Published online Apr 26, 2022. doi: 10.12998/wjcc.v10.i12.3834
Peer-review started: July 23, 2021
First decision: October 15, 2021
Revised: October 25, 2021
Accepted: March 4, 2022
Article in press: March 4, 2022
Published online: April 26, 2022
Processing time: 272 Days and 4.3 Hours
Use of liver allograft with hepatic hemangioma after in vivo resection of hemangioma in living donor liver transplantation (LDLT) has been previously reported. However, there are few reports describing ex vivo backtable resection of hemangioma from liver allografts in LDLT.
A 55-year-old male was evaluated as a donor for an 8-month-year old patient with acute hepatic failure due to biliary atresia. Pre-operative contrast enhanced computed tomography revealed a 9 cm hemangioma in segment 4 with vascular variations in the donor. During LDLT, an intra-operative intrahepatic cholangiography was performed to ensure no variation in the anatomy of the intrahepatic bile duct. After intra-operative pathological diagnosis, ex vivo backtable resection of the hemangioma was performed and the liver allograft was transplanted into the recipient. The donor’s and recipient’s post-operative course were uneventful. At the 2-year follow-up, the liver allograft showed good regeneration without any recurrence of hemangioma.
Liver allografts with hemangiomas are an acceptable alternative strategy for LDLT. Ex vivo backtable resection of hemangioma from the donor liver during pediatric LDLT is safe and feasible, and can effectively reduce the operative time and intra-operative bleeding for the donor.
Core Tip: It is of great significance to expand the liver donor pool due to the shortage of donor livers. In this paper, we describe how a discard left lobe of the liver with hemangioma after hepatectomy was fixed backtable to meet the criteria for transplantation. Subsequently, a successful liver transplantation was performed for a 2-year-old child with congenital biliary atresia by using this liver with satisfactory outcome. Two years of follow-up showed that the child recovered well with no significant complications.
- Citation: Li SX, Tang HN, Lv GY, Chen X. Pediatric living donor liver transplantation using liver allograft after ex vivo backtable resection of hemangioma: A case report. World J Clin Cases 2022; 10(12): 3834-3841
- URL: https://www.wjgnet.com/2307-8960/full/v10/i12/3834.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i12.3834
Liver transplantation has brought a paradigm shift in the management and outcomes of pediatric patients with liver failure. However, due to the shortage of donor livers, many children on organ transplant waiting lists die[1]. Use of marginal liver allografts to expand the donor pool can help reduce the waiting time[2]. One type of liver allograft from marginal donors includes the use of livers with benign tumors, such as hepatic hemangioma. Hepatic hemangiomas usually remain asymptomatic[3] and have a benign course[4]. According to previous reports, liver allograft with hepatic hemangioma or after resection of hemangioma can be safely transplanted[5-10]. Sanada et al[11] described the use of a living donor liver allograft after in vivo hemangioma resection. This case report indicates that the liver allograft can be safely used for liver transplantation after removal of the hemangioma. In pediatric patients, especially infants, a small liver allograft is required for liver transplantation. Thus, we proposed that we could use liver segments from patients with symptomatic hemangioma undergoing hepatectomy after backtable resection of the hemangioma for pediatric liver transplantation.
Herein, we describe the first case of pediatric living donor liver transplantation (LDLT) using a liver allograft following backtable resection of hemangioma.
An 8-month-old female infant presented to the emergency department with jaundice and high-grade fever. At the same time, a 55-year-old male was admitted at our center due to discomfort in the right upper abdomen.
The pediatric patient had jaundice with high fever for 3 days. The jaundice subsided after the Kasai operation and gradually worsened over a month, and by the time she was admitted to the hospital, she had altered sensorium.
The adult patient had discomfort in the right upper abdomen for one month. The upper abdominal discomfort was aggravated by heavy meals and relieved by fasting. He denied any recent fever, jaundice, allergy, chills, or changes in bowel habits.
The pediatric patient had undergone Kasai portoenterostomy at the age of one month. The adult patient denied any past illness.
They both did not have any addictions or any significant family history.
On clinical examination, there was a 6-cm postoperative scar in the abdomen due to previous Kasai portoenterostomy of the pediatric patient. The adult patient’s abdominal examination was unremarkable with no organomegaly.
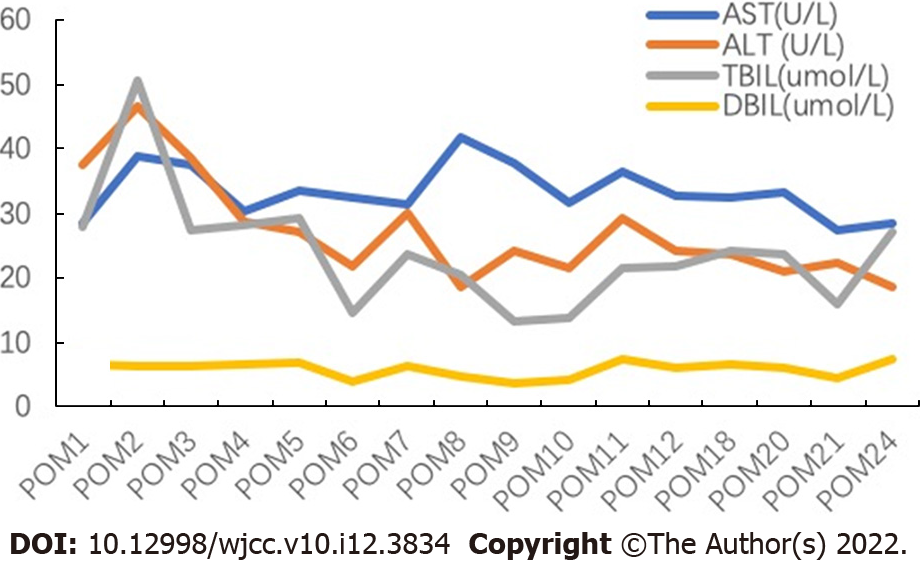
The pediatric patient’s liver function tests at admission were as follows: Serum total bilirubin = 120.5 μmol/L, direct bilirubin = 78.8 μmol/L, international normalized ratio = 1.37, aspartate aminotransferase = 153.2 U/L, and alanine aminotransferase = 119.9 U/L. Pre-operative liver functions of the adult patient were within the normal range and there was no evidence of coagulopathy.
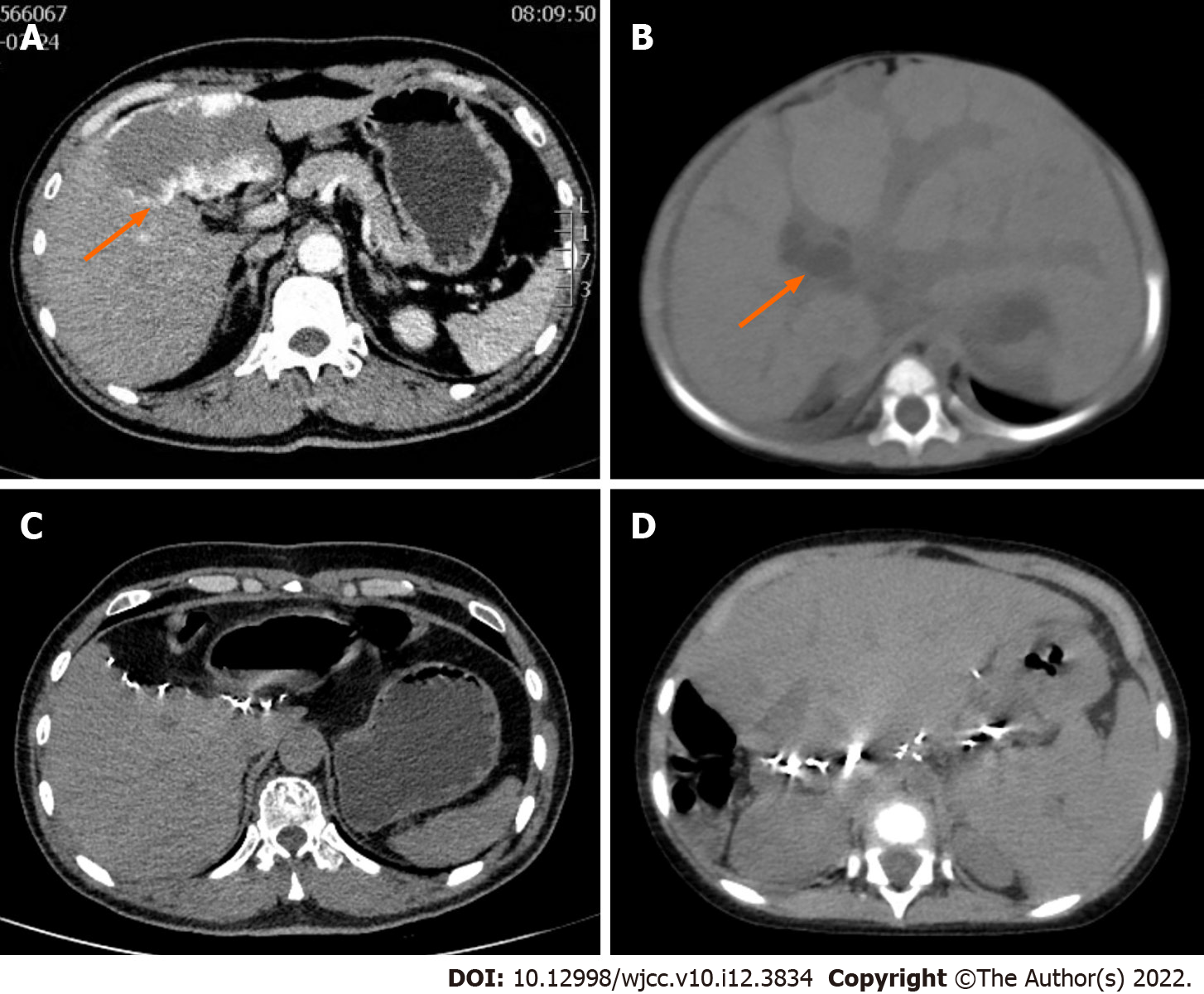
The CT of the pediatric patient showed dilated intrahepatic biliary system and diffuse hepatomegaly (Figure 1). Triple phase contrast enhanced computed tomography of the abdomen of the adult revealed a 9.0 cm × 5.8 cm hemangioma in segment 4 of the liver (Figure 1).
On the second day of hospitalization, the pediatric patient was diagnosed to have Child-Pugh grade C hepatic failure due to congenital biliary atresia with grade 2 hepatic encephalopathy. The adult was diagnosed as hemangioma in segment 4 of the liver.
The pediatric patient was listed for emergency liver transplantation. There was a strong possibility that the adult patient would develop ischemic necrosis of segments 2 and 3 if simple enucleation of the hemangioma was performed. We therefore performed a left hepatic lobectomy[6]. Since the liver parenchyma was normal and soft in consistency, we considered using segments 2 and 3 after excising the hemangioma of the resected left lobe as an allograft for the pediatric patient with acute liver failure presented above. After consulting the adult patient and his family, we obtained informed consent to donate the left hepatic lobe as an allograft. The therapeutic decision was approved by the Ethical Committee of the First Hospital of Jilin University.
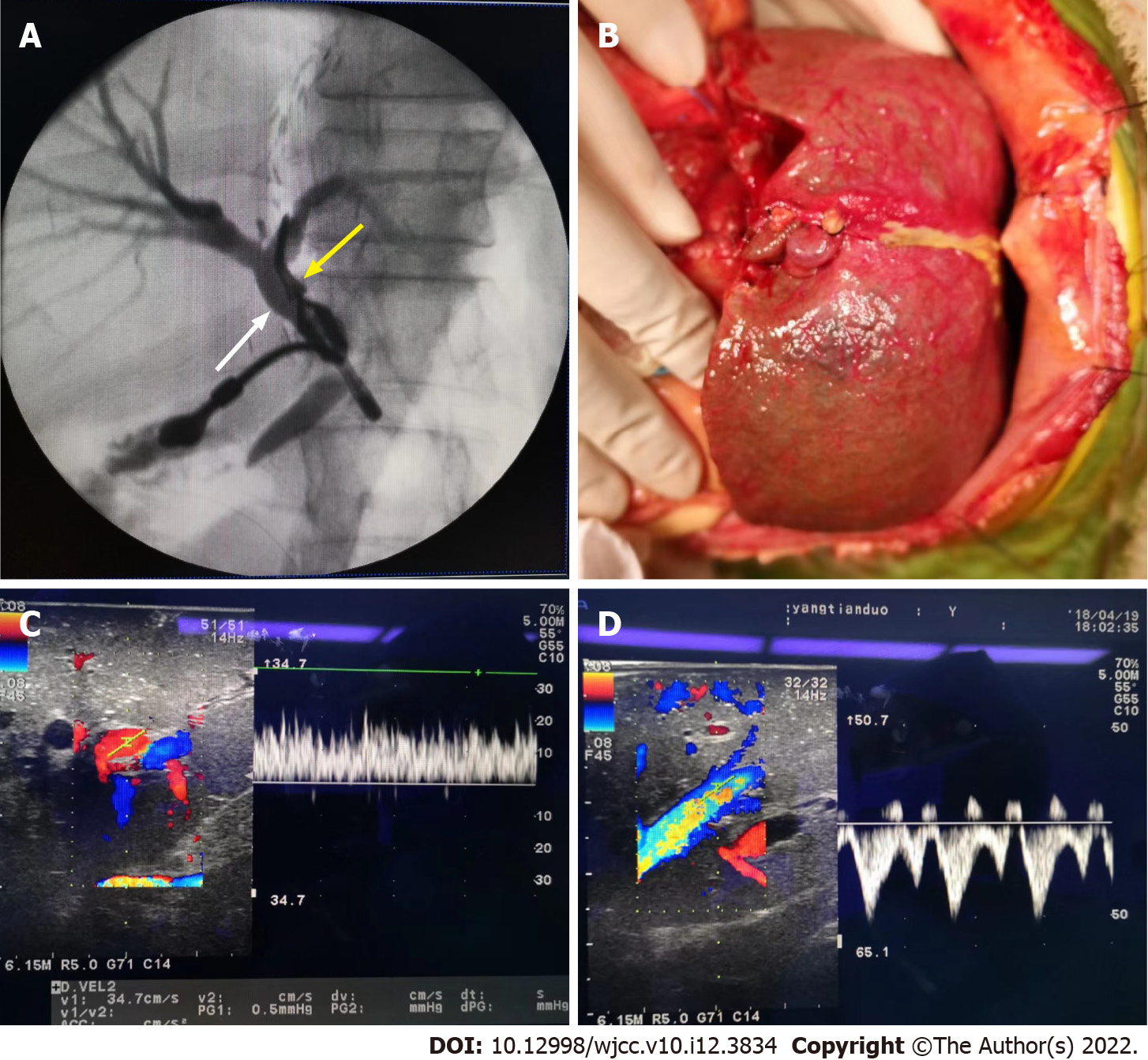
The left hepatic lobe was resected using the standard technique described previously[12]. We found a 9 cm diameter mass in the left hepatic lobe. Intra-operative ultrasonography and pathological examination confirmed the diagnosis of hemangioma. Intrahepatic cholangiography was conducted, and no intrahepatic bile duct anomaly was detected (Figure 2). After harvest of the left hepatic lobe, ex vivo resection of segment 4 of the graft and hepatic vein reconstruction was performed (Figure 3). The left hepatic vein of the donor liver and the left lateral marginal vein of the left lobe were opened and reconstructed to obtain a width of approximately 2.5 cm in order to avoid venous outflow obstruction[13]. The cold ischemic time was 4 h 17 minutes, and the estimated blood loss during the donor operation was 210 mL.
The liver allograft and recipient weighed 190 g and 8.7 kg, respectively, with a graft-to-recipient weight ratio (GRWR) of 2.1%. The liver allograft was implanted into the recipient using a piggyback orthotopic liver transplant procedure. Intra-operatively, the native liver was cirrhotic, 12 cm × 10 cm × 8 cm in size, yellow-green in color, and firm in consistency. There were multiple nodules of different sizes on the liver surface. There were no palpable emboli in the main portal vein and no obvious masses in the abdominal organs. The liver allograft was placed on the right side in the abdominal cavity of the recipient (Figure 2). First, the inferior vena cava of the recipient was anastomosed to the left hepatic vein of the donor liver by continuous suture in an inverted triangle pattern. Then, the donor and recipient portal veins were anastomosed with continuous valgus suture followed by anastomosis between the left hepatic artery of the donor and the recipient. After reperfusion, there was no bleeding from the resection site. Roux-en-Y hepaticojejunostomy was performed by anastomosing the left hepatic duct of the donor liver to the recipient Roux-en-Y jejunal limb in an end-to-side fashion under magnification. Intra-operative ultrasound revealed satisfactory blood flow of the liver allograft and no constriction of the hepatic vein (Figure 2). The operation time was 8 h, the warm ischemic time was 35 min, and the estimated blood loss was 150 mL.
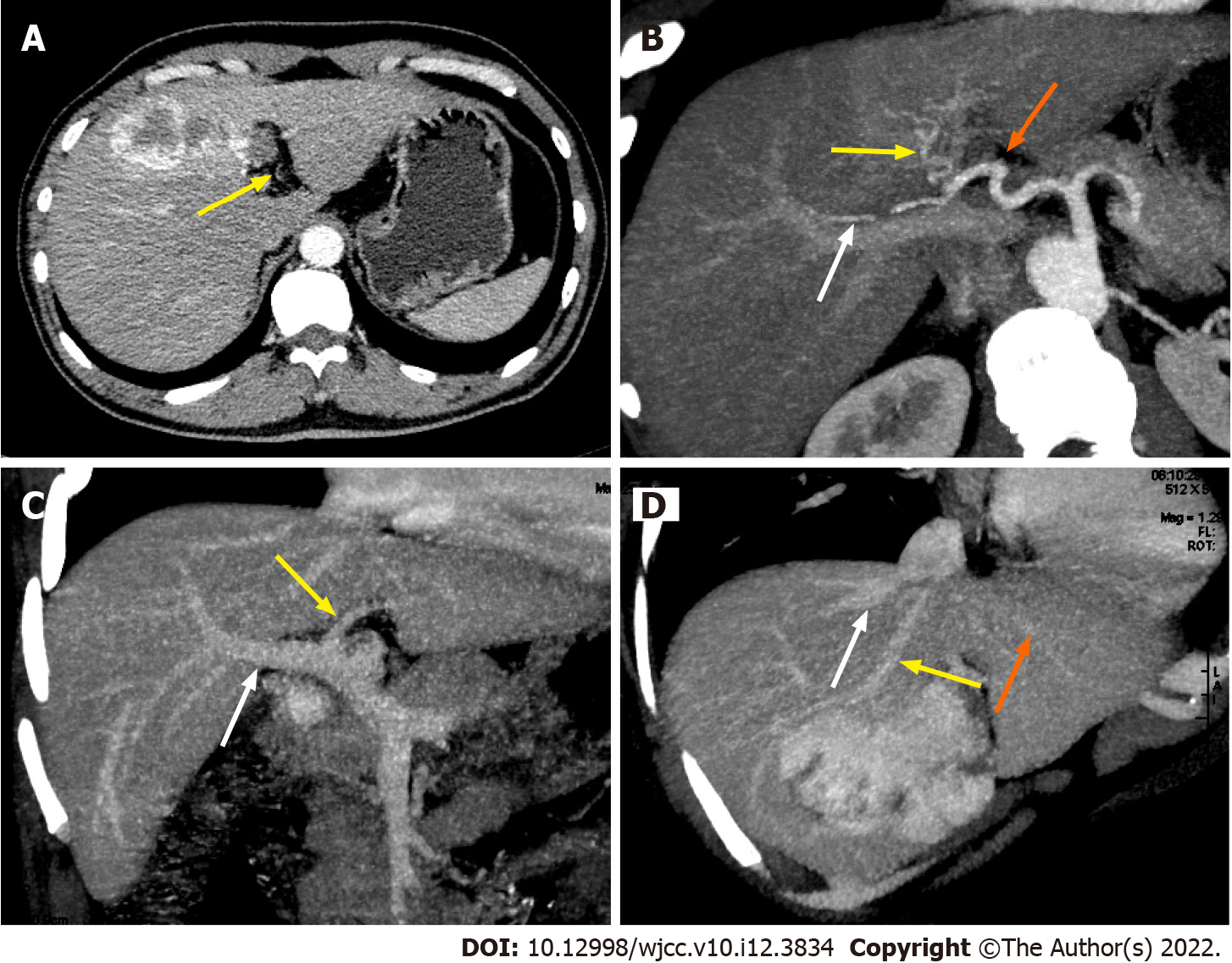
The post-operative course of the donor and the recipient was uneventful, and both were discharged from the hospital on post-operative days 12 and 35, respectively. Tacrolimus was used as an immunosuppressant up to two years after surgery. The follow-up of the recipient two years after the liver transplantation showed good liver function without any bile duct strictures (Figure 4). Abdominal CT of the donor at six months after surgery (Figure 1C) and that of the recipient at two years after surgery (Figure 1D) showed good regeneration of the liver without any recurrence of hemangioma.
Liver transplantation is the most effective treatment for end-stage liver disease[14]. The reported 1-year and 5-year survival rates are more than 90% and 70%, respectively[15]. However, organ scarcity is still the greatest limitation for patients in need of liver transplantation. Therefore, marginal liver allograft in liver transplantation, particularly in cases of benign tumors, has become an accepted alternative[16]. Use of liver allograft with hemangioma for liver transplantation has been previously reported[5-10]. In some cases, hemangiomas were not resected due to the risk of small-for-size syndrome, and follow-up of such cases showed that the volume of hepatic hemangioma decreased and the normal parenchymal volume increased with time, without the appearance of new hemangiomas. However, long-term follow-up of such cases has not been carried out. However, a small volume of liver allograft after hemangioma excision is sufficient for pediatric liver transplant and it can effectively avoid the possibility of lethal changes[11]. Thus, we propose that for pediatric patients, large hemangiomas in the liver allograft should be resected prior to transplantation.
Two surgical procedures for the resection of hemangiomas can be adopted, namely in vivo resection and backable resection during LDLT. In previous reports, in vivo resection was performed and found to be feasible, as it avoided intra-operative bleeding, bile leakage, and limited cold ischemic time compared to backtable resection[11]. However, in vivo resection prolongs the operative time and increases the risk of bleeding in donors, which can be potentially harmful. Backtable resection of hemangioma has only been reported in deceased donor livers[10,17]. With the advancements in liver transplantation techniques, complications of backtable liver resection, such as intra-operative bleeding, bile leakage, and prolonged cold ischemic time, can successfully be avoided[18]. Some of the important points for backtable resection include the use of an electric knife, electric bipolar, or ultrasonic knife during resection, and closure of all the orifices at the cut surface using hemo-lok clips and/or sutures. In the case presented here, we performed backtable resection, as in vivo resection of segment IV hemangioma was technically difficult and could have compromised donor safety.
Anatomical lobectomy is one of the most common operative approaches for the treatment of liver hemangioma[19]. After appropriate pre-operative evaluation, selected livers after hepatic lobectomy can be used for pediatric liver transplantation after the backtable resection of hemangioma and reconstruction of the liver allograft, as shown in the case presented here. This offers a novel strategy for enlarging the donor pool. Sanada et al[11] proposed a strategy to use liver allograft with hemangiomas in pediatric LDLT. If the estimated graft liver volume to standard liver volume (GV/SLV) ratio after the tumor resection is more than 40%, then the remnant liver after resection of the hemangioma can be used for transplantation. GRWR is also an important factor for pediatric LDLT, which should be more than 0.8%[20,21]. In the present case, the GV/SLV ratio was 76.84% and GRWR was 2.1%, which were safe and sufficient indicators for LDLT. In the follow-up period, the patient recovered well without any complications with good liver function at two years after the operation.
Liver allografts with hemangiomas can be used in LDLT. Ex vivo backtable resection of hemangioma during pediatric LDLT is a safe and feasible alternative to in vivo resection. Moreover, backtable resection can effectively shorten the operative time of the donor and reduce the risk of intra-operative bleeding during donor operation. Nevertheless, more cases are needed to confirm this method.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chae HB, South Korea S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Leung DH, Narang A, Minard CG, Hiremath G, Goss JA, Shepherd R. A 10-Year united network for organ sharing review of mortality and risk factors in young children awaiting liver transplantation. Liver Transpl. 2016;22:1584-1592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Badawy A, Kaido T, Uemoto S. Current Status of Liver Transplantation Using Marginal Grafts. J Invest Surg. 2020;33:553-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Vagefi PA, Klein I, Gelb B, Hameed B, Moff SL, Simko JP, Fix OK, Eilers H, Feiner JR, Ascher NL, Freise CE, Bass NM. Emergent orthotopic liver transplantation for hemorrhage from a giant cavernous hepatic hemangioma: case report and review. J Gastrointest Surg. 2011;15:209-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Yoon SS, Charny CK, Fong Y, Jarnagin WR, Schwartz LH, Blumgart LH, DeMatteo RP. Diagnosis, management, and outcomes of 115 patients with hepatic hemangioma. J Am Coll Surg. 2003;197:392-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 133] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Onishi Y, Kamei H, Imai H, Kurata N, Hori T, Ogura Y. Successful adult-to-adult living donor liver transplantation using liver allograft after the resection of hemangioma: A suggestive case for a further expansion of living donor pool. Int J Surg Case Rep. 2015;16:166-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Sun B, Mu X, Wang X. Successful adult-to-adult liver transplantation of an otherwise discarded partial liver allograft with a cavernous hemangioma: new strategy for expanding liver donor pool. Transpl Int. 2013;26:e79-e80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Nikeghbalian S, Kazemi K, Salahi H, Bahador A, Davari HR, Jalaeian H, Rasekhi AR, Nejatollahi SM, Gholami S, Malek-Hosseini SA. Transplantation of a cadaveric liver allograft with right lobe cavernous hemangioma, without back-table resection: a case report. Transplant Proc. 2007;39:1691-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Aucejo FN, Ortiz WA, Kelly D, Winans C, Vogt D, Eghtesad B, Fung JJ, Miller CM. Expanding the donor pool: safe transplantation of a cadaveric liver allograft with a 10 cm cavernous hemangioma--a case report. Liver Transpl. 2006;12:687-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Pacheco-Moreira LF, Enne M, Balbi E, Santalucia G, Martinho JM. Hemangioma at the liver section plane. Is it a contraindication for living donor liver transplantation? Surgery. 2005;138:113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Onaca N, Mizrahi S, Bar Nathan N, Burstein I, Mor E. Liver transplantation after backtable resection of giant hemangioma. Liver Transpl. 2005;11:851-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Sanada Y, Mizuta K, Urahashi T, Umehara M, Wakiya T, Okada N, Egami S, Hishikawa S, Fujiwara T, Sakuma Y, Hyodo M, Yasuda Y. Pediatric living donor liver transplantation using liver allograft with hemangioma. Ann Transplant. 2011;16:66-69. [PubMed] |
| 12. | Soejima Y, Shimada M, Suehiro T, Kishikawa K, Minagawa R, Hiroshige S, Ninomiya M, Shiotani S, Harada N, Sugimachi K. Feasibility of duct-to-duct biliary reconstruction in left-lobe adult-living-donor liver transplantation. Transplantation. 2003;75:557-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Fukuda A, Sakamoto S, Sasaki K, Narumoto S, Kitajima T, Hirata Y, Hishiki T, Kasahara M. Modified triangular hepatic vein reconstruction for preventing hepatic venous outflow obstruction in pediatric living donor liver transplantation using left lateral segment grafts. Pediatr Transplant. 2018;22:e13167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Rana A, Gruessner A, Agopian VG, Khalpey Z, Riaz IB, Kaplan B, Halazun KJ, Busuttil RW, Gruessner RW. Survival benefit of solid-organ transplant in the United States. JAMA Surg. 2015;150:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 356] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 15. | Diaz GC, Zerillo J, Singhal A, Hibi T, Vitale A, Levitsky J, Renz JF. Report of the 22nd Annual Congress of the International Liver Transplantation Society. Transplantation. 2017;101:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (1)] |
| 16. | Schmeding M, Sauer I, Hartwig K, Theruvath T, Pratschke J, Neuhaus R, Neuhaus P, Neumann UP. Aging of the liver graft and functional quality in the absence of recurrent disease: a 10 year histological follow-up. Ann Transplant. 2010;15:5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Mor E, Boccagni P, Thung SN, Sheiner PA, Emre S, Guy SR, Schwartz ME, Miller CM. Backtable resection of a giant cavernous hemangioma in a donor liver. Transplantation. 1995;60:616-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Ge J, Perito ER, Bucuvalas J, Gilroy R, Hsu EK, Roberts JP, Lai JC. Split liver transplantation is utilized infrequently and concentrated at few transplant centers in the United States. Am J Transplant. 2020;20:1116-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Li G, Mu X, Huang X, Qian X, Qin J, Tan Z, Zhang W, Xu X, Tan S, Zhu Z, Li W, Wang X, Sun B. Liver transplantation using the otherwise-discarded partial liver resection graft with hepatic benign tumor: Analysis of a preliminary experience on 15 consecutive cases. Medicine (Baltimore). 2017;96:e7295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Bell R, Pandanaboyana S, Upasani V, Prasad R. Impact of graft-to-recipient weight ratio on small-for-size syndrome following living donor liver transplantation. ANZ J Surg. 2018;88:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Dahm F, Georgiev P, Clavien PA. Small-for-size syndrome after partial liver transplantation: definition, mechanisms of disease and clinical implications. Am J Transplant. 2005;5:2605-2610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 472] [Article Influence: 23.6] [Reference Citation Analysis (0)] |