Published online Apr 26, 2022. doi: 10.12998/wjcc.v10.i12.3899
Peer-review started: August 30, 2021
First decision: November 7, 2021
Revised: November 16, 2021
Accepted: March 6, 2022
Article in press: March 6, 2022
Published online: April 26, 2022
Processing time: 233 Days and 23.9 Hours
Acute pancreatitis (AP) is an acute inflammatory process of the pancreas characterized by self-digestion of pancreatic tissue, which can trigger a systemic inflammatory response. Venous thrombosis, resulting from a hypercoagulable state, is a vascular complication of AP. AP complicated by pulmonary embolism (PE) is very rare, and the combined use of extracorporeal membrane oxygenation (ECMO) with a vascular interventional procedure for AP complicated by PE is even rarer.
A 32-year-old man with a history of obesity developed rapidly worsening AP secondary to hypertriglyceridemia. During treatment, the patient developed chest tightness, shortness of breath, and cardiac arrest. Computed tomography (CT) scans of his upper abdomen were consistent with pancreatitis. PE was identified by chest CT angiography involving the right main pulmonary artery and multiple lobar pulmonary arteries. The patient’s D-dimer level was significantly elevated (> 20 mg/L). The patient received high-frequency oxygen inhalation, continuous renal replacement therapies, anti-infective therapy, inhibition of pancreatic secretion, emergent endotracheal intubation, and advanced cardiac life support with cardiopulmonary resuscitation. Following both ECMO and a vascular interventional procedure, the patient recovered and was discharged.
PE is a rare but potentially lethal complication of AP. The early diagnosis of PE is important because an accurate diagnosis and timely interventional procedures can reduce mortality. The combined use of ECMO with a vascular interventional procedure for AP complicated by PE can be considered a feasible treatment method. A collaborative effort between multiple teams is also vital.
Core Tip: Acute pancreatitis (AP) complicated by pulmonary embolism (PE) is very rare and has a high mortality rate if not detected in time. We herein report a rare case of a 32-year-old male with AP complicated by PE. Following both extracorporeal membrane oxygenation and a vascular interventional procedure, the patient recovered and was discharged. We believe this paper describes a feasible treatment method for patients with PE, potentially aiding the clinical decision-making process.
- Citation: Yan LL, Jin XX, Yan XD, Peng JB, Li ZY, He BL. Combined use of extracorporeal membrane oxygenation with interventional surgery for acute pancreatitis with pulmonary embolism: A case report. World J Clin Cases 2022; 10(12): 3899-3906
- URL: https://www.wjgnet.com/2307-8960/full/v10/i12/3899.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i12.3899
Acute pancreatitis (AP) is an acute inflammatory process of the pancreas characterized by self-digestion of pancreatic tissue, triggering pancreatic edema, hemorrhaging, necrosis, and a systemic inflammatory response. The main etiopathogenesis of AP is gallstones, alcoholism, and hypertriglyceridemia[1]. Vascular complications of pancreatitis include mainly hemorrhaging resulting from arterial erosion or pseudoaneurysm formation, ischemic complications, and venous thrombosis (especially in the portal vein, splenic vein, and superior mesenteric vein) resulting from a hypercoagulable state[2,3].
Pulmonary embolism (PE) refers to the obstruction of the pulmonary arteries by clots that originate elsewhere in the body (e.g., by breaking out of the vein walls and traveling through the heart to the pulmonary arteries)[4]. AP complicated by PE is very rare. Currently, it is believed that the main cause of PE is systemic inflammation and blood hypercoagulability. PE has a high mortality rate; its 30-d all-cause mortality rate is 4.9% to 6.6%[5].
Extracorporeal membrane oxygenation (ECMO) is a treatment option for patients with severe acute respiratory distress syndrome (ARDS) with refractory hypoxemia[6]. We herein report a case of ARDS secondary to hypertriglyceridemia-related pancreatitis complicated by obstructive shock secondary to massive PE. The patient was ultimately successfully treated with venoarterial (VA) ECMO and pulmonary artery thrombolysis, thrombus aspiration, and mechanical thrombectomy.
A 32-year-old man was admitted to the hospital with a chief complaints of persistent upper abdomen pain accompanied by nausea and vomiting for 2 d. He developed chest tightness and shortness of breath twice during treatment.
The patient, who had a high-fat diet and heavy drinking habit, had experienced persistent upper abdomen pain radiating to his back for two days, accompanied by nausea and vomiting. He had no fever, dyspnea, cough, expectoration, chest pain, or other discomfort. He was diagnosed with AP. Five hours after admission, he developed chest tightness and shortness of breath. On day 4 of admission, his dyspnea gradually improved after treatment. Subsequently, the patient’s body temperature peaked and gradually decreased, his abdominal pain was significantly relieved, and his intestinal function was also restored. On day 12 of admission, the patient developed chest tightness and shortness of breath again, accompanied by profuse sweating and conscious indifference. Unfortunately, the patient suffered cardiac arrest.
The patient had a history of obesity [Body mass index (BMI): 33.8 kg/m2]. He denied a history of hypertension, diabetes, tuberculosis, thromboembolic disease, drug use, or any other medical disease.
The patient had been consuming about 160 g of alcohol every day for 10 years and had been smoking 20 cigarettes every day for 20 years. No family members had similar diseases.
The patient’s vital signs on admission were as follows: temperature: 37.2 °C; heart rate: 117 beats per minute; respiratory rate: 18 breaths per minute; and blood pressure: 118/78 mmHg. The entire abdomen was flat and soft. Tenderness was obvious in the upper abdomen, but there was no rebound pain. We could not hear the patient’s bowel sounds.
Five hours after admission, he developed chest tightness and shortness of breath for the first time. He developed a fever, and his body temperature fluctuated between 38.7 and 38.9 °C. His heart rate and respiratory rate quickened, fluctuating between 130 and 140 beats per minute and 30 and 37 breaths per minute, respectively. His blood pressure was normal. On day 4 of admission, the patient’s body temperature peaked and gradually decreased, and there was no tenderness or rebound pain in the upper abdomen.
On day 12 of admission, the patient developed chest tightness and shortness of breath again. The patient’s vital signs were as follows: heart rate, 130 beats per minute; respiratory rate, 29 breaths per minute; temperature, 38.7 °C; blood pressure, 129/79 mmHg; and pulse oximetry, 46%. Immediately afterward, the patient fell unconscious, his heart rate slowed, and his pulse oximetry continued to drop. Unfortunately, the patient suffered cardiac arrest. After 4 min of cardiopulmonary resuscitation, the patient achieved a return to spontaneous circulation, and his heart rate and oxygen saturation recovered to 143 beats per minute and 95%, respectively. Over the next few hours, the patient’s oxygen saturation levels decreased to 84%–92%, and his blood pressure started to decrease despite receiving up to 40 mcg/min of norepinephrine and 67 mg/h of dopamine hydrochloride. On day 18 of admission, the patient was conscious and had stable vital signs.
On admission, his amylase level was 201 U/L. A further examination revealed triglycerides of 26.57 mmol/L. Five hours after admission, his white blood cell count was 18.8 × 109/L with 87.6% neutrophils. His C-reactive protein level was high (314.3 mg/L). His pressure of arterial oxygen to fractional inspired oxygen concentration (PaO2/FiO2) was 263. Arterial blood gas (ABG) measurements showed a potential of hydrogen (pH) of 7.31, a partial pressure of oxygen of 87 mmHg, a partial pressure of carbon dioxide of 41 mmHg, bicarbonate level of 20.2 mmol/L and lactic acid level of 4.0 mmol/L at an oxygen flow rate of 3 L/min nasal cannula. Plasma dimerized plasmin fragment D (D dimer) levels were 1.55 mg/L. His brain natriuretic peptide and troponin levels were normal.
On day 12 of admission, his ABG measurements were as follows: pH: 7.18, pCO2: 74 mmHg, pO2: 63 mmHg, bicarbonate: 20.2 mmol/L and lactic acid: 2.6 mmol/L. His PaO2/FiO2 was 140. The patient’s D-dimer level was significantly elevated (> 20 mg/L) and began to decline until day 18 of admission (17.42 mg/L), and the D-dimer level was 2.65 mg/L at discharge. On day 16 of admission, the patient’s platelet count decreased (Lowest value: 23 × 109/L) but recovered to 112 × 109/L at discharge.
The test results for antinuclear antibody, anticardiolipin antibody, heparin-induced thrombocytopenia (HIT) and thrombophilia screen for antithrombin III, protein C, and protein S deficiency were negative. The results for factor VIII, factor IX, factor XI, plasminogen activity, and homocysteine were normal. The genetic test results for the prothrombin gene mutation, factor V Leiden, and the mutation of the methylenetetrahydrofolate reductase (MTHFR) gene were negative.
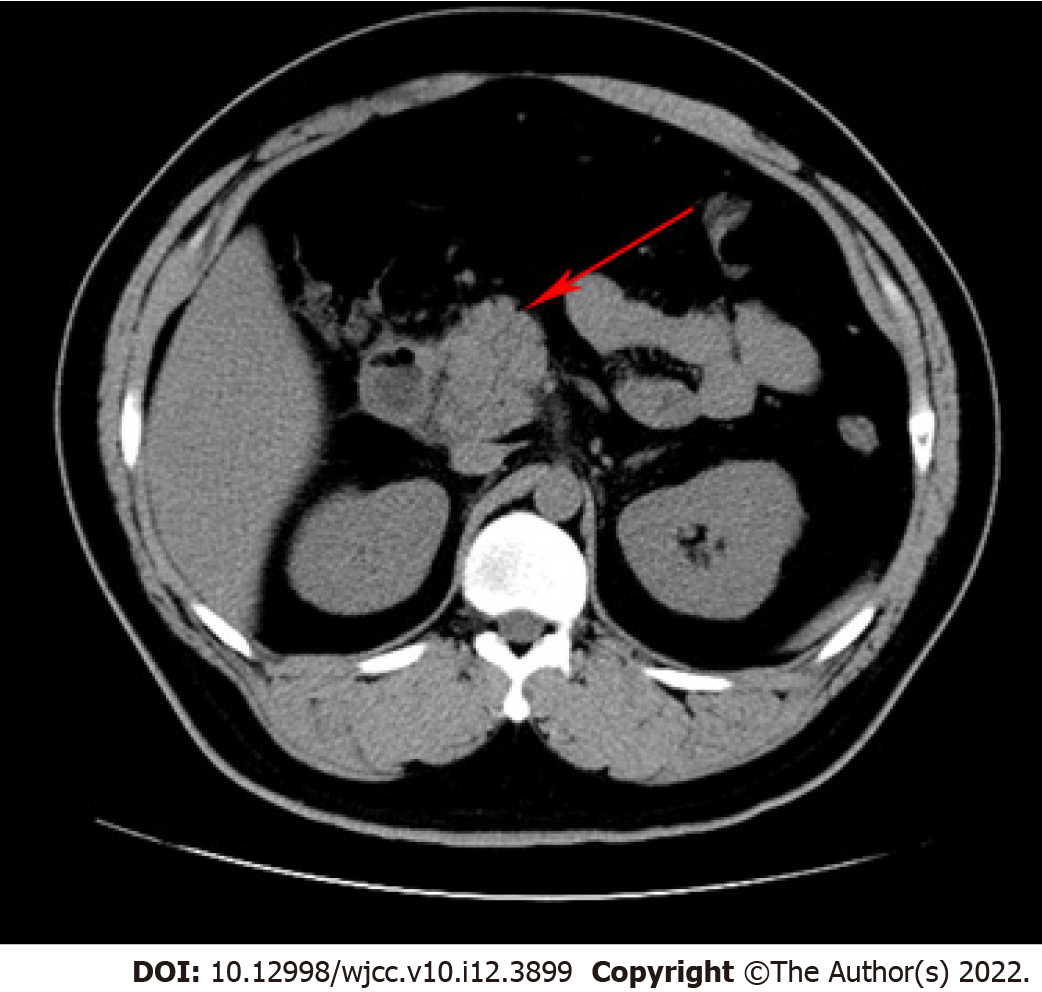
After admission, computed tomography (CT) scans of his upper abdomen were consistent with pancreatitis (Figure 1).
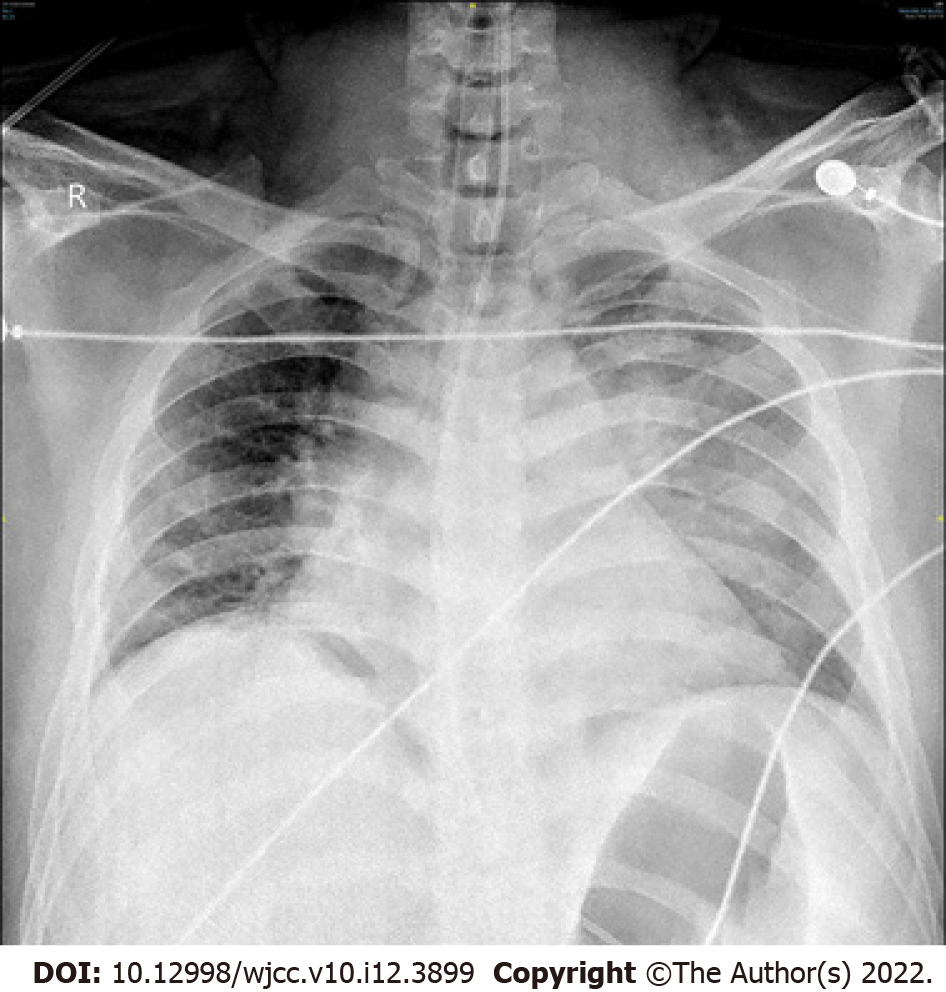
On day 12 of admission, the patient’s electrocardiogram (ECG) revealed sinus tachycardia with a heart rate of 145 beats per minute (Figure 2). Chest X-ray revealed exudative changes in the left lung (Figure 3). Doppler echocardiography revealed that his right atrium was enlarged, and his artery systolic pressure was 30 mmHg.
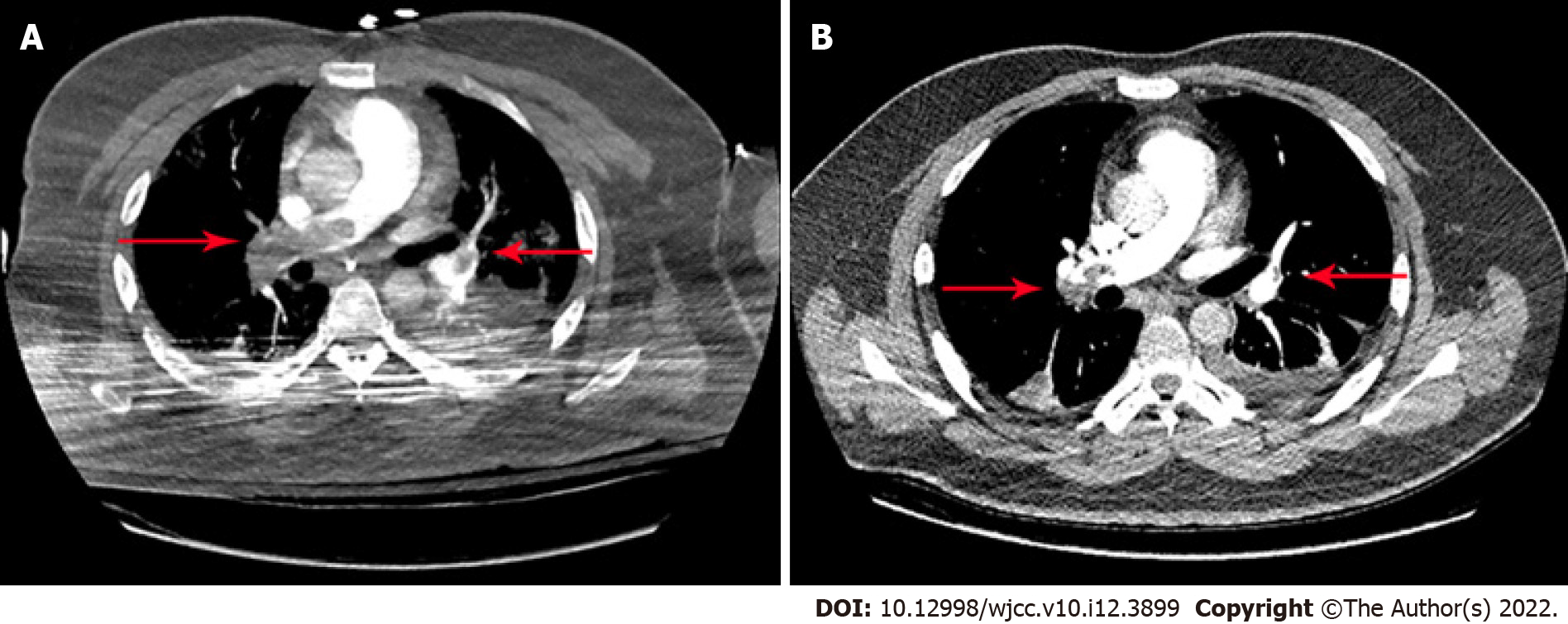
On day 14 of admission, PE was identified by chest CT angiography (CTA) involving the right main pulmonary artery and multiple lobar pulmonary arteries (Figure 4A). Color Doppler ultrasound of the upper limbs, lower limbs, and abdomen did not show thrombosis. Partial resolution of thrombosis was documented on follow-up chest CTA (Figure 4B).
The patient was eventually diagnosed with AP, acute PE, ARDS, and hypertriglyceridemia.
After admission, the patient was treated with somatostatin, antibiotics, proton pump inhibitors, low-molecular-weight heparin (LMWH), and fluid resuscitation. He was subcutaneously injected with 4100 iu of LMWH every 12 h.
Five hours after admission, the patient was immediately transferred to our intensive-care unit (ICU) for further treatment, including high-frequency oxygen inhalation, continuous renal replacement therapy, anti-infective therapy, inhibition of pancreatic secretion, and anticoagulant therapy. After the recovery of the patient’s intestinal function, a jejunal feeding tube was placed on day 8 of admission. Enteral nutrition was supported, and other treatments were continued, including anticoagulant therapy.
On day 12 of admission, when the patient sustained cardiac arrest, he underwent advanced cardiac life support with cardiopulmonary resuscitation and emergent endotracheal intubation, and 1 mg of adrenaline was injected intravenously every 3 min. We transferred the patient to the ICU again. He was placed on a ventilator and underwent bronchoalveolar lavage for sputum drainage, hormone administration for anti-inflammation, meropenem for anti-infection, and LMWH for anti-coagulation. After a discussion among multiple teams, the possibility of PE was considered, and his condition continued to worsen. The decision was made to cannulate him for VA ECMO (7-French braided antegrade arterial sheath, Femoral artery: 16-gauge, femoral vein: 22-gauge) approximately 3.5 h after his condition worsened, and the ECMO flow rate was 3.8 liters per minute. We performed VA ECMO insertion into the left femoral artery and the right femoral vein using the Seldinger technique to maintain blood pressure and oxygenation.
On day 14 of admission, Due to extensive thromboembolism and the inability to wean the patient from ECMO, interventional vascular surgery was performed, and pulmonary artery thrombolysis, thrombus aspiration, and mechanical thrombectomy were performed. On day 16 of admission, the patient was extubated.
After 35 d of hospitalization, including 4 d of ECMO therapy, the patient was discharged. Oral anticoagulation of rivaroxaban was continued. Partial resolution of thrombosis was documented on follow-up chest CTA. In three months of follow-up, the patient has not shown recurrence of AP or PE.
A combination of blood hypercoagulability, venous blood flow stasis, and vascular endothelial dysfunction is believed to trigger thrombosis[7]. AP is an inflammatory disease characterized by the self-digestion of pancreatic tissue, which can trigger a systemic inflammatory response[1]. PE is a rare complication of pancreatitis, and only a few cases have been reported[8-11]. The mechanism underlying the formation of a PE is currently believed to be as follows: (1) The cyst connected to the pancreatic duct penetrates the vascular system and releases pancreatic juice, which then triggers the formation of a thrombus secondary to vasculitis; (2) hypercoagulability occurs due to liver dysfunction, hypertyrosinemia (resulting in increased concentrations of fibrinogen and factor VIII), and cachexia; (3) the systemic inflammatory response secondary to AP damages the vascular endothelium, consequently affecting the endothelium-dependent acetylcholine relaxation reaction and causing the release of procoagulant substances and the activation of platelets, leading to blood hypercoagulability; (4) hyperlipidemia results in venous blood flow stasis; and (5) proteolytic damage plays a significant role in the development of pulmonary vascular injury after AP, such as pancreatic elastase[8,12].
Our patient was a young man who had been diagnosed with moderately severe acute hypertriglyceridemia-related pancreatitis accompanied by the accumulation of peripancreatic fluid but without pseudocysts. The patient did not have venous thrombosis in the lower extremities or other previous thrombotic diseases and had no history of recent surgery, trauma, or blood disease; however, the patient did have obesity and hyperlipidemia. Examinations revealed normal fibrinogen, normal antithrombin III, and normal protein C and S levels. Factor V Leiden and the MTHFR gene mutation were negative, and there was no evidence of atrial fibrillation on an ECG. As the patient did not have genetic or other acquired causes of PE, we concluded that the patient’s PE was secondary to AP. The predisposing factors for PE in this patient included systemic inflammatory response syndrome, hyperlipidemia-related venous blood flow stasis, long-term bed rest (12 d in bed), and obesity.
The symptoms of PE include dyspnea, chest pain, syncope, cough, and hemoptysis. However, most clinical presentations are non-specific; thus, PE is easily missed or misdiagnosed[10]. The patient experienced chest tightness, dyspnea, tachypnea, and tachycardia without chest pain or hemoptysis in the early stage. During the disease, the patient gradually developed ARDS and conscious indifference, and his D-dimer levels progressively increased. Thus, we suspected that the patient had PE. Once PE is suspected, the detection of related coagulation indicators, such as pulmonary artery pressure assessment, contrast-enhanced chest CT, and D-dimer must be completed in order to make the diagnosis as soon as possible. Our patient’s CTA showed thrombosis in the right main pulmonary artery and multiple lobar pulmonary arteries, which confirmed the diagnosis of PE. Once the diagnosis is confirmed, treatments, including anticoagulation alone, catheter-directed thrombolysis, systemic thrombolysis, catheter embolectomy, surgical embolectomy, and/or mechanical circulatory support, such as ECMO, should be started as soon as possible[13]. We therefore cannulated the patient for VA ECMO as soon as possible (i.e., only 3.5 h after his condition began worsening). However, it has been shown that treatment with ECMO alone may activate innate physiologic thrombolysis thus making it possible to achieve an improvement in the right ventricle and hemodynamic values[14]. It is presently believed that ECMO mainly provides hemodynamic and respiratory support for critically ill patients whose condition is too unstable to tolerate either surgical or catheter-based embolectomy[15]. The improvement of PE symptoms and the decrease in the D-dimer level are generally considered to be due to successful vascular interventional procedure. ECMO can improve oxygenation and remove CO2, thereby reducing the need for ventilator support (using low tidal volume and low airway pressure). This protective ventilation strategy for open lungs allows the lungs to rest, thereby increasing time available to treat the original disease[16]. ECMO has been used in the following clinical scenarios for PE patients[15,17,18]: (1) To rescue patients when thrombolytic treatment fails or as a temporary hemodynamic support prior to performing intervention; and (2) To treat patients with refractory cardiogenic shock or cardiac arrest. Our patient experienced cardiac arrest and was both hemodynamically unstable and unable to tolerate interventional surgery and, as a result, he underwent ECMO therapy. However, the patient was unable to be weaned from ECMO because of his extensive thromboembolism. In addition, due to the decreased platelet count and possible hemorrhagic conversion of pancreatitis, we had to perform interventional vascular surgery instead of systemic anticoagulant therapy.
Two doubts remain concerning the findings in this report. First, despite his receiving treatment with LMWH since admission and the lack of genetic factors related to thrombosis, PE still occurred in the present patient. There may be several reasons for this. The patient may have had predisposing factors for PE, including systemic inflammatory response syndrome, hyperlipidemia-related venous blood flow stasis, long-term bed rest (12 d in bed), and obesity. In addition, the dose of LMWH may have been insufficient, given that the patient’s BMI was 33.8 kg/m2. Second, the patient’s platelet count decreased during treatment, and the result of the HIT test was negative. The development of thrombocytopenia in severe infection and sepsis may occur as a result of the massive consumption of circulating platelets through interactions with immune cells[19].
In summary, this patient suffered from severe AP with acute PE, life-threatening ARDS, and cardiac arrest. Our gastroenterology team worked collaboratively with other teams, including the Critical Care ECMO team, who provided mechanical support, and the interventional vascular surgery team, who performed thrombolysis and mechanical thrombectomy. Consequently, the patient was successfully resuscitated, stabilized, and discharged.
AP complicated with PE is very rare, and its 30-d all-cause mortality rate is extremely high. This patient had multiple acute comorbidities, including PE, ARDS, thrombocytopenia, and obstructive shock, which complicated the treatment options and goals. The early diagnosis of PE is important, as an accurate diagnosis and timely interventional procedures can reduce mortality. The combined use of ECMO with a vascular interventional procedure for AP complicated by PE can be considered a feasible treatment method. A collaborative effort between multiple teams was vital.
The authors would like to thank the Critical Care ECMO team and interventional vascular surgery team.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kitamura K, Japan; Nyamuryekung'e M, Tanzania S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Crockett S, Falck-Ytter Y, Wani S, Gardner TB. Acute Pancreatitis Guideline. Gastroenterology. 2018;154:1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (2)] |
| 2. | Mendelson RM, Anderson J, Marshall M, Ramsay D. Vascular complications of pancreatitis. ANZ J Surg. 2005;75:1073-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 3. | Patel R, Choksi D, Chaubal A, Pipaliya N, Ingle M, Sawant P. Renal Vein and Inferior Vena Cava Thrombosis: A Rare Extrasplanchnic Complication of Acute Pancreatitis. ACG Case Rep J. 2016;3:e172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 4. | Goldhaber SZ, Morrison RB. Cardiology patient pages. Pulmonary embolism and deep vein thrombosis. Circulation. 2002;106:1436-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 5. | Jiménez D, de Miguel-Díez J, Guijarro R, Trujillo-Santos J, Otero R, Barba R, Muriel A, Meyer G, Yusen RD, Monreal M; RIETE Investigators. Trends in the Management and Outcomes of Acute Pulmonary Embolism: Analysis From the RIETE Registry. J Am Coll Cardiol. 2016;67:162-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 256] [Article Influence: 28.4] [Reference Citation Analysis (1)] |
| 6. | Rozencwajg S, Pilcher D, Combes A, Schmidt M. Outcomes and survival prediction models for severe adult acute respiratory distress syndrome treated with extracorporeal membrane oxygenation. Crit Care. 2016;20:392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (1)] |
| 7. | Wolberg AS, Rosendaal FR, Weitz JI, Jaffer IH, Agnelli G, Baglin T, Mackman N. Venous thrombosis. Nat Rev Dis Primers. 2015;1:15006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 244] [Article Influence: 24.4] [Reference Citation Analysis (1)] |
| 8. | Zhang Q, Zhang QX, Tan XP, Wang WZ, He CH, Xu L, Huang XX. Pulmonary embolism with acute pancreatitis: a case report and literature review. World J Gastroenterol. 2012;18:583-586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 9. | Herath HM, Kulatunga A. Acute pancreatitis complicated with deep vein thrombosis and pulmonary embolism: a case report. J Med Case Rep. 2016;10:182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 10. | Fu XL, Liu FK, Li MD, Wu CX. Acute pancreatitis with pulmonary embolism: A case report. World J Clin Cases. 2021;9:904-911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 11. | Dickens B, Bryant C, Gaillard J, Westphal N. ARDS and Massive Pulmonary Embolism: The Combined Use of Extracorporeal Membrane Oxygenation (ECMO) with Thrombolytics. Case Rep Crit Care. 2020;2020:1032629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Lungarella G, Gardi C, de Santi MM, Luzi P. Pulmonary vascular injury in pancreatitis: evidence for a major role played by pancreatic elastase. Exp Mol Pathol. 1985;42:44-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 13. | Rivera-Lebron B, McDaniel M, Ahrar K, Alrifai A, Dudzinski DM, Fanola C, Blais D, Janicke D, Melamed R, Mohrien K, Rozycki E, Ross CB, Klein AJ, Rali P, Teman NR, Yarboro L, Ichinose E, Sharma AM, Bartos JA, Elder M, Keeling B, Palevsky H, Naydenov S, Sen P, Amoroso N, Rodriguez-Lopez JM, Davis GA, Rosovsky R, Rosenfield K, Kabrhel C, Horowitz J, Giri JS, Tapson V, Channick R; PERT Consortium. Diagnosis, Treatment and Follow Up of Acute Pulmonary Embolism: Consensus Practice from the PERT Consortium. Clin Appl Thromb Hemost. 2019;25:1076029619853037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 188] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 14. | Maggio P, Hemmila M, Haft J, Bartlett R. Extracorporeal life support for massive pulmonary embolism. J Trauma. 2007;62:570-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 117] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 15. | Corsi F, Lebreton G, Bréchot N, Hekimian G, Nieszkowska A, Trouillet JL, Luyt CE, Leprince P, Chastre J, Combes A, Schmidt M. Life-threatening massive pulmonary embolism rescued by venoarterial-extracorporeal membrane oxygenation. Crit Care. 2017;21:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 152] [Article Influence: 19.0] [Reference Citation Analysis (1)] |
| 16. | Wang J, Wang Y, Wang T, Xing X, Zhang G. Is Extracorporeal Membrane Oxygenation the Standard Care for Acute Respiratory Distress Syndrome: A Systematic Review and Meta-Analysis. Heart Lung Circ. 2021;30:631-641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 17. | Oh YN, Oh DK, Koh Y, Lim CM, Huh JW, Lee JS, Jung SH, Kang PJ, Hong SB. Use of extracorporeal membrane oxygenation in patients with acute high-risk pulmonary embolism: a case series with literature review. Acute Crit Care. 2019;34:148-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 18. | Gangaraju R, Klok FA. Advanced therapies and extracorporeal membrane oxygenation for the management of high-risk pulmonary embolism. Hematology Am Soc Hematol Educ Program. 2020;2020:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | McDonald B, Dunbar M. Platelets and Intravascular Immunity: Guardians of the Vascular Space During Bloodstream Infections and Sepsis. Front Immunol. 2019;10:2400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (1)] |