Published online Apr 26, 2022. doi: 10.12998/wjcc.v10.i12.3842
Peer-review started: July 25, 2021
First decision: December 10, 2021
Revised: December 10, 2021
Accepted: March 4, 2022
Article in press: March 4, 2022
Published online: April 26, 2022
Processing time: 270 Days and 1 Hours
Kimura’s disease is an inflammatory disease that is usually found in the deep lymph nodes of the head and neck. While rare, It is most frequently seen in young men. The oral cavity and salivary glands may also be involved. There are no reports on tumor occurring in soft palate. We have encountered a case of Kimura’s disease in the soft palate of an elderly woman.
A 63-year-old elderly Chinese woman with a slowly growing mass in the upper jaw was referred to our service. A biopsy to the mass was taken after the patient was referred to our service. The tumor was diagnosed as benign. We performed cervical lymph node puncture and partial surgical excision of the lesion. The tumor, which showed signs of marked follicular hyperplasia with follicles surrounded by eosinophils and lymphocytes, was located within the soft palate. Kimura’s disease was diagnosed after histopathologic examination of the resected tissue. The etiology of Kimura’s disease is not fully understood. One current model includes T-cells involvement with cytokines also playing a role. The patient was without evidence for recurrence of partially resected area 6 mo later. This report shows that Kimura’s disease is not limited to the head, neck, and salivary gland lymph nodes. We present a case of a tumor in soft palate. This location adds another possible site for consideration during the differential diagnoses of a slowly growing mass.
The present case illustrates a characteristic description of Kimura’s disease. This case highlights the main differences between Kimura’s disease and angiolym
Core Tip: Kimura’s disease is an inflammatory disease mainly seen in young men and is most often found in the deep lymph nodes of the head and neck. We report a unique case of Kimura’s disease located in the soft palate of an old woman.
- Citation: Li W. Kimura's disease in soft palate with clinical and histopathological presentation: A case report. World J Clin Cases 2022; 10(12): 3842-3848
- URL: https://www.wjgnet.com/2307-8960/full/v10/i12/3842.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i12.3842
Kimura’s disease affects the deep cervical tissues with benign inflammation and involves the head, neck, and salivary gland lymph nodes. The oral cavity is sometimes involved. There are no reports on tumors occurring in the soft palate. This condition was first reported by Kim and Szeto[1] in 1937 and further characterized in 1948 by Kimura[2]. Young Asian men (especially in China and Japan) are most commonly affected and the peak age of onset is in the 30s[3]. The most common laboratory findings are elevated serum immunoglobulin E (IgE) levels accompanied by eosinophilia[3,4]. In this report we review a rare case of Kimura’s disease in the upper jaw in an elderly woman.
The case in our report was a 63-year-old woman farmer of the Han race who is 1.6-m in height and 40-kg in weight.
The patient was admitted to our hospital with a growing mass in her upper jaw.
The tumor developed over a period of 4 mo and was still growing slowly. The patient did not present with any pain or any sensory loss in the affected area. She did report discomfort in the upper jaw while eating, which impaired her diet. She denied trauma at the site.
Her medical history revealed chronic nephritis, bilateral submandibular lymphadenectomy and weight loss. No other comorbidities or relevant diseases were observed in her family.
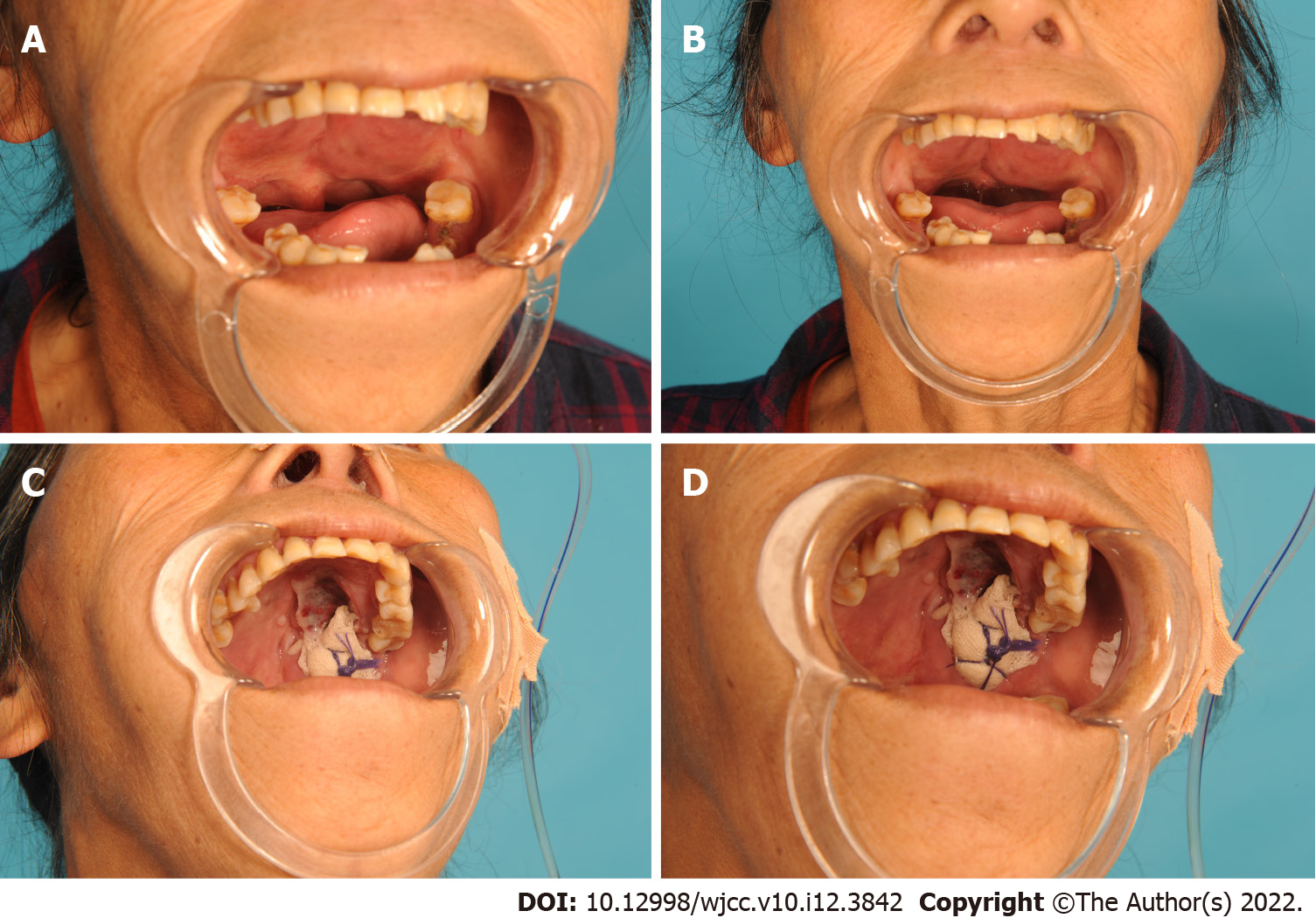
Physical examination discovered a red, intact mass that involved nearly the entire soft palate. The tumor exhibited bilateral symmetry in the upper jaw (Figure 1A and B). The patient’s oral hygiene was poor, with missing teeth in regions 31-32, 35-36, 38, 45-46, and 48.
The absolute value of eosinophils was 2.94 × 109/L, and the percentage of eosinophils was 39.50%. Blood tests indicated elevated peripheral blood eosinophilia. Renal function was normal without eosinophilia.
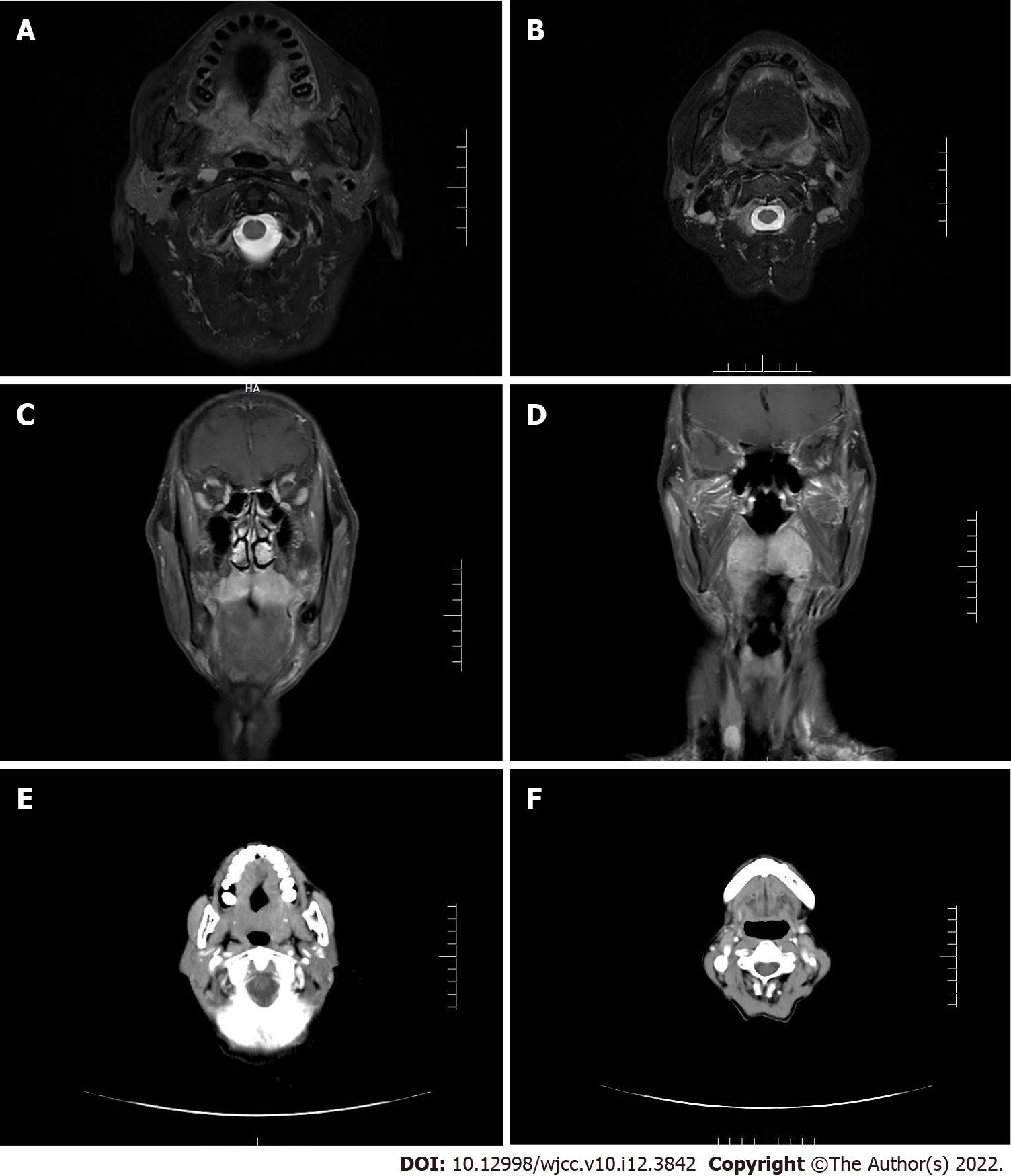
A magnetic resonance imaging (MRI) scan revealed a tumor in the upper jaw with bilateral symmetry and a size of 5 cm × 2 cm (Figure 2A-D). The soft palate was enlarged and the palatine tonsils exhibited swelling to the third degree. The tumor had a high retention of contrast agent although it did not appear to be a hemangioma. The tumor mainly infiltrated the soft tissue, without osseous destruction. There were enlarged cervical lymph nodes on both sides with multiple nodes between 1 and 2 cm in diameter. They were identifiable and symmetrical without suspicion of metastasis. The findings from radiology are consistent with a malignant lymphoma or sarcoma. A subsequent computed tomography (CT) was used to visualize the lesion (Figure 2E and F). Following contrast agent administration, the tumor was not enhanced compared to the adjacent tissues and it appeared hypodense. The cervical vessels appeared normal and had no obvious connection with the tumor. The lymph nodes failed to show characteristics of metastases. The CT results were consistent with a sarcoma or with a malignant lymphoma. Further examination of the patient failed to find evidence of any distant metastatic sites.
