Published online Apr 26, 2022. doi: 10.12998/wjcc.v10.i12.3814
Peer-review started: June 23, 2021
First decision: July 16, 2021
Revised: July 30, 2021
Accepted: March 4, 2022
Article in press: March 4, 2022
Published online: April 26, 2022
Processing time: 301 Days and 23.1 Hours
Aortoesophageal fistula (AEF) is a rare but life-threatening cause of upper gastrointestinal bleeding. Only a handful of cases of successful management of AEF caused by esophageal cancer have been reported. The purpose of this study is to report a case of AEF managed by endovascular aortic repair and review the relevant literature.
A 66-year-old man with upper gastroenterology bleeding presented at the Emergency Department of our hospital complaining of chest pain, fever and hematemesis for 6 h. He had vomited 400 mL of bright-red blood and experienced severe chest pain 6 h prior. He had a past medical history of advanced esophageal cancer. He received chemoradiotherapy but stopped 8 mo prior because of intolerance. A chest contrast computed tomographic scan revealed communi
Although AEF is a lethal condition, timely diagnosis and TEVAR may success
Core Tip: Aortoesophageal fistula (AEF) is a rare but life-threatening cause of upper gastrointestinal bleeding. However, only a few cases of successful management of AEF have been reported. Herein, we report the case of a 66-year-old man with life-threatening upper gastroenterology bleeding (UGB) caused by AEF who was successfully treated by thoracic endovascular aortic repair (TEVAR). The bleeding was controlled after TEVAR; he received antibiotics and was discharged. He died 2 mo later due to cancer progression. UGB caused by AEF is a lethal condition, but TEVAR is an effective treatment.
- Citation: Zhong XQ, Li GX. Successful management of life-threatening aortoesophageal fistula: A case report and review of the literature. World J Clin Cases 2022; 10(12): 3814-3821
- URL: https://www.wjgnet.com/2307-8960/full/v10/i12/3814.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i12.3814
Primary aortoesophageal fistula (AEF) is communication between the primary aorta and the esophagus. AEF is a rare but lethal cause of upper gastroenterology bleeding (UGB). As there is a significant risk of massive bleeding, sepsis and ultimately death, emergent and aggressive treatment is needed for AEF. Indeed, upper gastrointestinal bleeding in patients caused by malignant AEF is lethal unless immediate treatment is performed[1]. To date, AEF has not been fully described, and no treatment guidelines exist. Despite some studies about AEF caused by advanced esophageal cancer, the management of AEF due to esophageal cancer remains to be explored[2]. Thoracic endovascular aortic repair (TEVAR) is a good treatment for malignant AEF. Here, we present a case of UGB caused by malignant AEF that was successfully controlled by TEVAR. We detected AEF timely manner in this case. We emphasize that contrast-enhanced computer tomography (CT) scans should be performed immediately when a patient with esophageal cancer has hematemesis and chest pain. We searched PubMed for articles related to AEF associated with esophageal cancer written in English and reviewed these cases to gain a further understanding of the disease.
A 66-year-old male patient was admitted to the Emergency Department complaining of hematemesis and chest pain.
The 66-year-old male patient was admitted to the Emergency Department complaining of chest pain, fever and hematemesis for 6 h. He had vomited 400 mL of bright-red blood and experienced severe chest pain 6 h prior. The pain was retrosternal with radiation toward the back. The pain was sharp and could not be relieved. He also had fever as well as progressive dysphagia and weight loss. He did not undergo any surgical procedure to relieve the dysphagia.
The patient had previously been diagnosed with stage III (cT4N1M0) esophageal squamous-cell carcinoma (ESCC) 11 mo prior and was treated with chemoradiotherapy (CRT), including albumin-bound paclitaxel plus a radiation dose of 60 Gy. He stopped radiotherapy 10 mo prior and chemotherapy 8 mo prior because of intolerance. He did not undergo any surgical procedure.
There was no notable family history.
On initial evaluation, the patient had a temperature of 38.9 °C, a heart rate 110 beats per minute, a respiratory rate 20 breaths per minute, blood pressure of 92/50 mmHg and oxygen saturation at 98% on room air. The patient was conscious, and he complied with the physical examination. He had no abdominal tenderness. His bowel sounds occurred at a rate of 4 per minute.
Blood analysis revealed mild leukocytosis of 11.11 × 109/L, with predominant neutrophils (91.4%). The patient’s hemoglobin level was 83 g/L, the platelet count was normal, and serum C reactive protein was increased to 242.84 mg/L (normal range < 10.0 mg/L). The prothrombin time was 12.5 s (normal value 10.9 s). The partial thromboplastin time was normal, and d-dimers were increased to 6.42 mg/L FEU (normal range < 0.55 mg/L FEU). Blood urea nitrogen was 9.66 mmol/L (normal range 3.10-8.80 mmol/L).
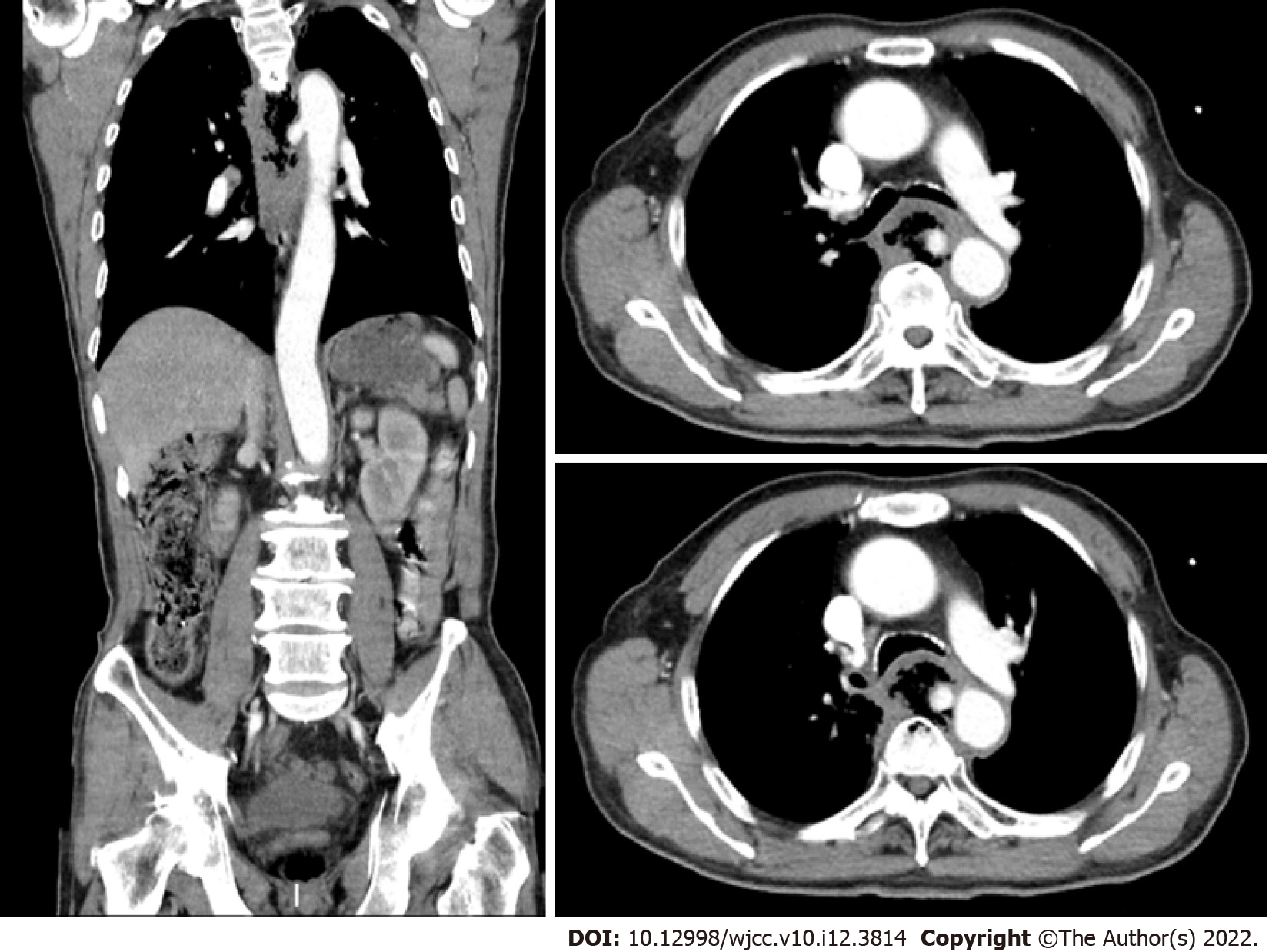
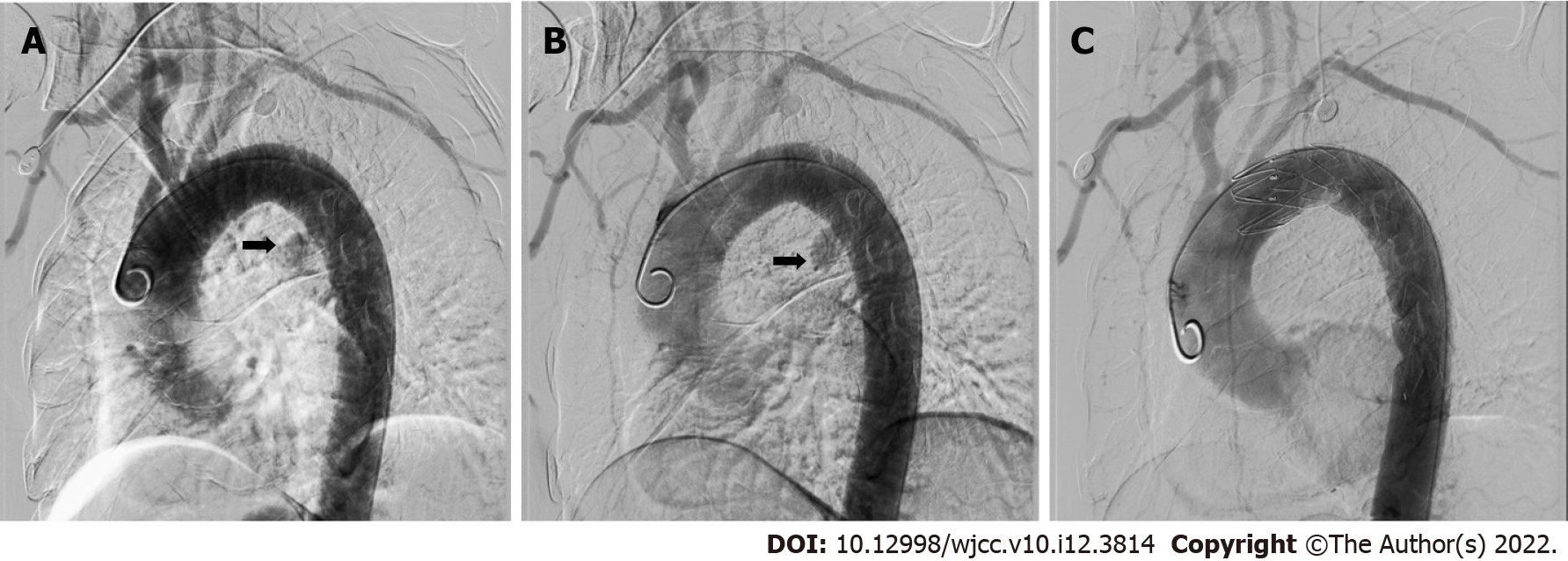
A computed tomographic scan revealed communication between the esophagus and descending aorta as well as a descending aortic pseudoaneurysm (Figure 1). Aortic angiography indicated AEF and descending aortic pseudoaneurysm (Figure 2A and B).
The final diagnosis was AEF.
Following admission to the Emergency Department, the patient immediately received fluid resuscitation. His blood pressure increased to over 90/60 mmHg. Conventionally, AEF is treated by open surgery. However, emergency open surgery has a high mortality of 55.5% for AEF[3]. Because of the unstable hemodynamics of the patient, emergency percutaneous TEVAR of the descending aorta was performed. Aortic angiography showed no aortic pseudoaneurysm or contrast agent leakage after TEVAR (Figure 2C). The bleeding stopped, and his vital signs were stable. The patient refused surgery or gastrostomy after TEVAR. As he had an infection, he received biapenem 0.3 g TID intravenously for 3 wk. He underwent oral fasting and parenteral nutrition treatment for 3 wk. Upon completion of the 3 wk of intravenous antibiotic therapy with biapenem, the patient was discharged from our hospital free of symptoms.
After TEVAR, aorta angiography showed that there was no aortic pseudoaneurysm or contrast agent leakage (Figure 2B). The bleeding was stopped, and his vital signs were stable after TEVAR. Three wk later, he was discharged stably after treatment with antibiotics. He died 2 mo after TEVAR due to cancer progression.
AEF is a rare cause of upper gastrointestinal bleeding. The incidence of AEF is approximately 0.07%[4]. However, the mortality of AEF is high[5,6]. The main etiologic factors of AEF include aneurysm (54.2%), foreign body (19.2%) and esophageal cancer (17%)[7,8]. Other causes of AEF include radiotherapy[9,10] and infection[11,12].
The typical sign of AEF is Chiari’s triad, which involves mid-thoracic pain, sentinel hemorrhage, and final exsanguination following a symptom-free interval[10]. However, the proportion of AEF patients with Chiari’s triad is not high[6].
Endoscopy is the first choice for investigating upper gastrointestinal bleeding, but UGB caused by AEF with esophageal cancer cannot always be diagnosed in a timely manner. There is also a risk of dislodging clots and causing fatal bleeding with endoscopy[13]. Previous studies have shown that most patients with stable circulation and an aortic gastrointestinal fistula had undergone gastroscopy, but only approximately 25% were diagnosed with an aortic gastrointestinal fistula in time[6].
Contrast-enhanced CT is very helpful in the diagnosis of aortic esophageal fistula and can clearly show the aorta, aortic mural gas, pseudo aortic fistula, or aortic fistula ruptured into the esophagus. Aortic angiography can diagnose aortic-esophageal fistula at an early stage, and thoracic aortic angiography can show thoracic aortic aneurysms. Contrast agent leaking into the esophagus indicates a diagnosis of AEF in a timely fashion. In the present case, an AEF was diagnosed by a contrast-enhanced CT scan at the time of presentation, and aortic angiography confirmed the diagnosis.
In the past 20 years, TEVAR has gradually become the first choice for AEF instead of surgery[2,14,15]. Since Parodi et al[16] first reported endovascular stent grafts to treat abdominal aortic aneurysms, this method has developed rapidly. TEVAR can prevent sudden death and gain time for surgical treatment, radiotherapy and chemotherapy[17,18]. To date, TEVAR is the only option for AEF caused by unresectable esophageal cancer[19]. Compared to surgery, TEVAR has many advantages for the treatment of AEF, such as a good hemostatic effect, no need to open the chest, less damage, no aortic clipping, and less cerebral ischemic damage. As Sasaki et al[19] reported, TEVAR can extend the survival time by an average of 4 mo for AEF caused by advanced esophageal cancer. Additionally, Mezzetto et al[20] performed prophylactic TEVAR in patients with esophageal cancer involving the aorta in preparation for future radiotherapy and chemotherapy. Overall, prophylactic TEVAR may be future trend but still needs more validation.
There are only 12 reports of 19 cases of primary AEF due to esophageal cancer treated by TEVAR in PubMed written in English[1,2,19,21-29]. Table 1 shows the clinical features of the patients in previous studies and those of the present case[1,2,19,21-29]. Of these 20 cases, 4 were women and 16 men. Their average age was 64.1 years (range 47-87 years). Most of the patients had hematemesis, and a few of them had chest pain. It is worth noting that a few of the patients had no special symptoms. Seventeen patients underwent enhanced CT. Enhanced CT immediately detected AEF in 10 of the cases (10/17, 58.8%). This indicates that when a patient has hematemesis, an enhanced CT scan should be performed. The location of the fistula was not mentioned for three cases. The fistula in 16 cases was located in the descending thoracic aorta; one was located in the aortic arch. All of these cases were treated with TEVAR. Two cases were treated with embolization of the aortic fistula (EMB) at the same time as TEVAR. One case was treated with an esophageal stent in addition to TEVAR. Among the 20 patients, 2 died on the day of TEVAR, whereas 4 remained alive during follow-up. The average survival time of the patients was 3.68 mo.
| Ref. | Age (yr) | Sex | Symptoms | Performed endoscopy | Definitive diagnosis by contrast CT | Location of fistula | Treatment | Cause of death | Prognosis |
| Kato et al[23] | 59 | Male | Hematemesis | No | No | DTA | TEVAR | Pneumonia | 6 mo, death |
| Ikeda et al[25] | 64 | Male | Massive upper intestinal bleeding | No | Yes | DTA | TEVAR | - | 6 mo, alive |
| Feezor et al[27] | 48 | Male | Hematemesis | No | Yes | DTA | TEVAR | Hemoptysis | 3 mo, death |
| Ghosh et al[26] | 47 | Male | Hematemesis | Yes | Yes | DTA | TEVAR+ esophageal stent | Cancer | 2 mo, death |
| Ishikawa et al[24] | 75 | Female | Hematemesis | Yes | No | DTA | TEVAR | Sepsis | 5 mo, death |
| Ishikawa et al[24] | 81 | Male | Chest discomfort, anorexia | Yes | No | DTA | TEVAR | - | 12 mo, alive |
| Ishikawa et al[24] | 66 | Female | No symptoms | No | Yes | DTA | TEVAR | - | 6 mo, alive |
| Dorweiler et al[28] | 60 | Female | Unknown | Unknown | Unknown | Unknown | TEVAR | Cancer cachexia | 7 mo, death |
| Dorweiler et al[28] | 71 | Male | Unknown | Unknown | Unknown | Unknown | TEVAR | Sepsis | 0 mo, death |
| Dorweiler et al[28] | 68 | Male | Unknown | Unknown | Unknown | Unknown | TEVAR | Sepsis/pneumonia | 3 mo, death |
| Wong et al[1] | 87 | Female | Hematemesis | Yes | Yes | DTA | TEVAR | Cancer | 3 mo, death |
| Wong et al[1] | 58 | Male | Hematemesis | Yes | No | DTA | TEVAR | Cancer | 4 mo, death |
| Nagata et al[22] | 58 | Male | Hypotension | Yes | Yes | DTA | TEVAR | Cancer | 2 mo, death |
| Sasaki et al[19] | 67 | Male | Hematemesis | Yes | No | DTA | TEVAR | Cancer | 4 mo, death |
| Iwabu et al[2] | 69 | Male | Bloody bowel discharge | No | No | DTA | TEVAR | Cancer | 7 mo, death |
| Guerrero et al[21] | 69 | Male | Hematemesis, chest pain, hypotension | Yes | Yes | DTA | TEVAR | Hematemesis | 0 mo, death |
| Chen et al[29] | 55 | Male | Hematemesis | Unknown | No | DTA | EMB+TEVAR | Cancer | 4 mo, death |
| Chen et al[29] | 53 | Male | Hematemesis | Unknown | Yes | DTA | EMB+TEVAR | Cancer | 7 mo, death |
| Chen et al[29] | 61 | Male | Hematemesis | Unknown | Yes | AA | TEVAR | - | 6 mo, alive |
| Our case | 66 | Male | Hematemesis and Chest pain | No | Yes | DTA | TEVAR | Cancer | 2 mo, death |
In our case, the patient had been diagnosed with unresectable esophageal cancer 11 mo prior. He was admitted to our hospital because of hematemesis and chest pain. His vital signs were not stable, with hypotension and fever. Contrast-enhanced CT was performed immediately on the admission, and the patient was diagnosed with AEF according to the results. Endoscopy is known to be the most sensitive and specific method for the diagnosis of UGB, though there is a risk of precipitating fatal hemorrhage in AEF[30]. As the patient was diagnosed with AEF by a contrast-enhanced CT scan, we did not perform endoscopy. We performed successful emergent TEVAR because of the patient’s hemodynamic instability and stopped his upper gastrointestinal bleeding. Coselli et al[31] reported that among patients with AEF, 60% died within the first 6 h when hematemesis began[29]. Management of patients with AEF requires accurate diagnosis in a timely manner[29]. In our case, timely diagnosis ensured prompt treatment and a good treatment effect. Overall, the importance of immediate contrast-enhanced CT scans for patients with AEF must be emphasized. Our patient underwent endoscopy 8 d after TEVAR, revealing esophageal stricture but no other sources of bleeding. Esophageal stent placement and gastrostomy were recommended, but the patient refused. The patient also refused surgery or gastrostomy after TEVAR. As previous studies report that long-term antibiotic treatment is necessary, the patient received antibiotic treatment for 3 wk until discharge from our hospital. Although he died 2 mo later due to cancer progression, TEVAR prevented his sudden death. This case indicates that TEVAR is effective for the treatment of AEF.
As previously reported, chemoradiotherapy is a major cause of AEF[32,33], and doses higher than 35 Gy are an important factor causing AEF[28,33]. In a retrospective study involving 116 patients, Kawakami et al[34] reported that CRP ≥ 1.00 mg/dL is a risk factor for esophageal fistula in ESCC treated with CRT. Thus, cancer inflammation is a factor for fistula development[35]. In the present case, the patient underwent chemoradiotherapy. He had fever, and his CRP level was high. AEF in this patient may be attributed to tumor invasion into the aorta, chemoradiotherapy and/or inflammation.
A patient with AEF may die due to infection, even when TEVAR is performed[23,24,28], and prevention of infection is a challenge after TEVAR. Some studies have suggested lifelong antibiotic suppression, which has many side effects, such as gut dysbiosis. At present, some antimicrobial coatings on the surface of biliary stents have been designed and found to be effective[36]. Additional studies are needed to explore whether antimicrobial coatings on the surface of stents can be applied in TEVAR. In summary, we should perform contrast-CT scans if a patient with esophageal cancer is vomiting bright -red blood or has chest pain, especially those with hemodynamic instability. When AEF is suspected, TEVAR should be performed without endoscopy. TEVAR is effective for AEF caused by esophageal cancer. Additional studies are needed to explore how to prevent infection after TEVAR.
Successful management of AEF is rare. Patients who have hematemesis with esophageal cancer should undergo contrast-CT scans in a timely manner. Timely diagnosis of AEF is very important. TEVAR is effective for AEF caused by esophageal cancer. Infection is a challenge after TEVAR.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Vosoughi F, Iran S-Editor: Chang KL L-Editor: A P-Editor: Chang KL
| 1. | Wong PC, Chan YC, Law Y, Keung Cheng SW. Emergency aortic stent-graft treatment for malignant aortoesophageal fistula. Asian Cardiovasc Thorac Ann. 2017;25:649-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 2. | Iwabu J, Namikawa T, Yokota K, Kitagawa H, Kihara K, Hirose N, Hanazaki K. Successful management of aortoesophageal fistula caused by esophageal cancer using thoracic endovascular aortic repair. Clin J Gastroenterol. 2020;13:678-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 3. | Kieffer E, Chiche L, Gomes D. Aortoesophageal fistula: value of in situ aortic allograft replacement. Ann Surg. 2003;238:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 4. | Voorhoeve R, Moll FL, de Letter JA, Bast TJ, Wester JP, Slee PH. Primary aortoenteric fistula: report of eight new cases and review of the literature. Ann Vasc Surg. 1996;10:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 5. | Saha BK. Few and Far Between: A Case of Aortoesophageal Fistula in Locally Advanced Esophageal Cancer. Am J Med Sci. 2021;361:e3-e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Saers SJ, Scheltinga MR. Primary aortoenteric fistula. Br J Surg. 2005;92:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 187] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 7. | Hollander JE, Quick G. Aortoesophageal fistula: a comprehensive review of the literature. Am J Med. 1991;91:279-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 234] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 8. | Xi EP, Zhu J, Zhu SB, Liu Y, Yin GL, Zhang Y, Zhang XM, Dong YQ. Surgical treatment of aortoesophageal fistula induced by a foreign body in the esophagus: 40 years of experience at a single hospital. Surg Endosc. 2013;27:3412-3416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Sivaraman SK, Drummond R. Radiation-induced aortoesophageal fistula: an unusual case of massive upper gastrointestinal bleeding. J Emerg Med. 2002;23:175-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Kawaguchi Y, Fu K, Morimoto T, Shimizu T, Izumi Y, Kawabe M, Miyazaki A. Aortoesophageal fistula after radiation therapy for esophageal cancer (with video). Gastrointest Endosc. 2011;74:922; discussion 922-922; discussion 923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Lee OJ, Kim SH. Aortoesophageal fistula associated with tuberculous mediastinitis, mimicking esophageal Dieulafoy's disease. J Korean Med Sci. 2002;17:266-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Shin HK, Choi CW, Lim JW, Her K. Two-stage Surgery for an Aortoesophageal Fistula Caused by Tuberculous Esophagitis. J Korean Med Sci. 2015;30:1706-1709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | McGinnis GJ, Holland JM, Thomas CR, Jr. , Nabavizadeh N. Massive hemorrhage following definitive esophageal chemoradiation: Teaching case of a fatal aortoesophageal fistula and lessons learned. Clin Case Rep. 2017;5:2074-2079. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Takeno S, Ishii H, Nanashima A, Nakamura K. Aortoesophageal fistula: review of trends in the last decade. Surg Today. 2020;50:1551-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 15. | Lichtenstein JB, Brar HS, Wang C, Lei L, Zhang W, Fan X. An aorto-oesophageal fistula treated with total arch repair combined with oesophageal repair. Interact Cardiovasc Thorac Surg. 2021;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Parodi JC, Criado FJ, Barone HD, Schönholz C, Queral LA. Endoluminal aortic aneurysm repair using a balloon-expandable stent-graft device: a progress report. Ann Vasc Surg. 1994;8:523-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 73] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Sinclair EM, Stevens JP, McElhanon B, Meisel JA, Santore MT, Chahine AA, Riedesel EL. Development and repair of aorto-esophageal fistula following esophageal button battery impaction: A case report. J Pediatr Surg Case Rep. 2021;66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Yadav A, Shrestha UK, Shrestha KR, Gurung D. Thoracic aortic aneurysm causing aorto-esophageal fistula-our experience with a rare disease. J Surg Case Rep. 2020;2020:rjaa242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Sasaki A, Egashira H, Tokoro S, Ichita C, Takizawa S, Tsukiyama T, Ogino H, Kawachi J, Shimoyama R, Kako M. Thoracic Endovascular Aortic Repair of Esophageal Cancer-Associated Aortoesophageal Fistula: A Case Report and Literature Review. Case Rep Oncol Med. 2018;2018:9851397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Mezzetto L, Scorsone L, De Pasqual CA, Weindelmayer J, Giacopuzzi S, de Manzoni G, Veraldi GF. Preliminary Experience with Prophylactic Thoracic Endovascular Aortic Repair in Patients Affected by Advanced Esophageal Cancer. Ann Vasc Surg. 2019;61:142-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Guerrero I, Cuenca JA, Cardenas YR, Nates JL. Hemorrhagic Shock Secondary to Aortoesophageal Fistula as a Complication of Esophageal Cancer. Cureus. 2020;12:e7146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Nagata T, Wang Y, Asanuma M. Emergency thoracic aortic stent grafting for aortoesophageal fistula in advanced esophageal cancer. J Card Surg. 2017;32:650-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Kato N, Tadanori H, Tanaka K, Yasuda F, Iwata M, Kawarada Y, Yada I, Takeda K. Aortoesophageal fistula-relief of massive hematemesis with an endovascular stent-graft. Eur J Radiol. 2000;34:63-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Ishikawa N, Maruta K, Oi M, Iizuka H, Kawaura H, Omoto T. Thoracic endovascular repair for aorto-esophageal fistula in patients with esophageal carcinoma: report of 3 cases. Vasc Endovascular Surg. 2013;47:65-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Ikeda Y, Morita N, Kurihara H, Niimi M, Okinaga K. A primary aortoesophageal fistula due to esophageal carcinoma successfully treated with endoluminal aortic stent grafting. J Thorac Cardiovasc Surg. 2006;131:486-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Ghosh SK, Rahman FZ, Bown S, Harris P, Fong K, Langmead L. Survival following Treatment of Aortoesophageal Fistula with Dual Esophageal and Aortic Intervention. Case Rep Gastroenterol. 2011;5:40-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Feezor RJ, Hess PJ, Lee WA. Endovascular treatment of a malignant aortoesophageal fistula. J Vasc Surg. 2009;49:778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Dorweiler B, Weigang E, Duenschede F, Pitton MB, Dueber C, Vahl CF. Strategies for endovascular aortic repair in aortobronchial and aortoesophageal fistulas. Thorac Cardiovasc Surg. 2013;61:575-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Chen C, Kim JW, Shin JH, Kwon Y, Kim J, Lee IJ. Management of life-threatening aortoesophageal fistula: experiences learned from eight patients. Acta Radiol. 2021;62:447-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Benson MJ, Rouse D, van Someren N, Wingate DL, Swain CP. Fatal hemorrhage from an aorto-esophageal fistula precipitated by flexible endoscopy. Gastrointest Endosc. 1991;37:193-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Coselli JS, Crawford ES. Primary aortoesophageal fistula from aortic aneurysm: successful surgical treatment by use of omental pedicle graft. J Vasc Surg. 1990;12:269-277. [PubMed] |
| 32. | Flores J, Shiiya N, Kunihara T, Yoshimoto K, Yasuda K. Aortoesophageal fistula: alternatives of treatment case report and literature review. Ann Thorac Cardiovasc Surg. 2004;10:241-246. [PubMed] |
| 33. | Parikh MP, Sherid M, Panginikkod S, Rawal HA, Gopalakrishnan V. Radiation therapy-induced aortoesophageal fistula: a case report and review of literature. Gastroenterol Rep (Oxf). 2016;4:165-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Kawakami T, Tsushima T, Omae K, Ogawa H, Shirasu H, Kito Y, Yoshida Y, Hamauchi S, Todaka A, Machida N, Yokota T, Yamazaki K, Fukutomi A, Onozawa Y, Yasui H. Risk factors for esophageal fistula in thoracic esophageal squamous cell carcinoma invading adjacent organs treated with definitive chemoradiotherapy: a monocentric case-control study. BMC Cancer. 2018;18:573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 35. | Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8581] [Cited by in RCA: 8313] [Article Influence: 489.0] [Reference Citation Analysis (0)] |
| 36. | Obermeier A, Würstle S, Tübel J, Stolte P, Feihl S, Lipovcic N, Lanzinger S, Mühlhofer H, Weber A, Schmid RM, Burgkart R, Schneider J. Novel antimicrobial coatings based on polylactide for plastic biliary stents to prevent post-endoscopic retrograde cholangiography cholangitis. J Antimicrob Chemother. 2019;74:1911-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |