Published online Apr 26, 2022. doi: 10.12998/wjcc.v10.i12.3849
Peer-review started: July 26, 2021
First decision: October 22, 2021
Revised: December 2, 2021
Accepted: March 6, 2022
Article in press: March 6, 2022
Published online: April 26, 2022
Processing time: 268 Days and 23.3 Hours
Anaplastic thyroid carcinoma (ATC), also called undifferentiated thyroid cancer, is the least common but most aggressive and deadly thyroid gland malignancy of all thyroid cancers[1]. It has poor prognosis, and is the leading cause of death from malignant thyroid tumors. The one-year survival rate is 20%, with a median overall survival (OS) of only 5 mo[2]. The aim of this report is to provide our experience in the diagnosis and treatment of ATC.
A patient with a thyroid mass underwent surgical treatment after developing symptoms of hoarseness. The resected tumor was pathologically diagnosed as ATC. Imaging examination revealed organ and lymph node metastasis. After multiple cycles of chemotherapy and local radiotherapy, the metastases were not relieved and gradually increased in size and new metastases appeared. The patient immediately received immunotherapy combined with targeted therapy. During treatment, immune-related adverse reactions occurred, which were improved after symptomatic treatment, and tolerated by the patient. The OS of the patient was more than 30 mo after immunotherapy combined with targeted therapy.
For metastatic ATC, surgical treatment, radiotherapy and chemotherapy have no significant effect on remission of the disease. However, immunotherapy has made a breakthrough in the treatment of ATC.
Core Tip: Anaplastic thyroid carcinoma (ATC) is a rare thyroid malignancy, it is extremely aggressive and has a very low survival rate. Immunotherapy combined with targeted therapy results in prolonged survival and good quality of life. We summarize the relevant clinical data and review the literature. This report aims to provide clinical experience in the treatment of an ATC patient by immunotherapy combined with targeted therapy.
- Citation: Ma DX, Ding XP, Zhang C, Shi P. Combined targeted therapy and immunotherapy in anaplastic thyroid carcinoma with distant metastasis: A case report. World J Clin Cases 2022; 10(12): 3849-3855
- URL: https://www.wjgnet.com/2307-8960/full/v10/i12/3849.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i12.3849
Anaplastic thyroid carcinoma (ATC) is an undifferentiated tumor which is derived from follicular epithelial cells of the thyroid. The annual incidence of ATC is 1-2/1 000000 and accounts for 1%-2% of all thyroid carcinomas[3]. The prognosis of patients is associated with age, socioeconomic status, distant metastases, surgery, chemotherapy, etc[4,5]. At present, there is no definite or standardized treatment for ATC in order to prolong the survival of patients. Treatments include surgery, radiation, chemotherapy, immunotherapy, and targeted therapy. Patients are often diagnosed at advanced stages of the disease and it is difficult to completely remove ATC by surgery[6]. As a result, the overall response rate is usually very low, so is the overall survival rate. Due to the poor prognosis and lack of treatment, molecular characterization of the tumor may provide more targets and provide patients with more therapeutic options[7]. We here summarize the clinical diagnosis and treatment of an ATC patient. The patient showed significant improvement in survival following targeted therapy and immunotherapy.
A 65-year-old man was admitted to hospital due to hoarseness, cough and expectoration for 3 mo.
He was found to have a thyroid mass on physical examination.
The patient had no relevant medical history.
There was no personal history of tobacco or alcohol consumption or any other family medical history.
Neck examination showed no abnormal sign.
Laboratory examination were unremarkable.
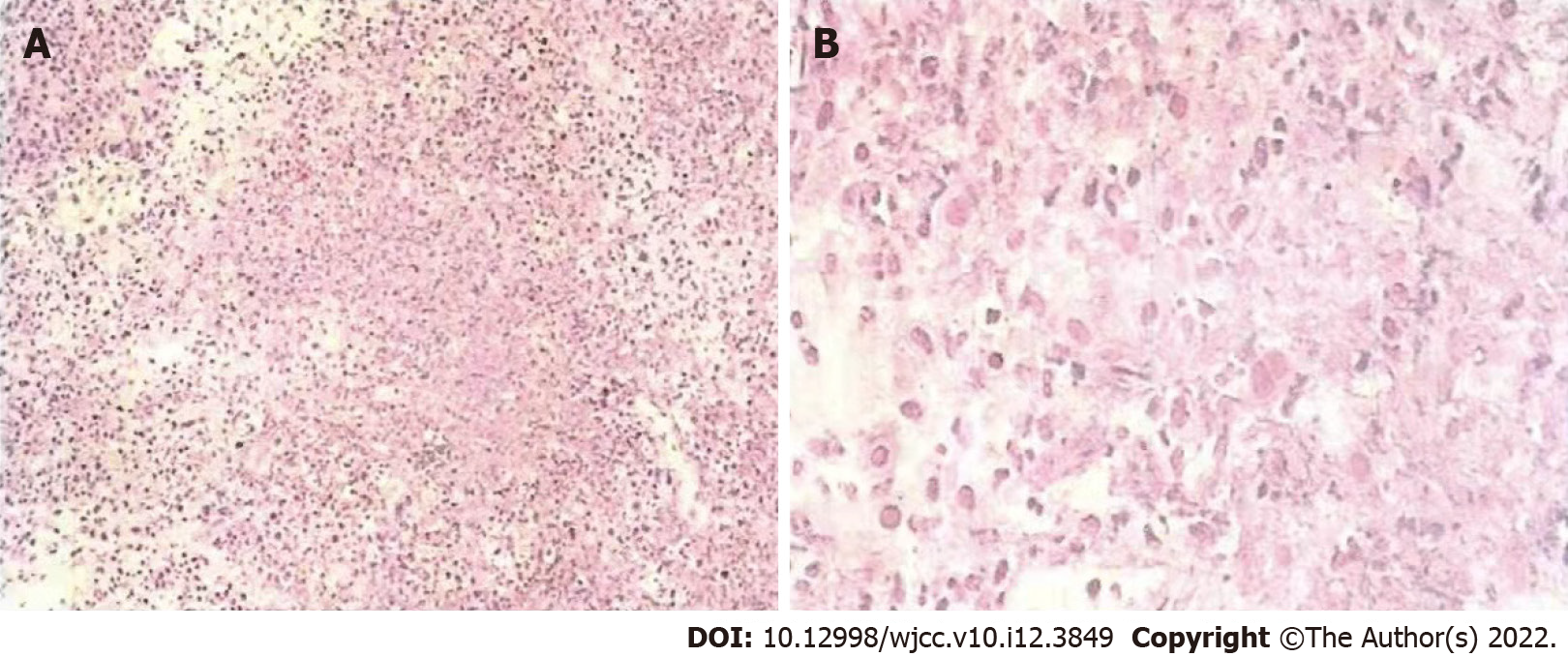
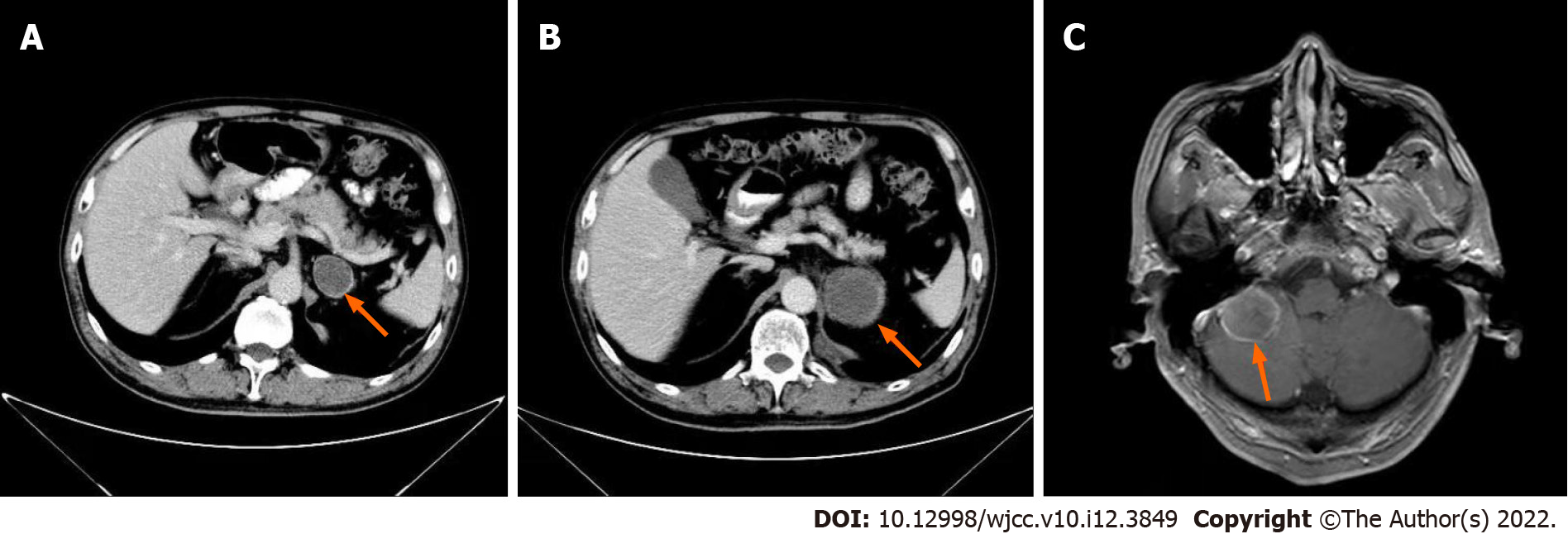
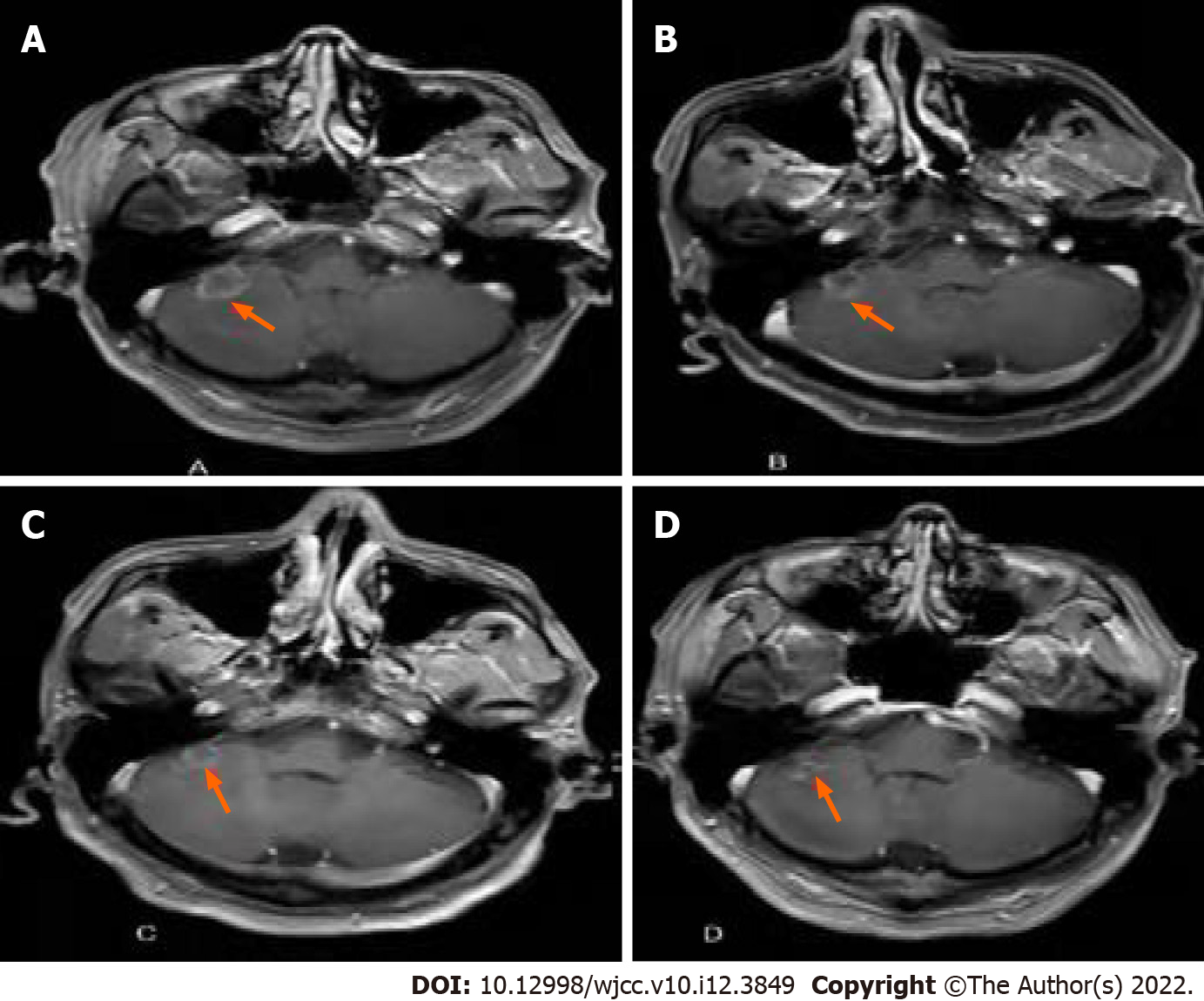
He was found to have thyroid nodules on thyroid ultrasound. And underwent thyroidectomy. Pathological diagnosis of the resected tissue suggested the possibility of ATC (Figure 1). Ten days later, positron emission tomography-computed tomography (PET-CT) showed multiple metastases in the right hilar and mediastinal lymph nodes, and left adrenal metastases (Figure 2). One month later, CT re-examination showed that mediastinal lymph node and left adrenal metastases were larger than before (Figure 3A). During chemotherapy, repeated CT showed that the mediastinal lymph nodes were not significantly changed, and left adrenal metastases were larger (Figure 3B). Brain magnetic resonance imaging (MRI) showed a cerebellar mass, which was considered to be metastasis (Figure 3C). In these two years of combination therapy, the results of regular review of brain MRI are as follows (Figure 4).
The patient was diagnosed with ATC with brain and adrenal metastases.
The patient received multiple cycles of chemotherapy after thyroidectomy. During this period, the patient received radiotherapy to the thyroid tumor bed and metastases, with a planned prescribed dose of 2.0 Gy/fraction × 28 fractions. After the appearance of brain metastases, He then received radiotherapy for cerebellar metastases at a prescribed dose of 3.0 Gy/fraction × 14 fractions. After recurrence of metastasis, nivolumab (240 mg every two weeks) was administered in combination with cabozantinib (40 mg) for two years. During the treatment, the patient experienced some immune-related adverse events (irAEs) and vascular endothelial growth factor receptor-tyrosine kinase inhibitor toxicity, mainly gastrointestinal toxicity and pneumonia, manifested as abdominal pain, diarrhea, cough and dyspnea, However, the patient developed arteriovenous thrombotic events, which were considered to be caused by cabozantinib, so the drug was discontinued. But the patient insisted on anti-programmed death-1 (PD-1) therapy for two years, during which time, after symptomatic treatment with glucocorticoids, the grade of irAEs could be controlled below grade 1[8]. After re-follow-up, the dose of nivolumab was changed to 200 mg once every three weeks.The whole treatment process of patients is shown in Table 1.
| Treatment mode | Starting and ending time | Efficacy |
| Surgery | August 30, 2017 | Tumor resection; Disease progression |
| Chemoradiotherapy | October, 2017-April, 2018 | Brain metastasis |
| Targeted therapy (Cabozantinib) | May, 2018-April, 2020 | Organ metastases gradually shrink |
| Immunotherapy (Nivolumab) | July, 2018- | Prolonged progression-free survival |
The patient's cerebellar and adrenal lesions were significantly reduced, and the corresponding symptoms also disappeared gradually. Clinical benefit was observed over 30 mo.
ATC has a high degree of malignancy and rapid progression, and its imaging diagnosis is difficult[9]. The positive rate of fine needle aspiration cytology is not high, and confirmation of the diagnosis mostly relies on postoperative pathology[10]. The patient in this report developed hilar and mediastinal lymph node and adrenal metastases, and was treated with chemotherapy, palliative radiotherapy and targeted therapy, but the disease was not controlled. He was subsequently treated with targeted therapy combined with immunotherapy. Nivolumab is a monoclonal antibody that can bind to the PD-1 receptor, which can block the interaction between PD-1 and PD-L1/PD-L2, alleviates inhibition of the PD-1 pathway-mediated immune response, and restores tumor-specific T cell immunity. This combination allows the tumor cells that are otherwise induced by T cells to be released, in order to re-identify the tumor cells and attack and kill them[11]. By targeted therapy, it means that appropriate anti-cancer drugs are used to precisely hit cancer cells, thereby killing tumor cells and preventing continued tumor growth[12]. The targeted anti-cancer drug can specifically select the carcinogenic site and combine with it to produce an effect in vivo, inducing apoptosis of tumor cells, but does not affect the normal tissue cells around the tumor; thus, it will not damage the normal cells of the body[13]. However, a single targeted therapy does have some limitations. Cancer cells can become resistant; the targets themselves change due to mutation, so that targeted therapy does not interact well with them[14]. Similarly, after long-term immunotherapy, tumor cells may undergo further variation, thereby evading the inhibition effect of immunotherapy. In addition, targeted therapies can also modulate immune responses. By analyzing the advantages and disadvantages of targeted drugs and immunotherapy, we have realized that these two therapies may have complementary effects in cancer treatment, and the combination of the two may increase the therapeutic effect[15]. In the present case, The resected tumor tissue was sent for immunohistochemical analysis and targeted next generation sequencing. However, only the RET gene was mutated. Treatment with Immunologicals may not have a significant therapeutic effect. So we used Cabozantinib. At that time, Nivolumab was relatively mature, more experiments were performed in other cancers, the safety was high, and furthermore, the subjective willingness of patients was strong, and the drugs were purchased and used on their own. the patient was treated with cabozantinib and nivolumab, and clinical benefit was seen over 30 mo. At present, the patient is alive and regularly followed up. In this patient, it was confirmed that immunotherapy and/or targeted therapy against PD-1 may be an effective treatment strategy for ATC, which can prolong survival of patients with ATC and may provide more treatment possibilities. Of course, there are still some issues to be resolved. While immunotherapy can relieve the disease and improve the patient survival, it will also cause immune-related adverse reactions. Therefore, to select a more appropriate treatment strategy according to individualized risk and benefit is important. However, it is expected that the survival rate of ATC can be improved by immune combined targeted therapy.
In this case report, it was confirmed that immunotherapy and/or targeted therapy against PD-1 may be an effective treatment strategy for ATC, which can prolong the survival of patients with this disease and provide more treatment possibilities. Finally, it is hoped that some treatment options can be better improved through immune combined targeted therapy to improve the survival rate of ATC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arias Ron D, Spain S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Huang LC, Tam KW, Liu WN, Lin CY, Hsu KW, Hsieh WS, Chi WM, Lee AW, Yang JM, Lin CL, Lee CH. CRISPR/Cas9 Genome Editing of Epidermal Growth Factor Receptor Sufficiently Abolished Oncogenicity in Anaplastic Thyroid Cancer. Dis Markers. 2018;2018:3835783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Capdevila J, Wirth LJ, Ernst T, Ponce Aix S, Lin CC, Ramlau R, Butler MO, Delord JP, Gelderblom H, Ascierto PA, Fasolo A, Führer D, Hütter-Krönke ML, Forde PM, Wrona A, Santoro A, Sadow PM, Szpakowski S, Wu H, Bostel G, Faris J, Cameron S, Varga A, Taylor M. PD-1 Blockade in Anaplastic Thyroid Carcinoma. J Clin Oncol. 2020;38:2620-2627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 213] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 3. | Molinaro E, Romei C, Biagini A, Sabini E, Agate L, Mazzeo S, Materazzi G, Sellari-Franceschini S, Ribechini A, Torregrossa L, Basolo F, Vitti P, Elisei R. Anaplastic thyroid carcinoma: from clinicopathology to genetics and advanced therapies. Nat Rev Endocrinol. 2017;13:644-660. [PubMed] |
| 4. | Bible KC, Kebebew E, Brierley J, Brito JP, Cabanillas ME, Clark TJ Jr, Di Cristofano A, Foote R, Giordano T, Kasperbauer J, Newbold K, Nikiforov YE, Randolph G, Rosenthal MS, Sawka AM, Shah M, Shaha A, Smallridge R, Wong-Clark CK. 2021 American Thyroid Association Guidelines for Management of Patients with Anaplastic Thyroid Cancer. Thyroid. 2021;31:337-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 383] [Article Influence: 95.8] [Reference Citation Analysis (0)] |
| 5. | Liao Y, Gao Y, Chang A, Li Z, Wang H, Cao J, Gu W, Tang R. Melatonin synergizes BRAF-targeting agent dabrafenib for the treatment of anaplastic thyroid cancer by inhibiting AKT/hTERT signalling. J Cell Mol Med. 2020;24:12119-12130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Tiedje V, Stuschke M, Weber F, Dralle H, Moss L, Führer D. Anaplastic thyroid carcinoma: review of treatment protocols. Endocr Relat Cancer. 2018;25:R153-R161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 7. | Abe I, Lam AK. Anaplastic Thyroid Carcinoma: Current Issues in Genomics and Therapeutics. Curr Oncol Rep. 2021;23:31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 8. | Ruste V, Goldschmidt V, Laparra A, Messayke S, Danlos FX, Romano-Martin P, Champiat S, Voisin AL, Baldini C, Massard C, Laghouati S, Marabelle A, Lambotte O, Michot JM. The determinants of very severe immune-related adverse events associated with immune checkpoint inhibitors: A prospective study of the French REISAMIC registry. Eur J Cancer. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 9. | Brauckhoff K, Biermann M. Multimodal imaging of thyroid cancer. Curr Opin Endocrinol Diabetes Obes. 2020;27:335-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Gharib H. Fine-needle aspiration biopsy of thyroid nodules: advantages, limitations, and effect. Mayo Clin Proc. 1994;69:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 302] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 11. | Roither B, Oostenbrink C, Schreiner W. Molecular dynamics of the immune checkpoint programmed cell death protein I, PD-1: conformational changes of the BC-loop upon binding of the ligand PD-L1 and the monoclonal antibody nivolumab. BMC Bioinformatics. 2020;21:557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Sawyers C. Targeted cancer therapy. Nature. 2004;432:294-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 806] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 13. | Albinali KE, Zagho MM, Deng Y, Elzatahry AA. A perspective on magnetic core-shell carriers for responsive and targeted drug delivery systems. Int J Nanomedicine. 2019;14:1707-1723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 14. | Butler EB, Zhao Y, Muñoz-Pinedo C, Lu J, Tan M. Stalling the engine of resistance: targeting cancer metabolism to overcome therapeutic resistance. Cancer Res. 2013;73:2709-2717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 15. | Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1085] [Cited by in RCA: 1146] [Article Influence: 88.2] [Reference Citation Analysis (0)] |