Published online Apr 26, 2022. doi: 10.12998/wjcc.v10.i12.3951
Peer-review started: November 16, 2021
First decision: December 27, 2021
Revised: January 4, 2022
Accepted: March 6, 2022
Article in press: March 6, 2022
Published online: April 26, 2022
Processing time: 156 Days and 5.9 Hours
Infectious mononucleosis (IM) is a disease caused by Epstein–Barr virus (EBV). EBV infection is common in children; however, it can cause IM in adults. Studies on recurrence of IM in adults after remission are limited.
We report a 28-year-old man who presented with IM-like symptoms with mild liver damage after initial remission of IM for 3 years. He was first diagnosed with IM and treated in 2015. Follow-up tests in 2016 and 2017 did not show any abnormalities. In November 2018, he presented with swelling of the tonsils. He was misdiagnosed with acute suppurative tonsillitis and treated for 5 d. No signs of improvement were observed. He was readmitted with recurrent fever, pharyngalgia, fatigue, and systemic muscle pain. Examinations revealed enlargement of the tonsils and cervical lymph nodes. Blood tests revealed elevated transaminase levels. Anti-EBV test was positive, indicating virus reactivation. IM recurrence was confirmed on the basis of laboratory tests and clinical manifestations. He was treated with antiviral, anti-infective, and hepatoprotective drugs and vitamin supplements. His condition improved and no abnormalities were observed during follow-up.
Recurrence of IM after remission is possible in adults; therefore, long-term follow-up and monitoring are essential.
Core Tip: Our case report demonstrates the possibility of infectious mononucleosis recurrence in cured adults after infection with Epstein–Barr virus (EBV). Because of the association between EBV infection and malignant diseases, long-term follow-up and monitoring are necessary.
- Citation: Zhang XY, Teng QB. Recurrence of infectious mononucleosis in adults after remission for 3 years: A case report. World J Clin Cases 2022; 10(12): 3951-3958
- URL: https://www.wjgnet.com/2307-8960/full/v10/i12/3951.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i12.3951
Epstein–Barr virus (EBV) is a member of the herpesvirus family. It is one of the most common human viruses, affecting nearly 90% of the adult population worldwide. In the majority of individuals, EBV infection occurs during childhood. However, if an adult is infected with EBV, the viral genome remains latent and establishes lifelong persistence in a small portion of memory B cells[1]. EBV infections in children are usually asymptomatic or mild; whereas, primary EBV infections in adolescents or adults are commonly characterized by infectious mononucleosis (IM). Typical manifestations of adult IM are fatigue, fever, pharyngitis and lymphadenopathy. In addition, liver damage due to elevated transaminase, jaundice, and hepatomegaly may also be observed. In rare cases, hemolytic anemia, thrombocytopenia, aplastic anemia, myocarditis, and neurological complications are observed. EBV is treated with several antiviral drugs that aim to inhibit the replication of EBV; however, these drugs have limited clinical success. Thus far, no drug has been approved for the treatment of EBV infection[2]. Supportive treatment is recommended for IM patients. EBV is known to be one of the main causes of nasopharyngeal cancer[3]. Usually, EBV is a harmless passenger residing in B cells. However, it may cause severe diseases, including hemophagocytic lymphohistiocytosis, and is associated with numerous human cancers. Most IM patients will attain remission without obvious sequelae and recover within 2 mo after disease onset. IM is a mild illness with better outcomes compared to other diseases related to adult EBV infections, such as hemophagocytic syndrome and malignancies[4].
Here, we report a patient who showed IM-like symptoms with mild liver damage at 3 years after initial IM remission. Typical symptoms were fever and pharyngitis, accompanied by elevated transaminase levels. Results of routine blood tests and EBV viral capsid antigen (VCA) antibody test (positive for VCA IgM antibodies) confirmed the recurrence of IM. Only a few cases of adult IM have been reported to date. Our case demonstrates the possibility of IM recurrence in adults after remission of EBV-related diseases, even in well-controlled patients. In addition, symptoms is similar to the primary manifestations (liver damage in our case) may also recur.
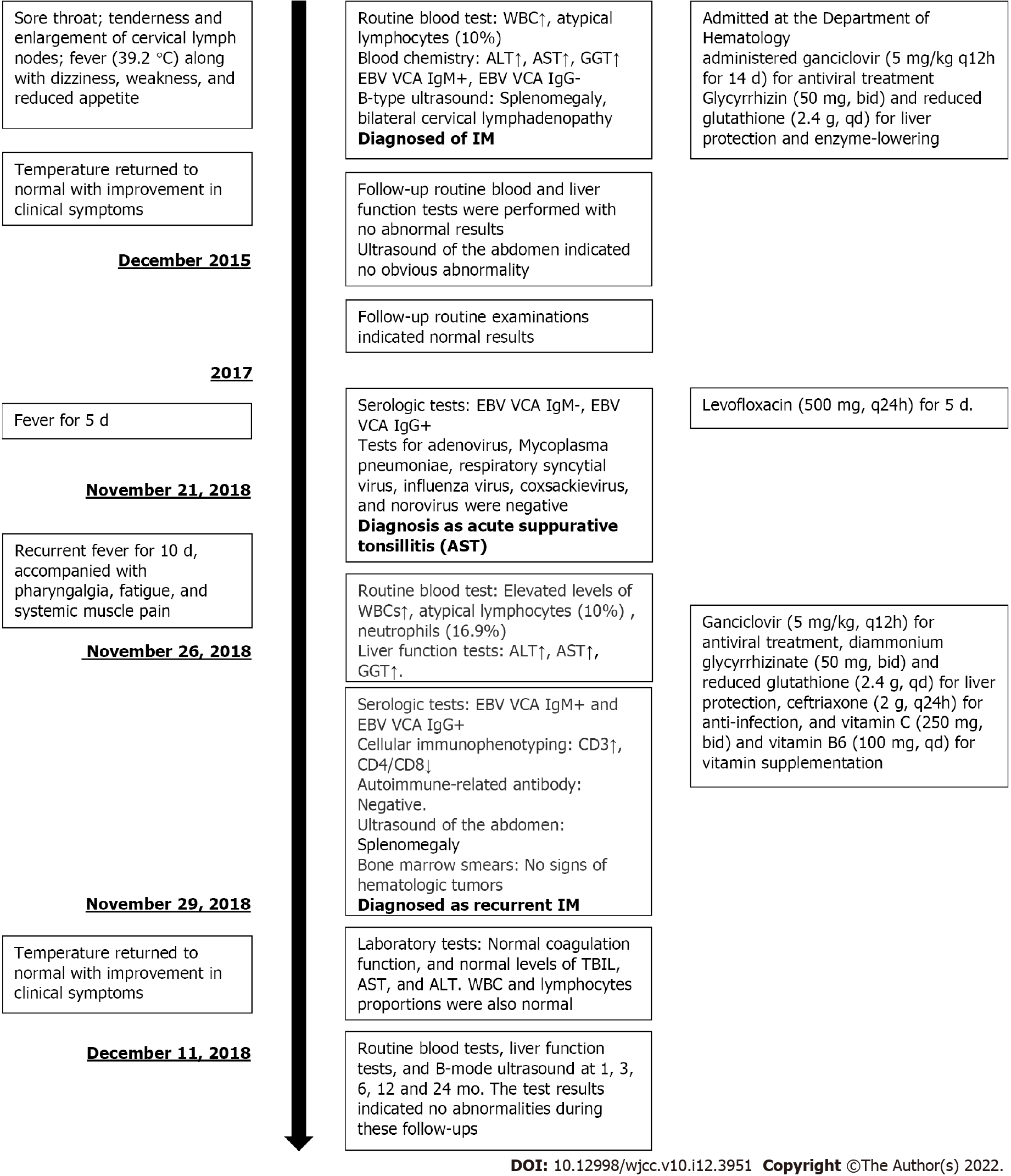
A 25-year-old male patient of Han nationality, who was admitted at the Department of Hematology, People’s Hospital of Quzhou, affiliated with the Zhejiang University School of Medicine on December 4, 2015 (Figure 1).
The patient complained of sore throat lasting from November 23, 2015, accompanied with tenderness and enlargement of cervical lymph nodes. One week later, he had fever (39.2 °C) along with dizziness, weakness, and reduced appetite.
The patient conveyed that he did not have any other disease or was consuming other drugs.
He denied a family history of disease.
Physical examination performed at admission demonstrated consciousness but with lassitude, pharyngeal hyperemia, swelling of the tonsils (Grade III, with yellow–white pus spots), and a palpable enlarged lymph node at the left submandibular region (1 cm × 1 cm in size, moderate hardness, smooth, and with satisfactory range of movement). Bilateral breath sounds in the lungs were coarse with the absence of dry or moist rales.
Routine blood test results revealed abnormal white blood cell (WBC) count, neutrophils, and atypical lymphocytes. Blood chemistry revealed highly elevated levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), -glutamyl transferase (GGT), alkaline phosphatase (AKP), lactate dehydrogenase (LDH) and moderately elevated values of total bilirubin and direct bilirubin. Elevated C-reactive protein (CRP) level was 4.5 mg/L. Positive results were obtained for EBV VCA IgM antibodies and negative results for EBV VCA IgG antibodies. Thyroid function [free triiodothyronine (T3), free thyroxine (T4), T3, T4, and thyroid-stimulating hormone] and tumor markers [carbohydrate antigen (CA) 724, -fetoprotein, carcinoembryonic antigen, total prostate-specific antigen (PSA), free PSA, CA199, and squamous cell carcinoma antigen] were normal.
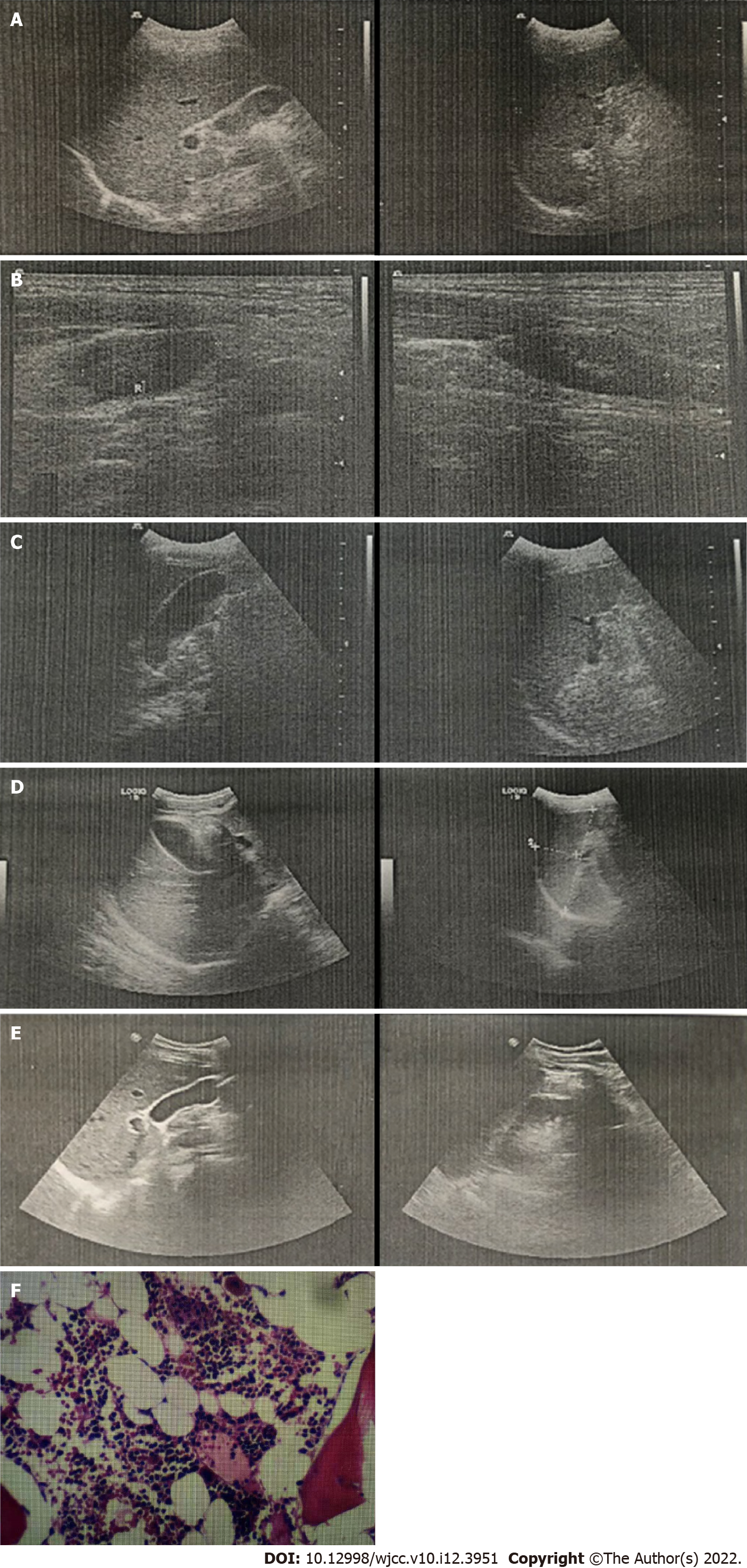
B-type ultrasound of the abdomen indicated splenomegaly (Figure 2A) and that of cervical lymph nodes indicated bilateral cervical lymphadenopathy (Figure 2B).
We considered a diagnosis of IM.
We administered ganciclovir (5 mg/kg q12h for 14 d) for antiviral treatment, as well as glycyrrhizin (50 mg, bid) and reduced glutathione (2.4 g, qd) for liver protection and enzyme-lowering on December 7, 2015.
After 2 wk of treatment, on December 22, 2015, the patient’s temperature returned to normal with improvement in clinical symptoms. No abnormal values were observed in blood test results except for the low level of neutrophils. However, slightly elevated levels were seen in liver function of ALT, AST, AKP and GGT. Abdominal ultrasound indicated a slightly enlarged spleen (Figure 2C). The patient was discharged on December 24, 2015. One week later, follow-up routine blood and liver function tests were performed with no abnormal results. In addition, ultrasound of the abdomen indicated no obvious abnormality (Figure 2D). In November 2016 and November 2017, the patient underwent follow-up routine examinations, which indicated normal results.
In November 2018, the patient visited the outpatient department (1 wk prior to readmission) and his physical examination showed swelling of the tonsils (Grade II, with pus spots). Routine blood tests revealed normal findings. Serological tests indicated negative result for EBV VCA IgM antibodies and positive result for EBV VCA IgG antibodies. Tests for adenovirus, Mycoplasma pneumoniae, respiratory syncytial virus, influenza virus, coxsackievirus, and norovirus were negative. Therefore, the outpatient department confirmed the diagnosis of acute suppurative tonsillitis and administered levofloxacin (500 mg, q24h) for 5 d; however, no signs of improvement were observed. On November 26, 2018, he was readmitted to the Department of Hematology of Medicine with recurrent fever (highest body temperature 39.9 °C) for 10 d, accompanied with pharyngalgia, fatigue, and systemic muscle pain. The patient was conscious at admission. Physical examinations revealed absence of yellowing of the skin or sclera; conjunctival congestion; enlarged lymph nodes that were palpable at the right neck with tenderness (size of a soybean, hard, with satisfactory range of movement); and enlarged tonsils (Grade III, with pus spots). Routine blood tests revealed slightly elevated WBC count (12.5 × 109/L) and atypical lymphocytes (10%) and the absolute lymphocyte count was 5.86 × 109/L and reduced neutrophils (16.9%). Liver function tests indicated elevated ALT (104.8 U/L), AST (66.6 U/L), and GGT (65.5 U/L) and normal levels of AKP (92.7 U/L). Serological tests were positive for both EBV VCA IgM and EBV VCA IgG antibodies. His EBV-DNA titer was < 1000 copies/mL and VCA-IgG was not detected, which were both lower than the lowest value that could be detected in the laboratory. Procalcitonin level was 0.22 g/L. Cellular immunophenotyping indicated elevated levels of CD3+ and CD8+ T cells, and decreased levels of CD4+/CD8+ T cells. Autoimmune-related antibody tests were also negative. Ultrasound of the abdomen indicated that the spleen was approximately 11.4 × 3.6 cm2 in size, with a homogeneous echogenic pattern (Figure 2E). Biopsy of bone marrow (Figure 2F) was sent to the Sir Run Run Shaw Hospital, Zhejiang University School of Medicine for examination. Results indicated active proliferative myeloid series with granulocytes at all stages of development (predominately myelocytes, metamyelocytes, band cells, and polymorphonuclear granulocytes); active proliferative erythroid series with discretely distributed nucleated cells; visible clusters of immature erythroid cells (with an irregular nucleus in some of the immature erythroid cells); and active proliferative megakaryocytes (no abnormality in counts or distribution). However, no abnormalities were observed in immunophenotyping and chromosomal analysis. As the patient had IM-like symptoms again after 3 years, we considered the possibility of chronic active EBV infection and recurrent IM in our diagnosis. His EBV-DNA titer and titers of VCA-IgG were both lower than the lowest value that could be detected in the laboratory, and there were no clinical symptoms that IM or other chronic diseases could not explain. Under the current medical conditions of our hospital and the guidelines[5], we considered that all the recent clinical evidence was insufficient for the diagnosis of chronic active EBV infection. Therefore, based on the clinical manifestations of the patient (lymphadenopathy and pharyngitis) and the laboratory test results (atypical lymphocytes ≥ 10% and positive EBV VCA antibodies), we confirmed the diagnosis of recurrent IM in the patient. We administered ganciclovir (5 mg/kg, q12h) for antiviral treatment, diammonium glycyrrhizinate (50 mg, bid) and reduced glutathione (2.4 g, qd) for liver protection, ceftriaxone (2 g, q24h) for anti-infection, and vitamin C (250 mg, bid) and vitamin B6 (100 mg, qd) for vitamin supplementation. From December 10, 2018 onwards, the patient’s temperature did not exceed 38°C. Results of laboratory tests indicated normal coagulation function, and normal levels of total bilirubin, AST and ALT. WBC and lymphocyte proportions were also normal. The patient’s condition improved and he was discharged on December 11. After discharge, the patient underwent routine blood tests, liver function tests, and B-mode ultrasound at 1, 3, 6, 12, and 24 mo. The test results indicated no abnormalities during these follow-ups.
IM is a rare disease in adults and has complex and nonspecific clinical manifestations; hence, it can be easily misdiagnosed. Therefore, detailed examinations are necessary for accurate diagnosis[6]. In this case report too, the patient was misdiagnosed with AST during IM recurrence. Previously, the patient was diagnosed with IM and received successful treatment. The follow-up tests also showed no abnormalities. However, 3 years later, he showed symptoms of enlarged tonsils, fever, pharyngalgia, fatigue, and muscle pain, along with mild liver damage. Laboratory test results indicated positive EBV VCA antibodies, which confirmed recurrence of IM. Once diagnosed, the patient was successfully treated with no further complications. This finding confirms the possibility of recurrence of IM in patients who are infected with EBV previously.
At present, most clinicians believe that the common symptoms of IM in adults are fever, sore throat, and muscle pain. The majority of patients aged < 20 years present these classic symptoms; however, the prevalence of nonspecific clinical features of abnormal liver function increases with age[7]. Prognosis of IM may be associated with age, body temperature at onset, and baseline disease. Age above 30 years may be a risk factor for onset of severe IM[8]. We reviewed relevant studies extracted from the Web of Science using “infectious mononucleosis” and “adult” as keywords and found eight case reports (Table 1). From these case reports, we observed that liver damage is the most frequently found complication in adults with IM. Our patient also had liver dysfunction mainly manifested as increased levels of aminotransferase. This finding was consistent with most of the cases that have been reported. In one case that mentioned recurrence[9], the patient was a 54-year-old middle-aged man with a history of recurrent IM-like symptoms for at least 1 year. However, in our case, the patient had his initial IM attack at the age of 25 (< 30 years), and he had been healthy without any baseline disease or immune system diseases. The patient was cured and discharged after 2 wk of treatment for the initial attack. Follow-up after discharge indicated no abnormalities in blood tests (atypical lymphocytes) or B-mode ultrasound. In addition, the patient did not have persistent or recurrent IM-like symptoms during the follow-up period. IM is usually associated with mild transaminitis and may induce acute hepatitis. However, liver damage is often mild and is characterized by a mild increase in transferase levels but not jaundice. For such patients, symptomatic treatments are administered. Older patients with IM have higher risks of liver dysfunction. The incidence rate of liver dysfunction is about 10% in younger patients; whereas, it could be as high as 30% in older patients, with clinical symptoms often being more severe[10]. EBV infections may induce hepatitis in susceptible individuals but are seldom associated with acute fulminant liver failure unless patients are undergoing transplantation or have immunodeficiency[11]. In addition, IM-induced liver damage may be associated with gene expression or mutations, resulting in a more severe clinical course[12]. The pathogenesis and immune mechanism of IM in the presence of acute or chronic hepatitis are unknown. However, infiltration of CD3+ and CD8+ T lymphocytes may be involved. With regard to IM hepatitis, CD8+ T cells or cytotoxic T lymphocytes infected with EBV may be present in the liver to release interferon β and tumor necrosis factor α to induce liver cell damage[13]. In summary, EBV-related liver damage is often self-limiting and resolves unnoticed. Hence, mildly elevated transferase levels during the early stages may be the only clinical manifestation.
| Ref. | Description of cases |
| Miyamoto et al[16], 1998 | This case report described an adult patient with chronic and active EBV infection. Hepatitis was prolonged after EBV infection |
| Cunha et al[17], 2017 | This case report described a 20-yr-old female patient diagnosed with IM and exhibiting the classic symptoms of infectious mononucleosis. However, she developed unexplained dyspnea and hypoxia. Further laboratory tests confirmed that she was coinfected with Mycoplasma pneumoniae. The report did not explain the reason for her hypoxia. She slowly recovered after respiratory quinolone therapy |
| Busch et al[18], 2014 | An 18-yr-old man was reported to have severe IM complicated with fulminant hepatic failure, splenic rupture, and esophageal necrosis |
| Kang et al[19], 2009 | Two cases were reported. The first case was a 20-yr-old man with acute hepatitis secondary to IM. The second case was a 24-yr-old woman with acute hepatitis secondary to IM concomitantly infected with hepatitis A |
| Higuchi et al[9], 2007 | A 54-yr-old man presented with fever, swelling of the oral mucosa and tongue, dispersed pulmonary infiltrations, systemic lymphadenopathy, and splenomegaly. He had a history of recurrent IM-like symptoms for at least 1 yr. Mantle cell lymphoma was diagnosed by biopsy of the cervical lymph node. Anti-EBV antibody titers indicated a reactivation of chronic infection with this virus |
| Goltzman et al[20], 2000 | This case report included two adult patients. Neither of them had the classic IM symptoms such as fever, pharyngitis, and lymphadenopathy, but both experienced complications of EBV infection |
| Akashi et al[21], 1993 | This report described the case of a 43-yr-old man with severe IM and HHV-6 coinfection, with a 7-d history of fever and 5-d history of progressive, generalized skin eruption. Liver dysfunction was present, with an increase in the levels of aspartate aminotransferase |
| Lawee et al[10], 2007 | A 22-yr-old female patient with mild EBV infection associated with mixed liver disease |
Recurrent IM after remission in adults has rarely been reported. The cause of IM recurrence in our patient was unknown. It is known that almost all individuals are infected with EBV at least once in their lifetime, which persists in a latent form throughout their life. EBV reactivation occurs mostly in immunocompromised individuals[14]. However, our patient reported no history of autoimmune disorder. During recurrence, laboratory tests indicated the absence of other pathogenic infections. The main clinical manifestations observed in our patient were mild liver dysfunction in addition to the classic symptoms of fever, fatigue, pharyngitis, and lymphadenopathy. Uncontrolled EBV infection can initiate autoimmune diseases in susceptible individuals, causing different symptoms and disease flare-ups that could lead to misdiagnosis[15]. Therefore, association of EBV with autoimmune disorders and malignancies confirms the importance of long-term monitoring in susceptible individuals.
The possibility of IM should be considered when patients aged 15–30 years show symptoms of persistent fever and sore throat. Moreover, for an accurate diagnosis, EBV VCA IgM antibody tests should be used to determine primary EBV infection. Our case report demonstrates the possibility of IM recurrence in cured adults after infection with EBV. Because of the association between EBV infections and malignant diseases, long-term follow-up and monitoring are necessary.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious diseases
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Al-Hadhrami R, Oman; Haque N, Bangladesh; Yoshida N, Japan S-Editor: Chen YL L-Editor: Kerr C P-Editor: Chen YL
| 1. | Babcock GJ, Decker LL, Freeman RB, Thorley-Lawson DA. Epstein-barr virus-infected resting memory B cells, not proliferating lymphoblasts, accumulate in the peripheral blood of immunosuppressed patients. J Exp Med. 1999;190:567-576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 240] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 2. | Pagano JS, Whitehurst CB, Andrei G. Antiviral Drugs for EBV. Cancers (Basel). 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 3. | Turunen A, Rautava J, Grénman R, Syrjänen K, Syrjänen S. Epstein-Barr virus (EBV)-encoded small RNAs (EBERs) associated with poor prognosis of head and neck carcinomas. Oncotarget. 2017;8:27328-27338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Luzuriaga K, Sullivan JL. Infectious mononucleosis. N Engl J Med. 2010;362:1993-2000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 359] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 5. | Okano M, Kawa K, Kimura H, Yachie A, Wakiguchi H, Maeda A, Imai S, Ohga S, Kanegane H, Tsuchiya S, Morio T, Mori M, Yokota S, Imashuku S. Proposed guidelines for diagnosing chronic active Epstein-Barr virus infection. Am J Hematol. 2005;80 64-69 [PMID:16138335 DOI: 10.1002/ajh.20398][. |
| 6. | Zhang X, Wang L, Zhang K, Duan J, Duan Q, Zhao J, Zhang S, Yan Y, Ma F. [Analysis of misdiagnosis and differential diagnosis of infectious mononucleosis in adults]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2010;24:1129-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Yu YP, Song P, An ZM, Zhou XG, Li F, Wang LP, Mei JG, Zhai YP. [Clinico-pathologic Characteristics of Adult Patients with Atypical Infectious Mononucleosis]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2016;24:1873-1878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Wemel AC, Mayet A, Bellier S, Bigaillon C, Rapp C, Ficko C. Severe infectious mononucleosis in immunocompetent adults. Med Mal Infect. 2017;47:540-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Higuchi M, Muta T, Karube KN, Eto T, Yamano Y, Ohshima K. Epstein-Barr virus-positive blastoid variant of mantle cell lymphoma in an adult with recurrent infectious mononucleosis-like symptoms: a case report. Int J Hematol. 2007;85:219-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Lawee D. Mild infectious mononucleosis presenting with transient mixed liver disease: case report with a literature review. Can Fam Physician. 2007;53:1314-1316. [PubMed] |
| 11. | Vento S, Guella L, Mirandola F, Cainelli F, Di Perri G, Solbiati M, Ferraro T, Concia E. Epstein-Barr virus as a trigger for autoimmune hepatitis in susceptible individuals. Lancet. 1995;346:608-609. [PubMed] |
| 12. | Guala A, Campra D, Marinelli I, Gaidano G, Pagani L. Are Gilbert's syndrome and liver involvement genetically linked in infectious mononucleosis? Pediatr Infect Dis J. 2003;22:1110-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Hara S, Hoshino Y, Naitou T, Nagano K, Iwai M, Suzuki K, Yamamoto K, Nagasaka T, Morishima T, Kimura H. Association of virus infected-T cell in severe hepatitis caused by primary Epstein-Barr virus infection. J Clin Virol. 2006;35:250-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Kerr JR. Epstein-Barr virus (EBV) reactivation and therapeutic inhibitors. J Clin Pathol. 2019;72:651-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 122] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 15. | Draborg AH, Duus K, Houen G. Epstein-Barr virus in systemic autoimmune diseases. Clin Dev Immunol. 2013;2013:535738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 16. | Miyamoto N, Nagayama R, Hosoi H, Wakashima M, Takikawa H, Miyake K, Yamanaka M, Shiga J. [A case of adult chronic active EB virus infection associated with prolonged hepatitis after the occurrence of infectious mononucleosis]. Nihon Shokakibyo Gakkai Zasshi. 1998;95:905-909. [PubMed] |
| 17. | Cunha BA, Herrarte Fornos S. Unexplained Dyspnea in a Young Adult with Epstein-Barr Virus Infectious Mononucleosis: Pulmonary Involvement or Co-Infection with Mycoplasma pneumoniae Pneumonia? J Clin Med. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Busch D, Hilswicht S, Schöb DS, von Trotha KT, Junge K, Gassler N, Truong S, Neumann UP, Binnebösel M. Fulminant Epstein-Barr virus - infectious mononucleosis in an adult with liver failure, splenic rupture, and spontaneous esophageal bleeding with ensuing esophageal necrosis: a case report. J Med Case Rep. 2014;8:35. [PubMed] |
| 19. | Kang MJ, Kim TH, Shim KN, Jung SA, Cho MS, Yoo K, Chung KW. Infectious mononucleosis hepatitis in young adults: two case reports. Korean J Intern Med. 2009;24:381-387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Goltzman G, Nagornov S, Horwitz M, Rapoport MJ. [Epstein-Barr virus infections in adults: a diagnostic challenge]. Harefuah. 2000;138:640-643, 711. [PubMed] |
| 21. | Akashi K, Eizuru Y, Sumiyoshi Y, Minematsu T, Hara S, Harada M, Kikuchi M, Niho Y, Minamishima Y. Brief report: severe infectious mononucleosis-like syndrome and primary human herpesvirus 6 infection in an adult. N Engl J Med. 1993;329:168-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 89] [Article Influence: 2.8] [Reference Citation Analysis (0)] |