patients with worse clinicopathological features. To our knowledge, no previous study investigated CD40L levels with the course of the disease, and only partial data are available from the study of Tada et al[11]. In that study, rectal cancer patients received neoadjuvant chemoradiotherapy prior to the surgical removal of the tumor, and CD40L was measured before and after the neoadjuvant treatment. They found that the post-treatment CD40L level of patients with a high response rate to the treatment was significantly lower, while no change was observed in those patients with low response rates[11]. Results of the current study confirmed the observations of those former studies[7,10,27] where circulating CD40L level was tendentiously higher in CRC patients than those of control patients. We also observed the highest measurements in Stage IV cancer and found that the CD40L level of CRC patients is basically constant with the course of the disease. The initial differences in CD40L levels between those patients with or without distant metastasis or thrombocytosis were observable throughout the whole course of the disease. The latter observation showed, that the CD40L level was the highest in those patients with more advanced disease. This should be the cause behind higher pre- and postoperative CD40L levels associated with shorter survival of patients, with high probability. Similar to our findings, the highest soluble CD40L levels have been observed in patients with distant metastases in squamous cancer or adenocarcinoma of the lung[29], in nasopharyngeal carcinoma[30], and in gastric cancer[31].
Approximately 95% of the soluble form of CD40L is thought to be derived from platelets[32,33]. Soluble CD40L level is strongly correlated with platelet count[34]. The highest levels can be measured in reactive thrombocytosis and essential thrombocythemia, while the lowest values can be measured in patients with low platelet count[34]. Thrombocytosis is associated with several cancers[2,3], and the platelet count is higher in patients with metastasis[2]. CD40L positively correlates with platelet count in patients with a high response rate to neoadjuvant chemoradiotherapy[11]. An assumption was made by Huang et al[27] that in cancer patients soluble CD40L is most probably derived from activated platelets than from T cells. However, this question was never further investigated.
Our data showed that CD40L level is positively correlated with several markers of (paraneoplastic) thrombocytosis, in particular with platelet count and interleukin-6. This strong connection persisted throughout the whole observation period. It has to be mentioned though that the stratification used in our survival models should have fully eliminated the significant effect of CD40L on CRC survival. However, we could not demonstrate this expected effect, which was observed, e.g., in the case of platelet count. This, together with the weaker explanatory powers observed in our linear models, suggests that the increase in CD40L levels is possibly not only influenced by (paraneoplastic) thrombocytosis alone. Increased CD40L production is known in various diseases characterized by general inflammation, like atherosclerosis, diabetes, or inflammatory bowel disease[35-38]. CRC can also be described as a disease known for its general inflammation[39], high interleukin-6 level[39], and inadequate T cell activation[27]. Furthermore, increased inflammation is also associated with metastasis[40,41]. The strong correlation between CD40L, interleukin-6, and metastases hints that the answer may be sought in the increased inflammation caused by the tumor or its metastases. To clarify this question, further investigations are needed.
Limitations of the current study were the relatively small sample size and the 57.4% follow-up rate, which did not allow us further analysis, e.g., subgroup analysis or stratifications in survival models of postoperative measurements. Heterogeneity of the study population also introduced some bias.
To summarize the results of the current study, our data suggested that, in line with some previous publications[7,10,27], the plasma CD40L level is significantly higher in CRC, and the highest levels could be observed in Stage IV cancer. CRC patients with thrombocytosis had significantly higher CD40L levels, and CD40L was strongly correlated with some of the parameters of paraneoplastic thrombocytosis. The CD40L level of patients did not changed during the disease. Results from our stratified survival models and their strong association with high interleukin-6 levels and distant metastases suggest that CD40L is not only dependent on platelet count/thrombocytosis. We hypothesize that the general inflammation caused by the tumor may also play a role in the CD40L elevation of CRC patients, with high probability. To clarify this hypothesis, further investigations are needed.
The role of CD40 ligand (CD40L) is controversial in colorectal cancer (CRC). Higher circulating CD40L levels of CRC patients are known, but their relationship with disease staging and local and distant metastasis is not clear.
To our knowledge, no previous study investigated the relationship between CD40L and CRC-related thrombocytosis. Furthermore, no study was conducted to observe if CD40L changes with the course of CRC.
To analyze the clinical characteristics and laboratory results of 106 CRC patients and evaluate CD40L, interleukin-6, thrombopoietin level, and platelet count changes with the course of the disease; and to evaluate their effect on patient survival.
CD40L and thrombopoietin were measured via enzyme-linked immunosorbent assay and interleukin-6 via electrochemiluminescence immunoassay. Measurements were conducted at the time of CRC diagnosis, at least 6 wk after primary tumor removal surgery, and at least 6 mo after primary tumor removal surgery.
CD40L of CRC patients was significantly higher in the presence of distant metastasis and/or thrombocytosis. CD40L was constant with the course of CRC, and all baseline differences persisted throughout the whole study. Both pre- and postoperative elevated CD40L were associated with poor overall and disease-specific survival of patients. The negative effect of CD40L on patient survival remained even after the stratification by thrombocytosis.
CD40L level of CRC patients does not change with the course of the disease. The CD40L level is strongly correlated with platelet count, interleukin-6, thrombocytosis, and the presence of distant metastases. The effect of CD40L on patient survival cannot be fully eliminated via stratification by thrombocytosis. This suggests that the circulating amount of platelets is not the only factor behind its elevation.
High plasma CD40L levels of CRC patients are with high probability not only dependent on circulating platelet count. General inflammation caused by the tumor could also contribute to CD40L elevation; therefore, further studies are required to clarify this question.
We are grateful to Viktor Madar-Dank for English proofreading.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Hungary
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Alvarez-Bañuelos MT, Mexico; Litvin A, Russia; Nagasawa M, Japan S-Editor: Chen YL L-Editor: Filipodia P-Editor: Chen YL
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55814] [Article Influence: 7973.4] [Reference Citation Analysis (132)] |
| 2. | Baranyai Z, Jósa V, Tóth A, Szilasi Z, Tihanyi B, Zaránd A, Harsanyi L, Szállási Z. Paraneoplastic thrombocytosis in gastrointestinal cancer. Platelets. 2016;27:269-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Stone RL, Nick AM, McNeish IA, Balkwill F, Han HD, Bottsford-Miller J, Rupairmoole R, Armaiz-Pena GN, Pecot CV, Coward J, Deavers MT, Vasquez HG, Urbauer D, Landen CN, Hu W, Gershenson H, Matsuo K, Shahzad MM, King ER, Tekedereli I, Ozpolat B, Ahn EH, Bond VK, Wang R, Drew AF, Gushiken F, Lamkin D, Collins K, DeGeest K, Lutgendorf SK, Chiu W, Lopez-Berestein G, Afshar-Kharghan V, Sood AK. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012;366:610-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 627] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 4. | Lin RJ, Afshar-Kharghan V, Schafer AI. Paraneoplastic thrombocytosis: the secrets of tumor self-promotion. Blood. 2014;124:184-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 5. | Laman JD, Claassen E, Noelle RJ. Functions of CD40 and its ligand, gp39 (CD40L). Crit Rev Immunol. 1996;16:59-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Korniluk A, Kemona H, Dymicka-Piekarska V. Multifunctional CD40L: pro- and anti-neoplastic activity. Tumour Biol. 2014;35:9447-9457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Wu Y, Wang L, He X, Xu H, Zhou L, Zhao F, Zhang Y. Expression of CD40 and growth-inhibitory activity of CD40 ligand in colon cancer ex vivo. Cell Immunol. 2008;253:102-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Zhou Y, Zhou SX, Gao L, Li XA. Regulation of CD40 signaling in colon cancer cells and its implications in clinical tissues. Cancer Immunol Immunother. 2016;65:919-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Pang X, Zhang L, Wu J, Ma C, Mu C, Zhang G, Chen W. Expression of CD40/CD40L in colon cancer, and its effect on proliferation and apoptosis of SW48 colon cancer cells. J BUON. 2017;22:894-899. [PubMed] |
| 10. | Dymicka-Piekarska V, Korniluk A, Gryko M, Siergiejko E, Kemona H. Potential role of soluble CD40 ligand as inflammatory biomarker in colorectal cancer patients. Int J Biol Markers. 2014;29:e261-e267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Tada N, Tsuno NH, Kawai K, Murono K, Nirei T, Ishihara S, Sunami E, Kitayama J, Watanabe T. Changes in the plasma levels of cytokines/chemokines for predicting the response to chemoradiation therapy in rectal cancer patients. Oncol Rep. 2014;31:463-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Lima PMA, Torres LC, Martins MR, da Matta MC, Lima JTO, de Mello MJG, da Silva LM, Cintra EB Jr, Lira CCR, da Fonte EJA, Forones NM. Soluble levels of sCD40L and s4-1BB are associated with a poor prognosis in elderly patients with colorectal cancer. J Surg Oncol. 2020;121:901-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Jardim DL, Rodrigues CA, Novis YAS, Rocha VG, Hoff PM. Oxaliplatin-related thrombocytopenia. Ann Oncol. 2012;23:1937-1942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Kilpatrick K, Shaw JL, Jaramillo R, Toler A, Eisen M, Sangaré L, Soff GA. Occurrence and Management of Thrombocytopenia in Metastatic Colorectal Cancer Patients Receiving Chemotherapy: Secondary Analysis of Data From Prospective Clinical Trials. Clin Colorectal Cancer. 2021;20:170-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Schwandt A, Denkinger M, Fasching P, Pfeifer M, Wagner C, Weiland J, Zeyfang A, Holl RW. Comparison of MDRD, CKD-EPI, and Cockcroft-Gault equation in relation to measured glomerular filtration rate among a large cohort with diabetes. J Diabetes Complications. 2017;31:1376-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 16. | Jessup J, Goldberg R, Asare E, Benson A, Brierley J, Chang G, Chen V, Compton C, De Nardi P, Goodman K, Gress D, Guinney J, Gunderson L, Hamilton S, Hanna N, Kakar S, Kosinski L, Negoita S, Ogino S, Overman M, Quirke P, Rohren E, Sargent D, Schumacher-Penberthy L, Shibata D, Sinicrope F, Steele S, Stojadinovic A, Tejpar S, Weiser M, Welton M, Washington M. Colon and Rectum. In: Amin M, Edge S, Greene F, Byrd D, Brookland R, Washington M, Gershenwald J, Compton C, Hess K, Sullivan D, Jessup J, Brierley J, Gaspar L, Schilsky R, Balch C, Winchester D, Asare E, Madera M, Gress D, Meyer L. AJCC Cancer Staging Manual. 8th ed. Chicago, IL, USA: Springer International Publishing, 2018: 251-274. |
| 17. | Shen H, Yang J, Huang Q, Jiang MJ, Tan YN, Fu JF, Zhu LZ, Fang XF, Yuan Y. Different treatment strategies and molecular features between right-sided and left-sided colon cancers. World J Gastroenterol. 2015;21:6470-6478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 133] [Cited by in RCA: 165] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 18. | R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2021. |
| 19. | Sekhon JS. Multivariate and Propensity Score Matching Software with Automated Balance Optimization: The Matching package for R. J Stat Soft. 2011;42:52. |
| 20. | Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team. Linear and Nonlinear Mixed Effects Models (R package version 3.1-149), 2020. |
| 21. | Therneau TM. A Package for Survival Analysis in R (R package version 3.1-8), 2020. |
| 22. | Holm S. A Simple Sequentially Rejective Multiple Test Procedure. Scand J Stat. 1979;6:65-70. |
| 23. | Bereznaya NM, Chekhun VF. Expression of CD40 and CD40L on tumor cells: the role of their interaction and new approach to immunotherapy. Exp Oncol. 2007;29:2-12. [PubMed] |
| 24. | Georgopoulos NT, Merrick A, Scott N, Selby PJ, Melcher A, Trejdosiewicz LK. CD40-mediated death and cytokine secretion in colorectal cancer: a potential target for inflammatory tumour cell killing. Int J Cancer. 2007;121:1373-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Richards DM, Sefrin JP, Gieffers C, Hill O, Merz C. Concepts for agonistic targeting of CD40 in immuno-oncology. Hum Vaccin Immunother. 2020;16:377-387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 26. | Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229:152-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 836] [Cited by in RCA: 1143] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 27. | Huang J, Jochems C, Talaie T, Anderson A, Jales A, Tsang KY, Madan RA, Gulley JL, Schlom J. Elevated serum soluble CD40 ligand in cancer patients may play an immunosuppressive role. Blood. 2012;120:3030-3038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 28. | Büning C, Krüger K, Sieber T, Schoeler D, Schriever F. Increased expression of CD40 ligand on activated T cells of patients with colon cancer. Clin Cancer Res. 2002;8:1147-1151. [PubMed] |
| 29. | Roselli M, Mineo TC, Basili S, Martini F, Mariotti S, Aloe S, Del Monte G, Ambrogi V, Spila A, Palmirotta R, D'Alessandro R, Davì G, Guadagni F, Ferroni P. Soluble CD40 ligand plasma levels in lung cancer. Clin Cancer Res. 2004;10:610-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Zhao P, Fang WJ, Chai L, Ruan J, Zheng Y, Jiang WQ, Lin S, Zhou SH, Zhang ZL. The prognostic value of plasma soluble CD40 ligand levels in patients with nasopharyngeal carcinoma. Clin Chim Acta. 2015;447:66-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Li R, Chen WC, Pang XQ, Hua C, Li L, Zhang XG. Expression of CD40 and CD40L in gastric cancer tissue and its clinical significance. Int J Mol Sci. 2009;10:3900-3917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Henn V, Slupsky JR, Gräfe M, Anagnostopoulos I, Förster R, Müller-Berghaus G, Kroczek RA. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1492] [Cited by in RCA: 1580] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 33. | Nagasawa M, Zhu Y, Isoda T, Tomizawa D, Itoh S, Kajiwara M, Morio T, Nonoyama S, Shimizu N, Mizutani S. Analysis of serum soluble CD40 ligand (sCD40L) in the patients undergoing allogeneic stem cell transplantation: platelet is a major source of serum sCD40L. Eur J Haematol. 2005;74:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Viallard JF, Solanilla A, Gauthier B, Contin C, Déchanet J, Grosset C, Moreau JF, Praloran V, Nurden P, Pellegrin JL, Nurden AT, Ripoche J. Increased soluble and platelet-associated CD40 ligand in essential thrombocythemia and reactive thrombocytosis. Blood. 2002;99:2612-2614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 102] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 35. | Danese S, Sans M, Fiocchi C. The CD40/CD40L costimulatory pathway in inflammatory bowel disease. Gut. 2004;53:1035-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 120] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 36. | Lin R, Liu J, Gan W, Yang G. C-reactive protein-induced expression of CD40-CD40L and the effect of lovastatin and fenofibrate on it in human vascular endothelial cells. Biol Pharm Bull. 2004;27:1537-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | El-Asrar MA, Adly AA, Ismail EA. Soluble CD40L in children and adolescents with type 1 diabetes: relation to microvascular complications and glycemic control. Pediatr Diabetes. 2012;13:616-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Seijkens T, Kusters P, Engel D, Lutgens E. CD40-CD40L: linking pancreatic, adipose tissue and vascular inflammation in type 2 diabetes and its complications. Diab Vasc Dis Res. 2013;10:115-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Long AG, Lundsmith ET, Hamilton KE. Inflammation and Colorectal Cancer. Curr Colorectal Cancer Rep. 2017;13:341-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 40. | Riedl JM, Posch F, Moik F, Bezan A, Szkandera J, Smolle MA, Kasparek AK, Pichler M, Stöger H, Stotz M, Gerger A. Inflammatory biomarkers in metastatic colorectal cancer: prognostic and predictive role beyond the first line setting. Oncotarget. 2017;8:96048-96061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Tuomisto AE, Mäkinen MJ, Väyrynen JP. Systemic inflammation in colorectal cancer: Underlying factors, effects, and prognostic significance. World J Gastroenterol. 2019;25:4383-4404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 125] [Cited by in RCA: 190] [Article Influence: 31.7] [Reference Citation Analysis (5)] |