Published online Apr 26, 2022. doi: 10.12998/wjcc.v10.i12.3936
Peer-review started: October 17, 2021
First decision: January 11, 2022
Revised: January 19, 2022
Accepted: March 6, 2022
Article in press: March 6, 2022
Published online: April 26, 2022
Processing time: 185 Days and 20.5 Hours
There is no consensus on the antithrombotic treatment strategy for patients with coronary artery ectasia (CAE).
This case reports the dynamic observation of a patient for 48 mo after a diagnosis of CAE with acute myocardial infarction (AMI). The first antithrombotic agents used were aspirin (100 mg/d) and clopidogrel (75 mg/d). During the sixth month of observation, a second AMI occurred involving the same culprit vessel; therefore, antithrombotic agents were changed to aspirin (100 mg/d) and ticagrelor (90 mg twice per day). Twelve months after the second AMI, an attempt to reduce the dosage ticagrelor failed; therefore the original dose was continued. The CAE was relatively stable during the following 4 years.
This case indicates that a combination of aspirin and ticagrelor may be more effective for CAE patients with AMI than aspirin and clopidogrel.
Core Tip: Here we present an antithrombotic strategy for patients with coronary artery ectasia as follows: (1) For patients with acute coronary syndrome (ACS), dual antiplatelet therapy (DAPT) is recommended with the combination of aspirin and ticagrelor; further, more aggressive treatment combining DAPT with an anti-coagulant agent should be considered for patients with a high risk of thrombosis; and (2) ACS patients after 12 mo or patients without ACS should be routinely placed on aspirin as primary prevention, and DAPT would be a better choice if the patient does not have a high risk of bleeding.
- Citation: Liu RF, Gao XY, Liang SW, Zhao HQ. Antithrombotic treatment strategy for patients with coronary artery ectasia and acute myocardial infarction: A case report. World J Clin Cases 2022; 10(12): 3936-3943
- URL: https://www.wjgnet.com/2307-8960/full/v10/i12/3936.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i12.3936
Coronary artery ectasia (CAE) is defined as the dilatation of coronary arteries to a diameter ≥ 1.5 times the normal adjacent segment. The prevalence of CAE during coronary angiography (CAG) was found to be 0.9%-5.3%, and more than 50% of CAE cases had co-existing coronary heart disease (CHD)[1]. The principal manifestations of CAE include angina, acute myocardial infarction (AMI), arrhythmia, and sudden death[2]. CAE is characterized by extensive destruction of the musculoelastic elements in the coronary artery wall of unknown etiology and pathogenesis[3]. In daily practice, a substantial portion of patients with CAE are first diagnosed in the emergency department. Swaye and Valenta reported that the prevalence of AMI in the CAE population was higher than in the CHD and other non-CAE populations[4,5]. This supports the idea that thrombotic complications are prone to occur to some degree in patients with CAE; however, there is no consensus concerning the treatment of CAE, specifically an antithrombotic strategy. In practice, most medications are recommended according to CHD, and for patients with poor response to medications, coronary artery interventional therapy and coronary artery bypass graft should be considered[6]. In this study we observed a patient with CAE and AMI for 48 months to explore the antithrombotic strategy.
A 60-year-old Chinese male patient complained of intermittent chest tightness and chest pain for 4 mo, with worsening symptoms 3 d prior to admission into Beijing Friendship Hospital.
No special notes.
The patient had a history of hypertension, hyperlipidaemia, and diabetes mellitus.
The patient quit smoking 5 years ago, and occasionally consumes a small amount of alcohol. He has a family history of hypertension.
The patient’s pulse rate was 95 beats per minute, blood pressure was 131/90 mmHg (17.4/12.0 KPa), height was 165cm, weight was 86 kg, and body mass index was 31.59 kg/m2. There were no obvious positive cardiovascular signs or other other system signs.
Emergency tests showed peripheral blood myocardial injury markers were significantly increased: Creatine kinase MB fraction was 17.70 ng/mL (reference 0.00-6.60 ng/mL), troponin I was 3.731 ng/mL (reference 0.000-0.030 ng/mL), and troponin T was 0.370 ng/mL (reference 0.000-0.017 ng/mL). The routine blood tests, routine urine tests, renal and hepatic function were normal. The thromboelastogram showed that the patient had no clopidogrel resistance.
The emergency electrocardiogram showed clear Q waves in lead III and lead avF. Echocardiography showed the left ventricular ejection fraction was 69%, and there were no abnormalities in any of the heart valves.
ST-segment elevated myocardial infarction (STEMI), heart function Killip grade I.
After admission the following medications were administered: antiplatelet agents, including aspirin (aspirin enteric-coated tablet, 100 mg/d) and clopidogrel (clopidogrel hydrogen sulphate tablet, 75 mg/d), low-molecular-weight heparin (LMWH, enoxaparin sodium 6000 IU, twice per day for 1 wk), statin (rosuvastatin calcium tablet, 10 mg per night), beta-blocker (metoprolol tartrate tablet, 25 mg twice per day) and angiotensin receptor blocker (ARB, losartan potassium tablet, 50 mg/d). The chest pain was gradually relieved.
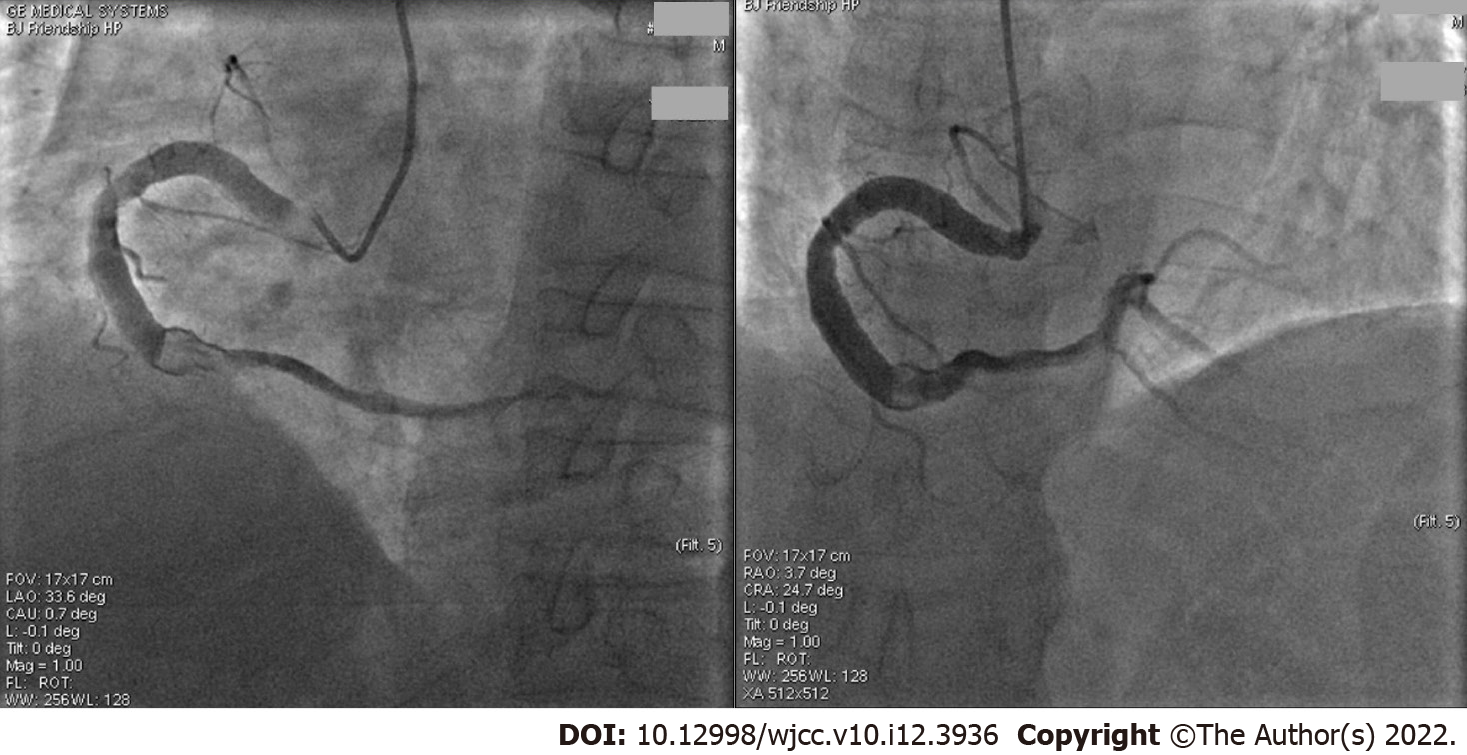
The first coronary angiography (CAG) was performed 3 d after admission to the hospital and showed aneurysmal dilation in the entire right coronary artery (RCA), with a diameter of 6.60 mm (Figure 1), which is four folds larger than the 5 French catheter and more than 1.70 fold larger than the normal RCA[7]. The middle of the RCA presented with 70%-80% limited stenosis, a thrombus shadow after the second turning point, and a grade 3 thrombolysis in myocardial infarction (TIMI) forward blood flow. In addition, part of the left anterior descending (LAD) coronary artery showed ectatic changes and was classified as Markis type II CAE[3]. The middle and distal parts of the LAD coronary artery had 40%-50% segmental stenosis, with a TIMI grade 3 forward blood flow. The left circumflex coronary artery was normal.
No additional percutaneous coronary intervention (PCI) was performed because the symptoms were relieved and coronary blood flow was unobstructed. The second CAG (Figure 2) was performed 2 wk later and showed that the RCA thrombus had disappeared, with a TIMI grade 3 forward blood flow. All of the medications mentioned previously, except LMWH, were continued.
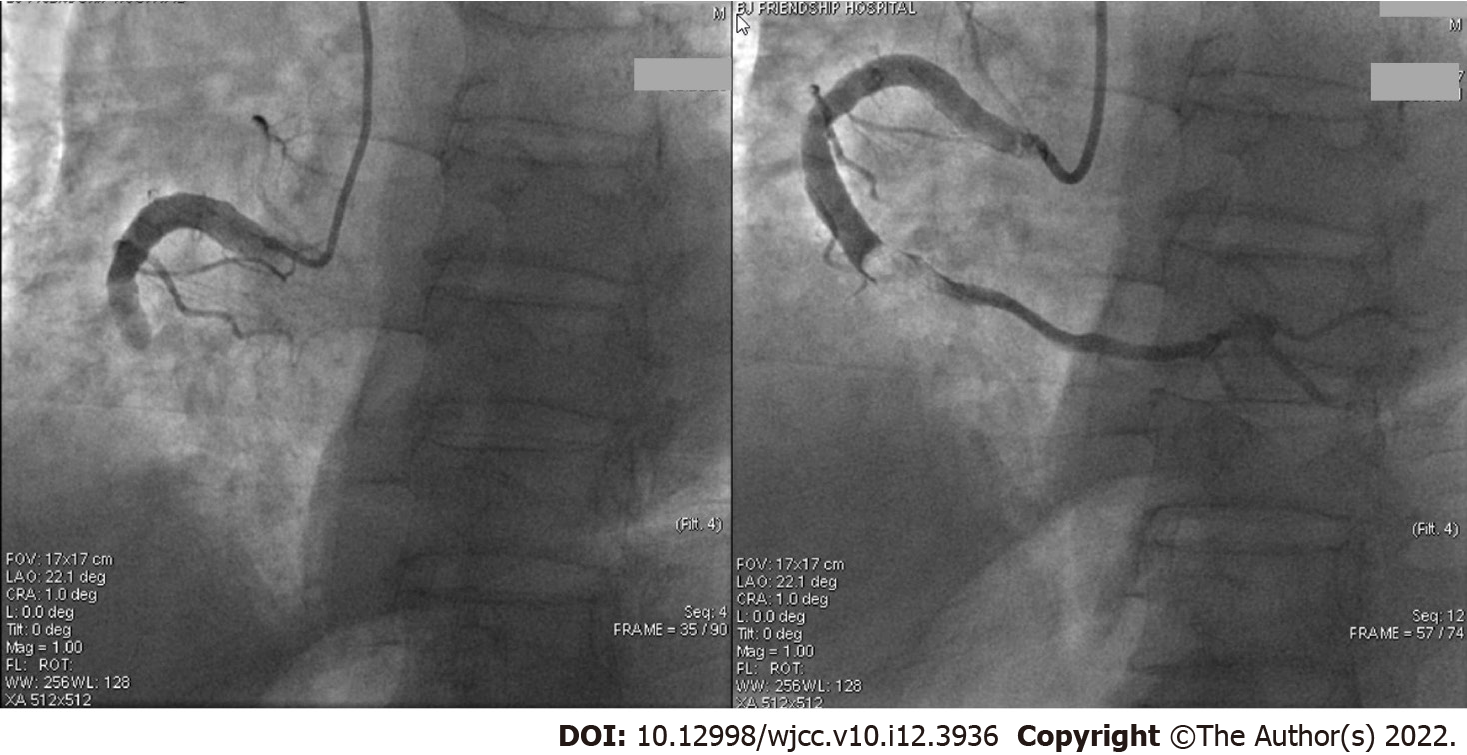
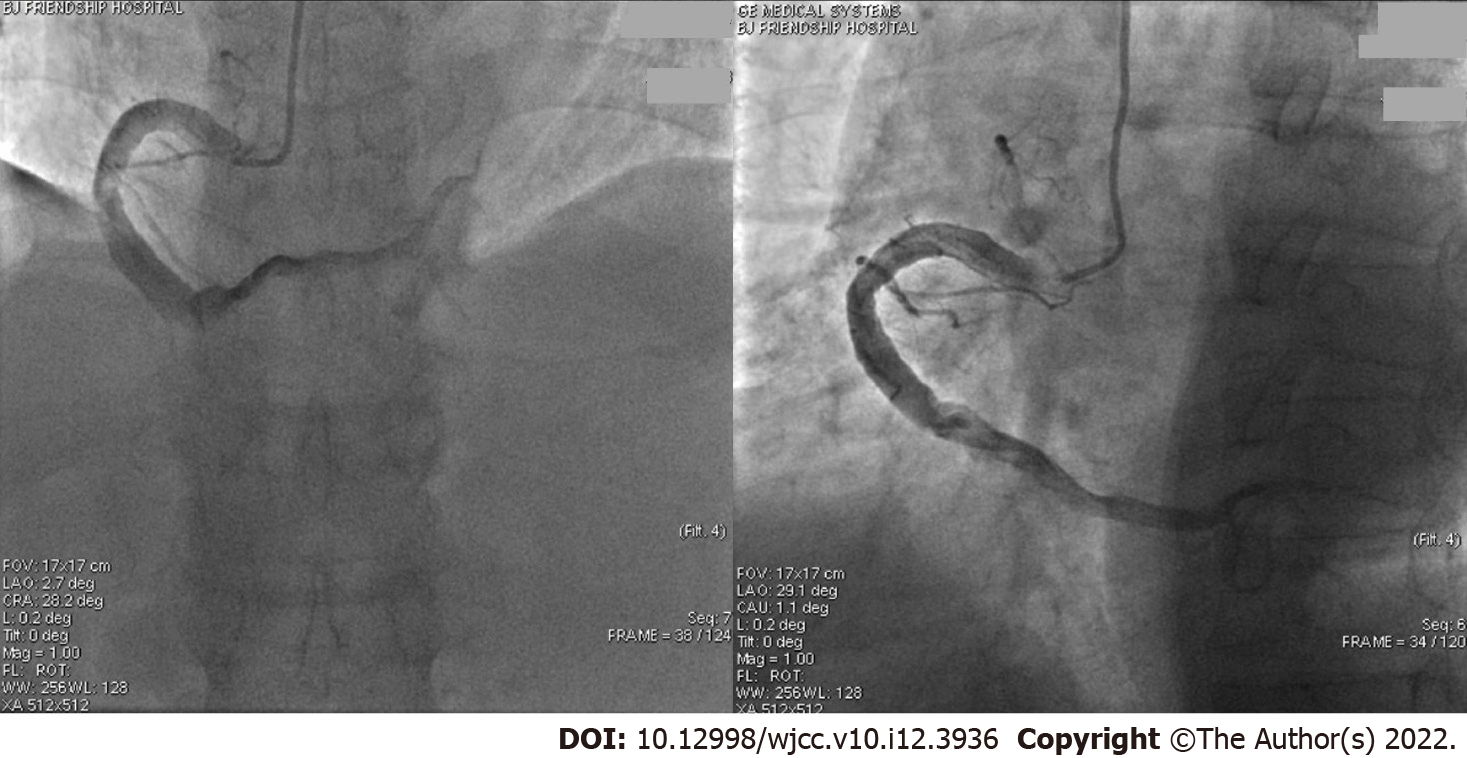
In the fifth month, the patient presented with sudden chest pain associated with palpitations, sweating, and dyspnoea. Three hours later emergency electrocardiogram showed the ST-segment increased by 0.1-0.2 mv in leads II, III, and aVF. Troponin I was 0.019 ng/mL, creatine kinase MB fraction was 1.80 ng/mL, and troponin T was < 0.010ng/mL. A STEMI was again diagnosed and an emergency CAG (Figure 3, the third CAG) showed that the middle RCA had a 100% occlusion with a thrombus shadow. Thrombus aspiration was performed and a small amount of white flocculent thrombus was extracted. The thromboelastogram showed no resistance for aspirin and clopidogrel. After discussions within the PCI group, clopidogrel was replaced by ticagrelor, and if the combination of aspirin and ticagrelor could not prevent thrombotic events, then an oral anticoagulation agent could be considered. Thus, we began treatment with aspirin (100 mg/d), ticagrelor (ticagrelor tablet, 90 mg twice per day), and 1 wk of LMWH. The other medications were continued as before.
In the sixth month the patient developed slight chest tightness without obvious ECG changes. A fourth CAG (Figure 4) was performed which showed that the middle of the RCA was slightly blurred. There were no obvious changes in other sites. The chest tightness was considered a side effect of ticagrelor. The medication was not changed, and the overall condition remained stable. About 12 mo after starting ticagrelor (eighteenth month of dynamic observation) the dosage was reduced from 90 mg twice per day to 45 mg twice per day due to nasal haemorrhage. Several days later chest pain reoccured frequently, so the dosage of ticagrelor was returned to 90 mg twice a day, and several later attempts at reducing the dosage were not successful. Of the total 48 mo of dynamic observation, the combination of aspirin and ticagrelor was observed for 42 mo; during this time the patient’s condition was stable and no obvious chest pain was observed (Table 1).
| Observation time | Complaint | Cardiac injury markers | Main diagnosis | Culprit vessel | Antithrombotic agents |
| 0 mo | Chest pain | Positive | STEMI | Thrombus in RCA | Aspirin (100 mg 1/d), clopidogrel (75 mg 1/d), LMWH for 1 wk |
| 1 mo | None | Decreased | CHD | No obvious thrombosis in RCA | Aspirin (100 mg 1/d), clopidogrel (75 mg 1/d) |
| 5 mo | Chest pain | Positive | STEMI | 100% occluded, thrombus in RCA | Aspirin (100 mg 1/d), clopidogrel (75 mg 1/d), LMWH for 1 wk |
| 6 mo | chest tightness | Negative | UAP | Middle of RCA was slightly blurred | Aspirin (100 mg 1/d), ticagrelor (90 mg 2/d) |
| 18 mo | None | Negative | CHD | - | Aspirin (100 mg 1/day), ticagrelor (45 mg 2/d) |
| 48 mo | None | Negative | CHD | - | Aspirin (100 mg 1/d), ticagrelor (90 mg 2/d) |
It should be emphasized that a complete treatment for CAE patients was essential by comprehensively evaluating applications of medical agents including anti-thrombotic agents, statin, ARB/angiotensin-converting enzyme inhibitor, beta-blocker, calcium channel blocker and antianginal agent, PCIs including thrombosis aspiration, coronary angioplasty, coronary stent implantation and so on, as well as surgical operations including dilated coronary artery resection, folding, transplantation and distal grafts ligation[1,2,6]. For the anti-thrombotic agents, in this case the patient was diagnosed with CAE and AMI; however, the combination of aspirin and clopidogrel could not prevent a second AMI. Clopidogrel was replaced with ticagrelor when the patient was in relatively stable condition; however, every attempt to reduce the dosage of ticagrelor was associated with recurrent chest pain which disappeared after reinstatement of the original dosage. Thus, the combination of aspirin and ticagrelor worked better than the combination of aspirin and clopidogrel after AMI despite the fact that there was no clopidogrel resistance according to the thromboelastogram. Our individual experience in this case needs to be verified by future trials with a larger sample size. The use of anti-coagulation agents also needs to be explored and discussed in the future.
This case involved three questions which confused us in everyday clinical practice.
The first question is “Do patients with CAE need antithrombotictic therapy?” As mentioned in the introduction, a large proportion of patients with CAE, especially older patients, have co-existing CHD, and CAE was more likely to present with thrombotic complications than patients with CHD[4,5]. The expansion sites were often the sites of thrombus formation (culprit vessels), without significant changes in atherosclerosis and stenosis[6]; the thrombus was often large because of the existence of local abnormal coronary blood flow[8]. Several studies found that patients with CAE had a large peripheral blood mean platelet volume which is also the reason for antiplatelet therapy[9]. Additionally, our previous research team showed that the prevalence of AMI in patients with CAE who received antiplatelet treatment was significantly lower than in patients with CAE who did not receive antiplatelet treatment (15.2 vs 34.7%, P = 0.020)[10]. Thus, in general CAE is associated with a higher thrombotic risk, and most treatments proposed for CAE are based on CHD treatments and these patients should be placed routinely on aspirin at least as a tool of primary prevention[6].
The second question is “Is aspirin enough for CAE patients?” The antithrombotic medicines included antiplatelet agents and anticoagulation agents. There is no consensus on what is the best strategy, single antiplatelet therapy (SAPT), dual antiplatelet therapy (DAPT), or a combination of antiplatelet and anti-coagulant therapy. The idea of adenosine diphosphate receptor inhibitors in combination with aspirin has not yet been thoroughly studied in clinical trials. Previous studies showed similar adverse event rates in patients with and without CAE[4,5,11,12], while other studies revealed higher adverse events in patients with CAE[2,13,14]. Hart et al[15] suggested that long-term warfarin therapy should be implemented to decrease the risk of coronary thrombus formation and its deleterious consequences, while aspirin would be sufficient to manage asymptomatic CAE. Other researchers noted that larger coronary aneurysms require aggressive treatment through a combination of antiplatelet and anti-coagulant therapy[16]. According to our limited experience, most CAE were associated with CHD, and the AMI in CAE was an arterial thrombotic event; thus, DAPT would be a better choice as the baseline medications if the patient did not have a high risk of bleeding. More evidence is needed to justify the antithrombotic strategies.
Regarding the third question, “How do we determine the antithrombotic strategy for patients with CAE and AMI?” The success rate of reperfusion treatment in patients with CAE who have coronary artery thrombotic events is lower than in patients with non-CAE AMI. A large thrombus may also increase the no-reflow phenomenon, distal embolization, and stent thrombosis[17-19]. A recently published systematic review summarized that the addition of anticoagulant treatment seemed to be more effective than DAPT alone for patients with CAE and ACS, the authors suggested that anticoagulant treatment must be considered if SAPT/DAPT fail to provide adequate protection against the recurrence of ACS, especially in patients with CAE who did not have other obvious stenotic lesions[20]. Current guidelines for CHD state that for patients with ACS, DAPT should be continued for 12 mo unless there are contraindications, such as a high risk of bleeding[21]. In this study, a relatively conservative treatment strategy was followed with DAPT therapy, which was not combined with anticoagulants because of the risk of bleeding, and the results indicate that the combination of aspirin and ticagrelor could prevent thrombotic events, while the combination of aspirin and clopidogrel did not. In the PLATO trial, ticagrelor proved to be superior to clopidogrel in patients with ACS irrespective of planned or unplanned invasive management, but the side effects of ticagrelor included dyspnoea (the chest tightness of this patient was considered a side effect) and a higher bleeding rate[22]. Thus, the combination of aspirin and ticagrelor was recommended as the baseline treatment for patients with CAE and ACS. The more aggressive treatment of DAPT combined with an anticoagulant agent should be considered in patients with a higher risk of thrombosis but it would significantly increase the risk of bleeding.
For patients with CAE and AMI, during the first 12 mo after AMI, the antithrombotic strategy of a combination of aspirin (100 mg/d) and ticagrelor (90 mg twice per day) might be more effective than the combination of aspirin (100 mg/d) and clopidogrel (75 mg/d). In addition, 12 mo after AMI DAPT should be considered baseline treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: El-Serafy AS, Egypt; Tumminello G, Italy; Vyshka G, Albania S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Kataoka Y, Doi T. Coronary artery ectasia: Importance of its risk stratification and management. Int J Cardiol. 2021;322:43-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Kawsara A, Núñez Gil IJ, Alqahtani F, Moreland J, Rihal CS, Alkhouli M. Management of Coronary Artery Aneurysms. JACC Cardiovasc Interv. 2018;11:1211-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 245] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 3. | Markis JE, Joffe CD, Cohn PF, Feen DJ, Herman MV, Gorlin R. Clinical significance of coronary arterial ectasia. Am J Cardiol. 1976;37:217-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 366] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 4. | Swaye PS, Fisher LD, Litwin P, Vignola PA, Judkins MP, Kemp HG, Mudd JG, Gosselin AJ. Aneurysmal coronary artery disease. Circulation. 1983;67:134-138. [RCA] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 676] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 5. | Valente S, Lazzeri C, Giglioli C, Sani F, Romano SM, Margheri M, Comeglio M, Gensini GF. Clinical expression of coronary artery ectasia. J Cardiovasc Med (Hagerstown). 2007;8:815-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Khedr A, Neupane B, Proskuriakova E, Jada K, Kakieu Djossi S, Mostafa JA. Pharmacologic Management of Coronary Artery Ectasia. Cureus. 2021;13:e17832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Ho JS, Cannaday JJ, FitzGerald SJ, Leonard D, Finley CE, Wade WA, Reinhardt DB, Ellis JR, Barlow CE, Haskell WL, Defina LF, Gibbons LW, Cooper KH. Relation of Coronary Artery Diameters With Cardiorespiratory Fitness. Am J Cardiol. 2018;121:1065-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Yokokawa T, Ujiie Y, Kaneko H, Seino Y, Kijima M, Takeishi Y. Lone aspiration thrombectomy without stenting for a patient with ST-segment elevation myocardial infarction associated with coronary ectasia. Cardiovasc Interv Ther. 2014;29:339-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Abe S, Nemoto N, Sasaki M. Sister-chromatid exchange induction by indirect mutagens/carcinogens, aryl hydrocarbon hydroxylase activity and benzo[alpha]pyrene metabolism in cultured human hepatoma cells. Mutat Res. 1983;109:83-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Liang S, Zhang Y, Gao X, Zhao H, Di B, Sheng Q, Liu R. Is Coronary Artery Ectasia a Thrombotic Disease? Angiology. 2019;70:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Boles U, Zhao Y, Rakhit R, Shiu MF, Papachristidis A, David S, Koganti S, Gilbert T, Henein MY. Patterns of coronary artery ectasia and short-term outcome in acute myocardial infarction. Scand Cardiovasc J. 2014;48:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Zhang Y, Huang QJ, Li XL, Guo YL, Zhu CG, Wang XW, Xu B, Gao RL, Li JJ. Prognostic Value of Coronary Artery Stenoses, Markis Class, and Ectasia Ratio in Patients with Coronary Artery Ectasia. Cardiology. 2015;131:251-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Warisawa T, Naganuma T, Tomizawa N, Fujino Y, Ishiguro H, Tahara S, Kurita N, Nojo T, Nakamura S. High prevalence of coronary artery events and non-coronary events in patients with coronary artery aneurysm in the observational group. Int J Cardiol Heart Vasc. 2016;10:29-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Doi T, Kataoka Y, Noguchi T, Shibata T, Nakashima T, Kawakami S, Nakao K, Fujino M, Nagai T, Kanaya T, Tahara Y, Asaumi Y, Tsuda E, Nakai M, Nishimura K, Anzai T, Kusano K, Shimokawa H, Goto Y, Yasuda S. Coronary Artery Ectasia Predicts Future Cardiac Events in Patients With Acute Myocardial Infarction. Arterioscler Thromb Vasc Biol. 2017;37:2350-2355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 15. | Qin Y, Tang C, Ma C, Yan G. Risk factors for coronary artery ectasia and the relationship between hyperlipidemia and coronary artery ectasia. Coron Artery Dis. 2019;30:211-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Baman TS, Cole JH, Devireddy CM, Sperling LS. Risk factors and outcomes in patients with coronary artery aneurysms. Am J Cardiol. 2004;93:1549-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 145] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Erden I, Erden EC, Ozhan H, Karabulut A, Ordu S, Yazici M. Outcome of primary percutaneous intervention in patients with infarct-related coronary artery ectasia. Angiology. 2010;61:574-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Feng T, Yundai C, Hongbin L, Lian C, Zhijun S, Jun G, Qinhua J, Tao Z. Evaluation neointimal coverage in patients with coronary artery aneurysm formation after drug-eluting stent implantation by optical coherence tomography. Int J Cardiovasc Imaging. 2013;29:1677-1683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Eitan A, Roguin A. Coronary artery ectasia: new insights into pathophysiology, diagnosis, and treatment. Coron Artery Dis. 2016;27:420-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Pranata R, Yonas E, Chintya V, Alkatiri AA. Is Anticoagulant Necessary in Patients with Coronary Artery Ectasia Presenting with Acute Coronary Syndrome? Int J Angiol. 2019;28:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, Jüni P, Kastrati A, Kolh P, Mauri L, Montalescot G, Neumann FJ, Peticevic M, Roffi M, Steg PG, Windecker S, Zamorano JL. [2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS.]. Kardiol Pol. 2017;75:1217-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA; PLATO Investigators, Freij A, Thorsén M. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4952] [Cited by in RCA: 5166] [Article Influence: 322.9] [Reference Citation Analysis (0)] |