A biopsy of the mass was performed after the patient was referred to our group, and the lesion was diagnosed as a benign tumor. We recommended cervical lymph node puncture and partial surgical excision of the lesion. Cervical lymph node biopsy by puncture showed visible lymphocytes. With the help of the Davis' opener, we removed part of the tumor located in the left soft palate. After complete hemostasis, the wound was packed with gauze and wrapped under pressure (Figure 1C and D). Multiple biopsies were performed during surgery, and examined after immediate sectioning.
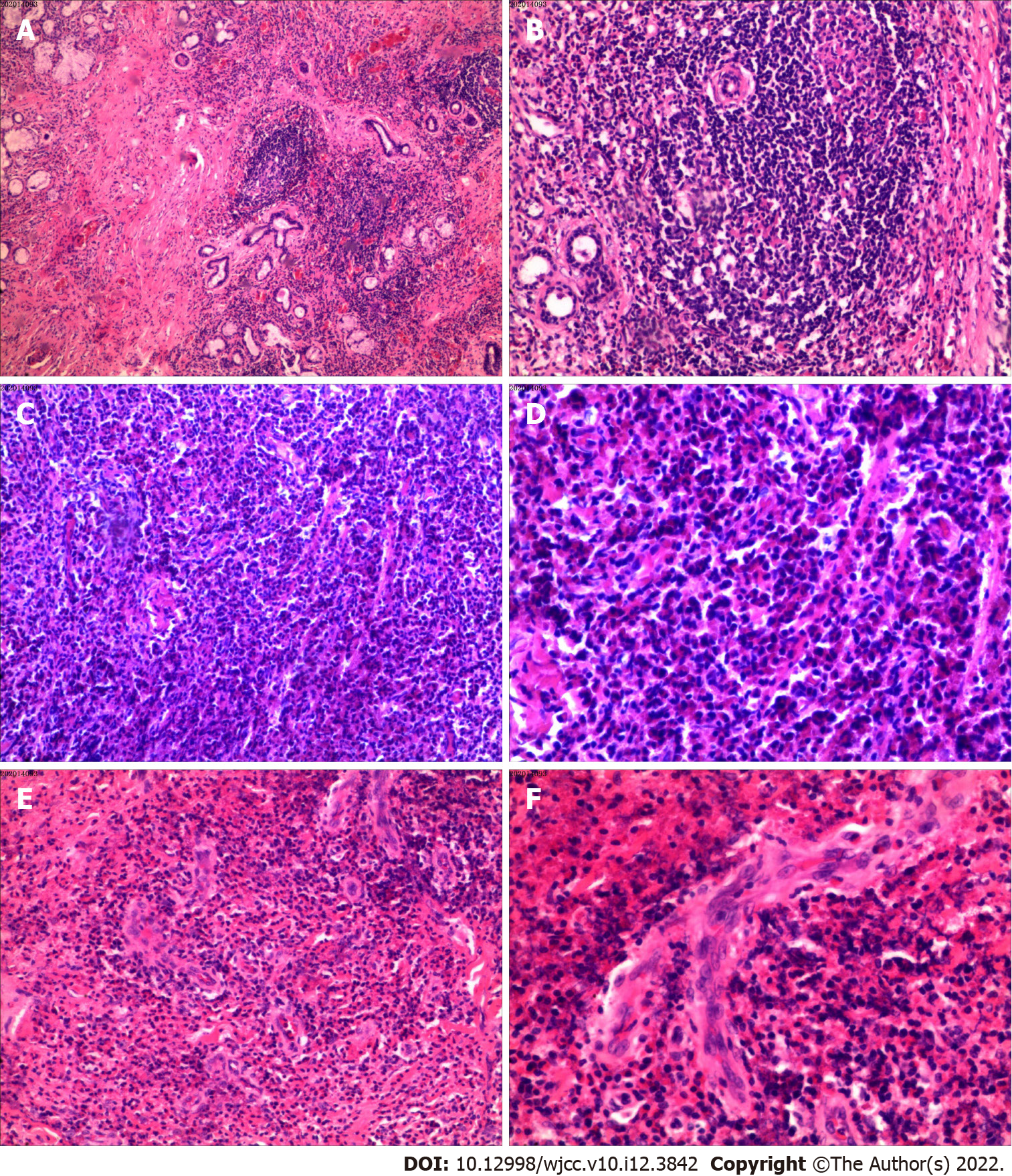
The results of histology and immunohistochemistry were consistent with angiomatosis with an inflammatory pseudotumor and many eosinophil cells. We found no evidence of malignancy. Final histopathologic examination diagnosed angiomatosis with inflammatory cells (Figure 3).
The eosinophilic inflammation determined our final diagnosis of Kimura’s disease.
The discomfort of eating was alleviated, and tumor growth was suppressed. We recommend patient follow-up and close observation.
Our patient was under physical examination 6 mo after resection, which resolved without evidence of recurrence of the partially resected area.
Kim and Szeto initially described the tumor in 1937 and Kimura’s disease was more widely recognized after the 1948 report of a systematic examination by Kimura[2]. This is a rare disease that can present as angiolymphoid hyperplasia accompanied by lymphadenopathy, elevated serum IgE, and eosinophilia observed in the peripheral blood[5]. Kimura’s disease is usually seen in men of Asian extraction in their third decade of life[6]. Kimura’s disease is rare and little reliable information on the incidence of Kimura’s disease is available. The disease is normally associated with deep subcutaneous tissue or with the patient’s salivary glands[5]. However, our patient had a lesion in the soft palate of the upper jaw. The soft palate can also originate the tumor, although two cases of Kimura’s disease have been reported to arise from the hard palate[5,7]. Other organs that can be affected are the kidney[8], orbital structures[9], and there are reports of axillary or inguinal lymph nodes being affected[3].
High serum IgE levels often a feature of Kimura’s disease[10]. Reports have suggested that normal and moderately elevated levels of IgE may have prognostic significance and help predict the aggressiveness of the tumor[6]. Our patient failed to show elevated serum IgE levels and she was disease free for more than four months after surgery; consistent with low IgE levels associcating with less agressive disease.
Differential diagnosis of this patient required us to consider other lymphatic diseases; angioimmunoblastic T-cell lymphoma, Hodgkin’s lymphoma, parasitic lymphadenitis, Langerhans cell histiocytosis, and, most importantly, angiolymphoid hyperplasia with eosinophilia (ALHE)[3,6]. Kimura’s disease and ALHE are often confused due to both their overlapping clinical and histopathological results. ALHE is more often seen in Western patients in their in the third, fourth, or fifth decades. The small reddish-brown cutaneous nodules of ALHE are more superficial than the lesions seen with Kimura’s disease. The ALHE patients do not present with elevated serum IgE, lymphadenopathy, nor do they have eosinophilia[11]. The basic biological difference is that ALHE is a blood vessel neoplasm while Kimura’s disease is a chronic inflammatory disease[3,12]. Young Asian men are most likely to develop Kimura’s disease that usually manifests as a single or multiple masses located in the subcutaneous tissue and/or in the salivary glands. The lesion is usually asymptomatic. Regional lymph nodes are often involved and serum eosinophilia and elevated IgE are characteristic of the condition. ALHE, by contrast, is usually seen in middle-aged women and presents as multiple small papules or nodules with erythematous and itching. The nephrotic syndromes like membranous glomerulonephritis and mesangioproliferative are more closely related to Kimura’s disease than they are to ALHE. In addition, systemic manifestations, like eosinophilia found with Kimura’s disease, are rarely found in patients with ALHE[13].
While the etiology of Kimura’s disease is still unclear, one hypothesis includes immune system pathogenesis with both Th2 and Tc1 cells involved. There are two recent publications that have reported multiple T-cell alterations that are seen with Kimura’s disease[3,14]. Other researchers[15,16] indicate that certain cytokines play a predominant role as well[17]. Future high-quality and sufficient sample studies should further evaluate these findings. ALHE’s pathogenesis also remains unknown. Some investigators have hypothesized that the damage results from the vascular tumor. Others speculate that they might represent a reaction to injury of vascular tissue; for example, trauma to the skin, persistent viral infection such as with a human T-lymphotropic virus or herpes virus 8, or a hormonal imbalance[18].
Kimura’s disease histology is characterized follicular hyperplasia that includes follicles surrounded by large numbers of eosinophils, lymphocytes, and mast cells. The lymphoid follicles are themselves hyperplastic and they include conspicuous germinal centers. We also observed the presence of fibrosis and sclerosis and the presence of vascular proliferation (Figure 3), consistent with a previous report[19]. By contrast, ALHE histology includes both abnormal vascular proliferation with diffuse lymphocyte infiltration and eosinophils. The abnormal vasculature is composed of capillaries that are clustered around atypical arterial or venous vessels that are dilated and have a protruded endothelium and exhibit rounded and occasionally angular nuclei. The endothelial cells can contain one or multiple cytoplasmic vacuoles. Lymphoid follicles are rare or absent in most cases. Histological examination found a diffuse inflammatory infiltrate with profuse vascular proliferation, endothelial cells with noticeable vacuoles, an eosinophil infiltrate, and predominant vascular processes. Kimura’s disease has different histological characteristics, with lymphoid follicles and with a greater average number of eosinophils and ALHE does not show cytoplasmic vacuoles[20]. While the literature comparing Kimura’s disease with ALHE is inconclusive, the clinical, epidemiological, and histological characteristic from the present case, and data from relevant publications, show that there are many differences between the two diseases.
The present case illustrates a characteristic description of Kimura’s disease and expands the phenotypic spectrum of the rare disease. The findings in our report also highlight the main differences between Kimura’s disease and angiolymphoid hyperplasia with eosinophilia.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Dentistry, oral surgery and medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kotelevets SM, Russia; Nasiri A, Saudi Arabia; Omiyale AO, United Kingdom S-Editor: Ma YJ L-Editor: Filipodia CL P-Editor: Ma YJ
| 1. | Kim HT, Szeto C. Eosinophilic hyperplastic lymphogranuloma, comparison with Mikulicz’s disease. Chin Med J. 1937;23:699-700. |
| 2. | Kimura T, Yoshimura S, Ishikawa E. On the unusual granulation combined with hyperplastic changes of lymphatic tissue. Trans Soc Pathol Jpn. 1948;37:179-180. |
| 3. | Abuel-Haija M, Hurford MT. Kimura disease. Arch Pathol Lab Med. 2007;131:650-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Chim CS, Shek Wh, Liang R, Kowng YL. Kimura's disease: no evidence of clonality. Br J Ophthalmol. 1999;83:880-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Helander SD, Peters MS, Kuo TT, Su WP. Kimura's disease and angiolymphoid hyperplasia with eosinophilia: new observations from immunohistochemical studies of lymphocyte markers, endothelial antigens, and granulocyte proteins. J Cutan Pathol. 1995;22:319-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Iwai H, Nakae K, Ikeda K, Ogura M, Miyamoto M, Omae M, Kaneko T, Yamashita T. Kimura disease: diagnosis and prognostic factors. Otolaryngol Head Neck Surg. 2007;137:306-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Terakado N, Sasaki A, Takebayashi T, Matsumura T, Kojou T. A case of Kimura's disease of the hard palate. Int J Oral Maxillofac Surg. 2002;31:222-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Müller-Richter UD, Moralis A, Reuther T, Kochel M, Reichert TE, Driemel O. Kimura's disease in a white man. Head Neck. 2011;33:138-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Dixit MP, Scott KM, Bracamonte E, Dixit NM, Schumacher MJ, Hutter J, Nagle R. Kimura disease with advanced renal damage with anti-tubular basement membrane antibody. Pediatr Nephrol. 2004;19:1404-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Prabhakaran VC, Sachdev A, Cheung D, Fletcher A, Brown LJ, Sampath R. Kimura disease of the eyelid: a clinicopathologic study with electron microscopic observations. Ophthalmic Plast Reconstr Surg. 2006;22:495-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Chen H, Thompson LD, Aguilera NS, Abbondanzo SL. Kimura disease: a clinicopathologic study of 21 cases. Am J Surg Pathol. 2004;28:505-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 197] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 12. | Armstrong WB, Allison G, Pena F, Kim JK. Kimura’s disease: two case reports and a literature review. Ann Otol Rhinol Laryngol. 1998;107:1066-1071. |
| 13. | Don DM, Ishiyama A, Johnstone AK, Fu YS, Abemayor E. Angiolymphoid hyperplasia with eosinophilia and vascular tumors of the head and neck. Am J Otolaryngol. 1996;17:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Esteves P, Barbalho M, Lima T, Quintella L, Niemeyer-Corbellini JP, Ramos-ESilva M. Angiolymphoid hyperplasia with eosinophilia: A Case Report. Case Rep Dermatol. 2015;7:113-116. |
| 15. | Chim CS, Fung A, Shek TW, Liang R, Ho WK, Kwong YL. Analysis of Clonality in Kimura's Disease. Am J Surg Pathol. 2002;26:1083-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Chen QL, Li CX, Shao B, Gong ZC, Liu H, Ling B, Abasi K, Hu LL, Wang B, Yin XP. Expression of the interleukin-21 and phosphorylated extracellular signal regulated kinase 1/2 in Kimura disease. J Clin Pathol. 2017;70:684-689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Motegi S, Hattori M, Shimizu A, Abe M, Ishikawa O. Elevated serum levels of TARC/CCL17, Eotaxin-3/CCL26 and VEGF in a patient with Kimura's disease and prurigo-like eruption. Acta Derm Venereol. 2014;94:112-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 18. | Hosoki K, Hirayama M, Kephart GM, Kita H, Nagao M, Uchizono H, Toyoda H, Senba Y, Imai Y, Komada Y, Ihara T, Fujisawa T. Elevated numbers of cells producing interleukin-5 and interleukin-10 in a boy with Kimura disease. Int Arch Allergy Immunol. 2012;158 Suppl 1:70-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Reddy PK, Prasad AL, Sumathy TK, Shivaswamy KN, Ranganathan C. An overlap of angiolymphoid hyperplasia with eosinophilia and Kimura’s disease: successful treatment of skin lesion with cryotherapy. Indian J Dermatol. 2015;60:216. [PubMed] |
| 20. | Chong WS, Thomas A, Goh CL. Kimura's disease and angiolymphoid hyperplasia with eosinophilia: two disease entities in the same patient: case report and review of the literature. Int J Dermatol. 2006;45:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |