Published online Apr 26, 2022. doi: 10.12998/wjcc.v10.i12.3801
Peer-review started: June 4, 2021
First decision: September 1, 2021
Revised: September 26, 2021
Accepted: March 6, 2022
Article in press: March 6, 2022
Published online: April 26, 2022
Processing time: 321 Days and 10 Hours
Nontuberculous mycobacterium (NTM) refers to all mycobacteria except Mycobacterium tuberculosis and Mycobacterium leprae, also known as environmental Mycobacterium. The patients with lung cancer and NTM are somewhat special; the two diseases are inevitably influenced by each other. It brings difficulties and challenges to the choice of treatment. Recently, cancer immunotherapy has been considered one of the pillars for the treatment of lung cancer. However, the clinical experience in the application of immune checkpoint inhibitors is scarce for lung cancer patients with pulmonary tuberculosis, and lung cancer with NTM is even more rare. Although it ameliorates lung cancer, immunotherapy with immune checkpoint inhibitors presents complications of infectious diseases, including tuberculosis and NTM.
A 61-year-old male patient visited a doctor in May 2019. His admitting diagnoses were: (1) Cancer of the left lung with a pathological diagnosis of poorly differentiated non-small cell carcinoma, likely poorly differentiated adenocarcinoma, clinical stage IIIb (T3N3M0); and (2) Mycobacterium fortuitum (M. fortuitum) infection. We chose to proceed with pembrolizumab treatment. After two treatment cycles, a chest computed tomography scan showed a new irregular subpleural mass in the anterior segment of the left upper lobe of the lung, a reduction in the mediastinal enlarged lymph node, and no other obvious changes. Next, an ultrasound-guided biopsy of the new tumor was performed. Pathological examination showed that a large number of carbon particles were deposited in the alveolar tissue with histiocyte reaction and multinucleated giant cell formation. The tuberculosis (TB) specialist suggested that anti-TB therapy be combined with continued antitumor treatment. The patient continued to be treated with pembrolizumab. After 14 cycles, the lesion shrunk by 79%, there was no recurrence of M. fortuitum infection, and there were no intolerable adverse reactions.
We have observed that in cases of lung cancer complicated with M. fortuitum infection, oppor
Core Tip: The clinical experience in the application of immune checkpoint inhibitors is scarce for lung cancer patients with pulmonary tuberculosis, and lung cancer with nontuberculous mycobacterium (NTM) is even more rare. We present the case of a patient who had both lung cancer and NTM. NTM was stable, and the tumors shrank after treatment with immune checkpoint inhibitors. It provides some reference for the treatment of coexistent lung cancer with NTM.
- Citation: Zhang CC, Chen P. Anti-programmed death 1 antibody in the treatment of coexistent Mycobacterium fortuitum and lung cancer: A case report. World J Clin Cases 2022; 10(12): 3801-3807
- URL: https://www.wjgnet.com/2307-8960/full/v10/i12/3801.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i12.3801
Nontuberculous mycosis (NTM) is a lung disease characterized by exposure to mycobacteria other than Mycobacterium tuberculosis (M. tuberculosis) and M. leprae. NTM has clinical manifestations similar to pulmonary tuberculosis (TB), and the number of patients with pulmonary NTM is increasing worldwide[1]. Mycobacterium fortuitum (M. fortuitum) accounts for 5.9% of all NTM cases[2]. Lung cancer is one of the most frequent malignant tumors, and it has a very high morbidity and mortality. Lung cancer and NTM lung disease share some common predisposing factors (e.g., smoking, atmosphere pollution), and coexistence of these two diseases is not uncommon. Diagnosis and treatment are more complex for patients with lung cancer and NTM. Immune checkpoint inhibitors (ICIs) have become generalized for use as anticancer therapeutics in many types of cancer. Many previous trials have reported that ICIs provide clinical benefits in patients with non-small cell lung cancer (NSCLC)[3,4]. Although ICIs are often effective in NSCLC, unique adverse events are known to occur during treatment, including skin rash, hepatotoxicity, and endocrine disturbances; these events are termed immune-related adverse events. Among the several types of immune-related adverse events, cases of infectious diseases have been increasing steadily[5]. ICI immunotherapy can also cause TB and other infectious diseases as well as noninfectious immune-related complications. However, it remains unknown whether ICI immunotherapy can cause a recurrence of TB or NTM and whether ICI immunotherapy is an appropriate option for patients with both lung cancer and NTM. Here, we report a case with no recurrence of M. fortuitum following pembrolizumab treatment for unresectable stage IIIb NSCLC.
A 61-year-old male patient visited a doctor in May 2019 and was admitted with intermittent chest pain for 4 mo, anorexia and fatigue for 1 mo, and chest tightness for 20 d.
The patient had a history of coronary heart disease (12 years), hypertension (more than 10 years), and a 40-year history of smoking 40 cigarettes per day.
His left lung sounds were a bit quieter than normal.
Carcinoembryonic antigen on July 2, 2019 was 29.86 ng/mL, and pathology diagnosis (via bron
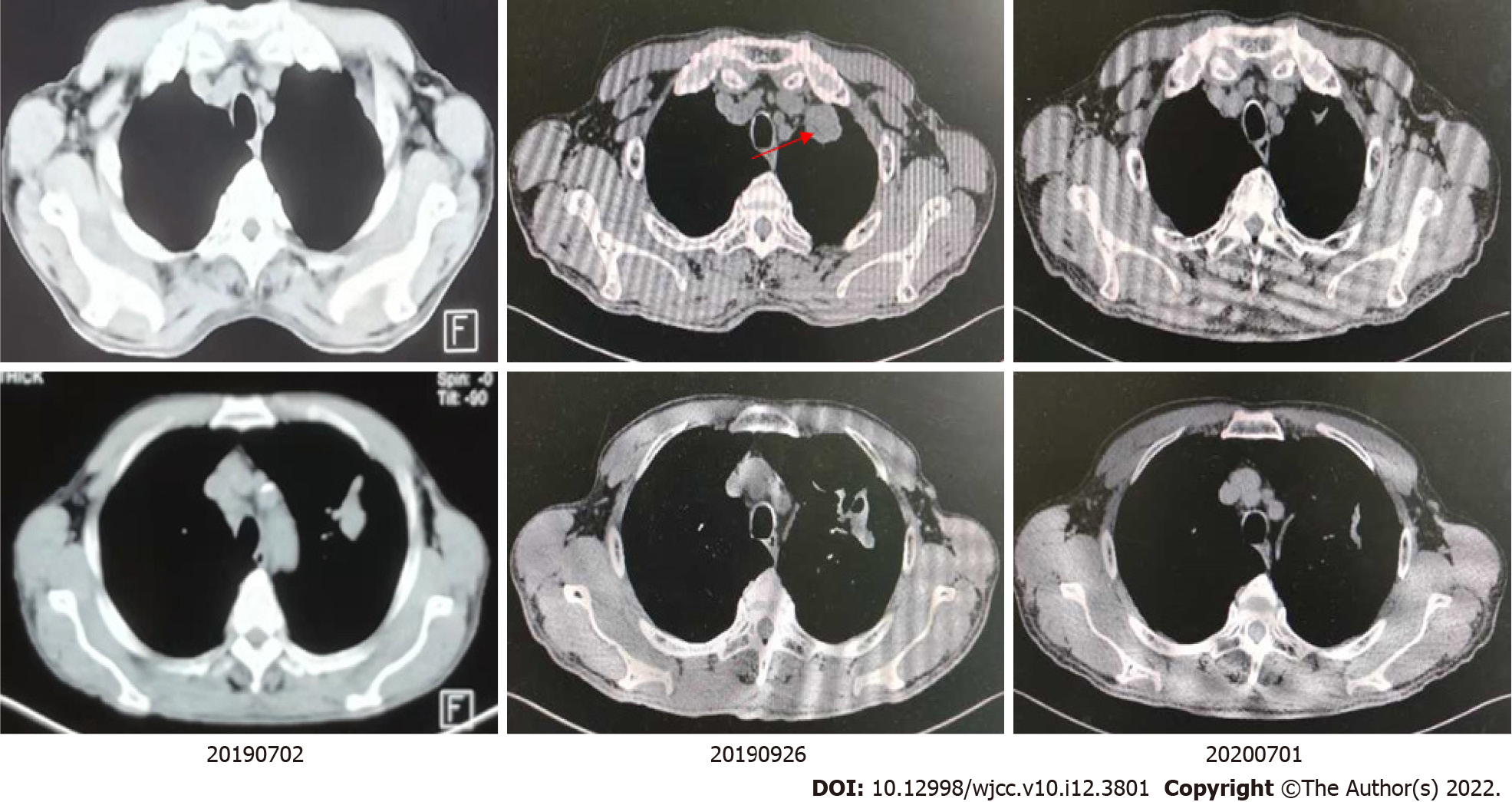
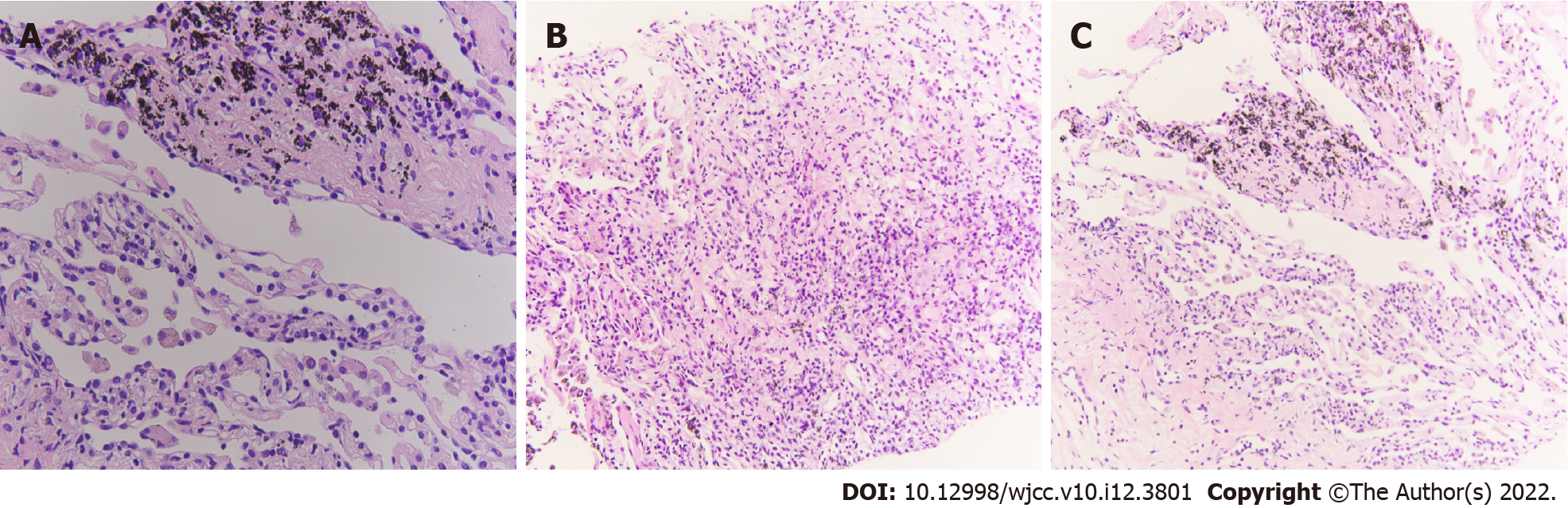
A chest computed tomography (CT) scan showed a mass shadow in the left upper lung with uneven density, carcinoembryonic antigen was measured to be 23.99 ng/mL, and sputum smear results were positive for TB. A biopsy was performed via bronchoscope (left apex), which identified a few blood vessels and fibrous tissues that were accompanied by carbon dust deposition. Species identification found Mycobacteria in bronchoalveolar lavage fluid [M. fortuitum (+)]. The patient was administered anti-TB drugs (isoniazid, moxifloxacin, and clarithromycin). A positron emission tomography-CT on July 2, 2019 identified: (1) An irregular mass in the posterior segment of the left superior lobe tip (3.0 cm × 2.3 cm). The mass was hypermetabolic with obstructive atelectasis and inflammation; and (2) Multiple enlarged lymph nodes in the right supraclavicular fossa, the left hilum, and the mediastinum (anterior to the trachea, aortopulmonary window, and inferior tracheal protuberance), with abnormal metabolism (the largest enlarged lymph node was 2.1 cm in diameter).
After consulting with a TB specialist, the patient, and his family, we chose to proceed with pembrolizumab treatment.
The admitting diagnoses were: (1) Cancer of the left lung with a pathological diagnosis of poorly differentiated NSCLC, likely poorly differentiated adenocarcinoma, clinical stage IIIb (T3N3M0); and (2) M. fortuitum infection.
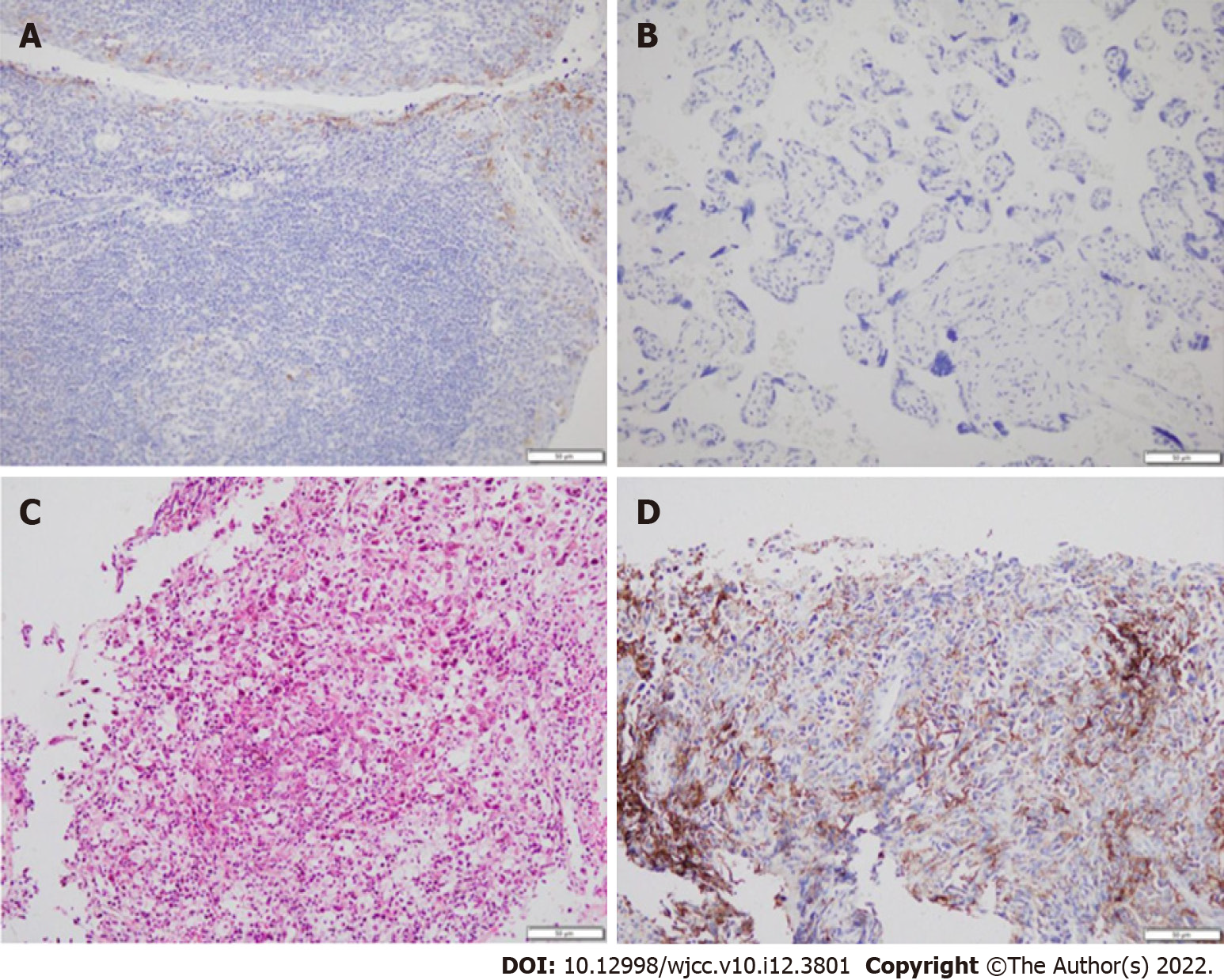
The National Comprehensive Cancer Network guidelines recommend that immunotherapy alone can be chosen as a first-line treatment for patients with PD-L1 expression ≥ 50%. After consulting with a TB specialist, the patient, and his family, we chose to proceed with pembrolizumab treatment (200 mg, intravenous infusion, every 3 wk). After two treatment cycles, a chest CT showed a new irregular subpleural mass in the anterior segment of the left upper lobe of the lung (Figure 2), a reduction in the mediastinal enlarged lymph node, and no other obvious changes. Next, an ultrasound-guided biopsy of the new tumor was performed. Pathological examination showed that a large number of carbon particles were deposited in the alveolar tissue with histiocyte reaction and multinucleated giant cell formation; some areas of fibrous tissue showed hyperplasia of collagen (Figure 3).
The chest CT and biopsy received consultation from a TB specialist, who diagnosed the patient with “pulmonary M. fortuitum infection,” thought to be caused by opportunistic infection. The mediastinal lymph nodes were smaller than those anterior, indicating that antitumor therapeutics were probably effective. The patient was receiving anti-TB treatment, and therefore the new subpleural mass in the anterior segment of the left superior lobe may have been induced by anti-TB therapy. The TB specialist suggested that anti-TB therapy be combined with continued antitumor treatment.
The patient continued to be treated with pembrolizumab (200 mg, every 3 wk). After 14 cycles, the lesion shrunk by 79%, there was no recurrence of M. fortuitum infection, and there were no intolerable adverse reactions. We next organized a multidisciplinary consultation in which doctors from a TB hospital indicated that the TB was stable and that anti-TB drugs could be reduced. Radiologists recommended that the patient be followed up by radical chest radiotherapy. At present, chest radiotherapy has been completed. Chest CT examination showed the lung cancer in a stable condition, and no recurrence of M. fortuitum infection was found. At the time of publication, progression-free survival reached more than 21 mo. We will continue to follow up the patient.
The treatment of NTM is a very difficult and lengthy process. Although NTM has high resistance to anti-TB drugs, it is still commonly treated using anti-TB drugs such as isoniazid, rifampicin, ethambutol, pyrazinamide, and streptomycin. In addition to high drug resistance, treatment can be complicated by physical factors and other problems[6]. Active TB is often detected during traditional cytotoxic chemotherapy because chemotherapy can lead to immunosuppression. Patients with lung cancer complicated with TB are primarily male patients with smoking history, and the most common pathological tumor type in this group is adenocarcinoma[7]. Therefore, the opportunity to use molecular-targeted treatments is relatively small, and this means it is difficult to treat patients with both lung cancer and NTM.
Immunotherapy and immune-checkpoint inhibitors (programmed cell death protein 1 or PD-L1 inhibitors) administered as monotherapy or in combination with chemotherapy have recently exhibited breakthrough progress in the treatment of advanced NSCLC[8,9]. Programmed cell death protein 1 or PD-L1 inhibitors lead to the activation of cytotoxic T cells, which promotes immune-mediated cancer cell recognition and destruction. In theory, the increased immune response to foreign antigens (cancer cells or infection factors) can lead to a high inflammatory response at a site of persistent infection[10]. However, existing clinical trials of immunosuppressive checkpoint inhibitors in advanced lung cancer generally exclude patients with active TB (NTM) infection. Therefore, little is known about the clinical use of immunosuppressive checkpoint inhibitors in these patients.
In Japan, Fujita et al[11] reported a lung cancer patient with pulmonary TB that developed into acute pulmonary TB after treatment with nivolumab. Kato et al[12] published the first report of TB reactivation during durvalumab therapy after radical chemoradiotherapy for stage III NSCLC. A study by Fujita et al[13] explained that “Immunotherapy with immune checkpoint inhibitors (ICIs), while ameliorating lung cancer, can cause infectious diseases, including TB, in addition to immune-related noninfectious complications.”
However, in 2018, Ishii et al[14] published a case report entitled “Improvement of Mycobacterium abscessus Pulmonary Disease after Nivolumab Administration in a Patient with Advanced Non-Small Cell Lung Cancer.” The patient in this report was initially diagnosed as stage IIIB, and there were no sensitive mutations. Therefore, there were no opportunities for surgical treatment or molecule-targeted treatments. Radiotherapy and chemotherapy could be administered until the M. fortuitum infection was better controlled.
In the absence of other anti-cancer strategies, pembrolizumab was chosen as a treatment after reviewing results of the immunological index detection, upon suggestion of a TB specialist, and after adequate communication with the patient and his family. After two treatment cycles, symptoms of M. fortuitum infection vanished completely. The patient’s physique was obviously improved, and tumor marker expression also decreased. However, imaging results showed an irregular soft tissue mass in the anterior segment of the left upper lobe after two cycles. The identification of this new mass was surprising, and it was initially unclear whether this was an indicator of lung cancer disease progression or M. fortuitum recurrence.
Improved physique, absence of M. fortuitum symptoms, and decreased tumor marker expression did not support disease progression or M. fortuitum recurrence. Therefore, we completed an ultrasound-guided biopsy of the new mass and consulted a TB expert. The expert suspected that this new lump was caused by opportunistic infection and may have been induced by anti-TB treatment. Although it has been reported that ICI treatment can lead to TB recurrence[15], the patient’s physical condition (anemia, hypoproteinemia, and fervescence all disappeared) had improved, and recurrence of NTM was not considered. We think this is caused by treatment (anti-TB treatment or antitumor therapy). Therefore, he recommended the continuation of antitumor therapy combined with anti-TB treatment. After treatment with pembrolizumab for 14 cycles, the patient continued to show improvements and also received radical radiation. At present, the radiotherapy has ended, and progression-free survival has exceeded 21 mo with no recurrence of M. fortuitum lung disease.
We have observed that in cases of lung cancer complicated with M. fortuitum infection, opportunistic pathogen infection recurrence can be overcome, and immunotherapy is most beneficial when TB doctors and oncologists cooperate to closely observe dynamic changes in M. fortuitum and lung cancer. Treatment should be maintained with low dosage anti-TB drugs after general anti-TB chemotherapy for 1 year; this may prevent opportunistic pathogens infection recurrence during immunotherapy. While the results achieved in this case are indeed promising, the effectiveness of ICIs for treating M. fortuitum infection requires confirmation by further randomized clinical trials.
We owe thanks to the patient and his family. We thank the staff at Tianjin Medical University Cancer Institute and Hospital.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Immunology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abed A, Australia S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ Jr, Winthrop K; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3867] [Cited by in RCA: 4192] [Article Influence: 232.9] [Reference Citation Analysis (0)] |
| 2. | Qin ZH, Jing Y, Du YQ, Song XM, Zhang LX. Spectrum and drug resistance analysis of 339 strains of nontuberculosis mycobacteria isolated from clinical practice. Zhongguo Fanglao Zazhi. 2020;42:630-633. |
| 3. | Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, Cortinovis DL, Leach J, Polikoff J, Barrios C, Kabbinavar F, Frontera OA, De Marinis F, Turna H, Lee JS, Ballinger M, Kowanetz M, He P, Chen DS, Sandler A, Gandara DR; OAK Study Group. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2868] [Cited by in RCA: 3705] [Article Influence: 463.1] [Reference Citation Analysis (0)] |
| 4. | Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G Jr, Srimuninnimit V, Laktionov KK, Bondarenko I, Kubota K, Lubiniecki GM, Zhang J, Kush D, Lopes G; KEYNOTE-042 Investigators. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1597] [Cited by in RCA: 2411] [Article Influence: 401.8] [Reference Citation Analysis (0)] |
| 5. | Fujita K, Kim YH, Kanai O, Yoshida H, Mio T, Hirai T. Emerging concerns of infectious diseases in lung cancer patients receiving immune checkpoint inhibitor therapy. Respir Med. 2019;146:66-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 6. | Zhu X, Ma YK, Yu GW. The research advances in the classification of Mycobacterium tuberculosis. Gansu Keji. 2021;37:160-162. |
| 7. | Hu Y, Yang X, Nie L, Zhao D, An J, Li B. [Analysis of Clinical Characteristics and Driver Genes in 405 Patients with Lung Cancer Complicated with Tuberculosis]. Zhongguo Fei Ai Za Zhi. 2020;23:337-342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 8. | Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O'Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR; KEYNOTE-024 Investigators. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375:1823-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5948] [Cited by in RCA: 7525] [Article Influence: 836.1] [Reference Citation Analysis (0)] |
| 9. | Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins RG, Stevenson JP, Jalal SI, Panwalkar A, Yang JC, Gubens M, Sequist LV, Awad MM, Fiore J, Ge Y, Raftopoulos H, Gandhi L; KEYNOTE-021 investigators. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17:1497-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 877] [Cited by in RCA: 1157] [Article Influence: 128.6] [Reference Citation Analysis (0)] |
| 10. | Ho JC, Leung CC. Management of co-existent tuberculosis and lung cancer. Lung Cancer. 2018;122:83-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 11. | Fujita K, Terashima T, Mio T. Anti-PD1 Antibody Treatment and the Development of Acute Pulmonary Tuberculosis. J Thorac Oncol. 2016;11:2238-2240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 12. | Kato Y, Watanabe Y, Yamane Y, Mizutani H, Kurimoto F, Sakai H. Reactivation of TB during administration of durvalumab after chemoradiotherapy for non-small-cell lung cancer: a case report. Immunotherapy. 2020;12:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Fujita K, Yamamoto Y, Kanai O, Okamura M, Nakatani K, Mio T. Development of Mycobacterium avium Complex Lung Disease in Patients With Lung Cancer on Immune Checkpoint Inhibitors. Open Forum Infect Dis. 2020;7:ofaa067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Ishii S, Tamiya A, Taniguchi Y, Tanaka T, Abe Y, Isa SI, Tsuyuguchi K, Suzuki K, Atagi S. Improvement of Mycobacterium abscessus Pulmonary Disease after Nivolumab Administration in a Patient with Advanced Non-small Cell Lung Cancer. Intern Med. 2018;57:3625-3629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Anastasopoulou A, Ziogas DC, Samarkos M, Kirkwood JM, Gogas H. Reactivation of tuberculosis in cancer patients following administration of immune checkpoint inhibitors: current evidence and clinical practice recommendations. J Immunother Cancer. 2019;7:239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |