Published online Apr 26, 2022. doi: 10.12998/wjcc.v10.i12.3729
Peer-review started: December 14, 2021
First decision: January 26, 2022
Revised: February 11, 2022
Accepted: March 6, 2022
Article in press: March 6, 2022
Published online: April 26, 2022
Processing time: 128 Days and 3.1 Hours
Microvascular tissue reconstruction is a well-established, commonly used technique for a wide variety of the tissue defects. However, flap failure is associated with an additional hospital stay, medical cost burden, and mental stress. Therefore, understanding of the risk factors associated with this event is of utmost importance.
To develop machine learning-based predictive models for flap failure to identify the potential factors and screen out high-risk patients.
Using the data set of 946 consecutive patients, who underwent microvascular tissue reconstruction of free flap reconstruction for head and neck, breast, back, and extremity, we established three machine learning models including random forest classifier, support vector machine, and gradient boosting. Model per
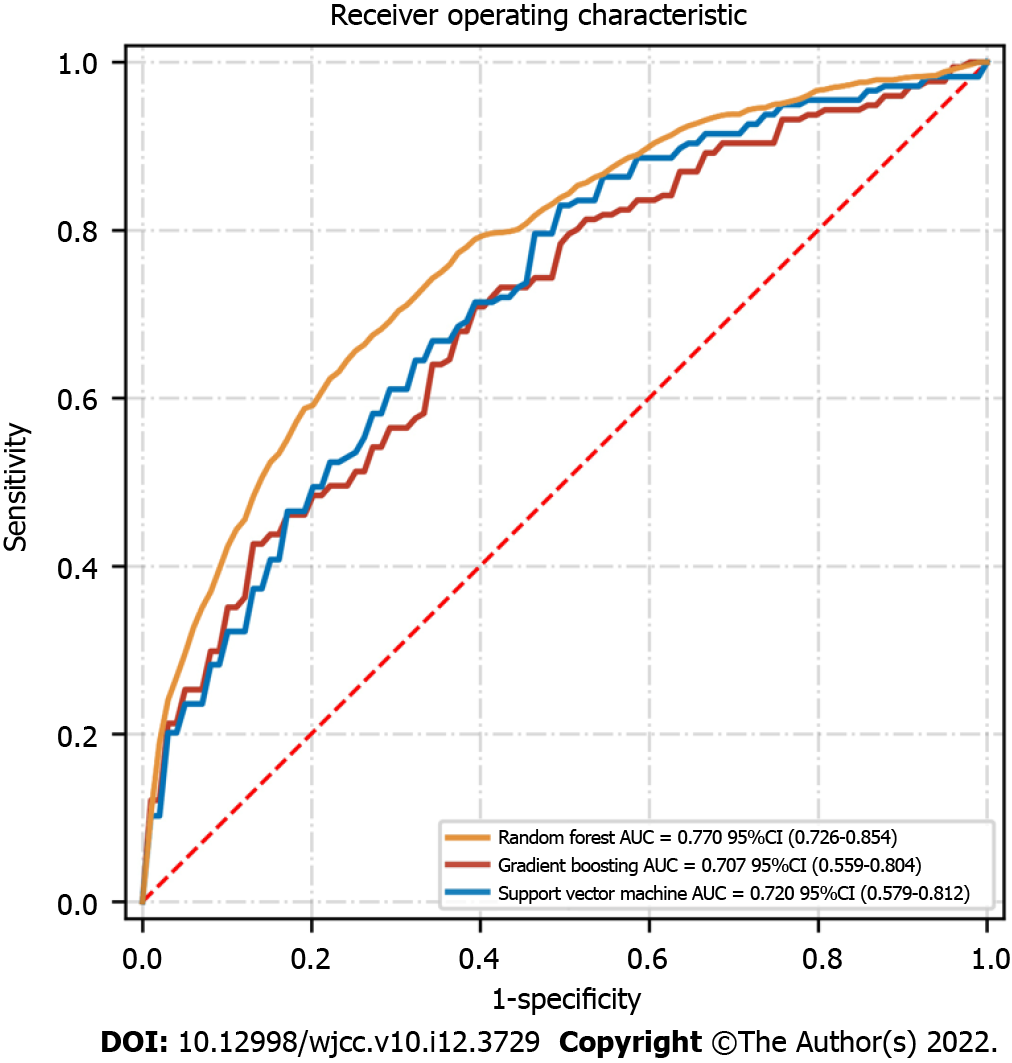
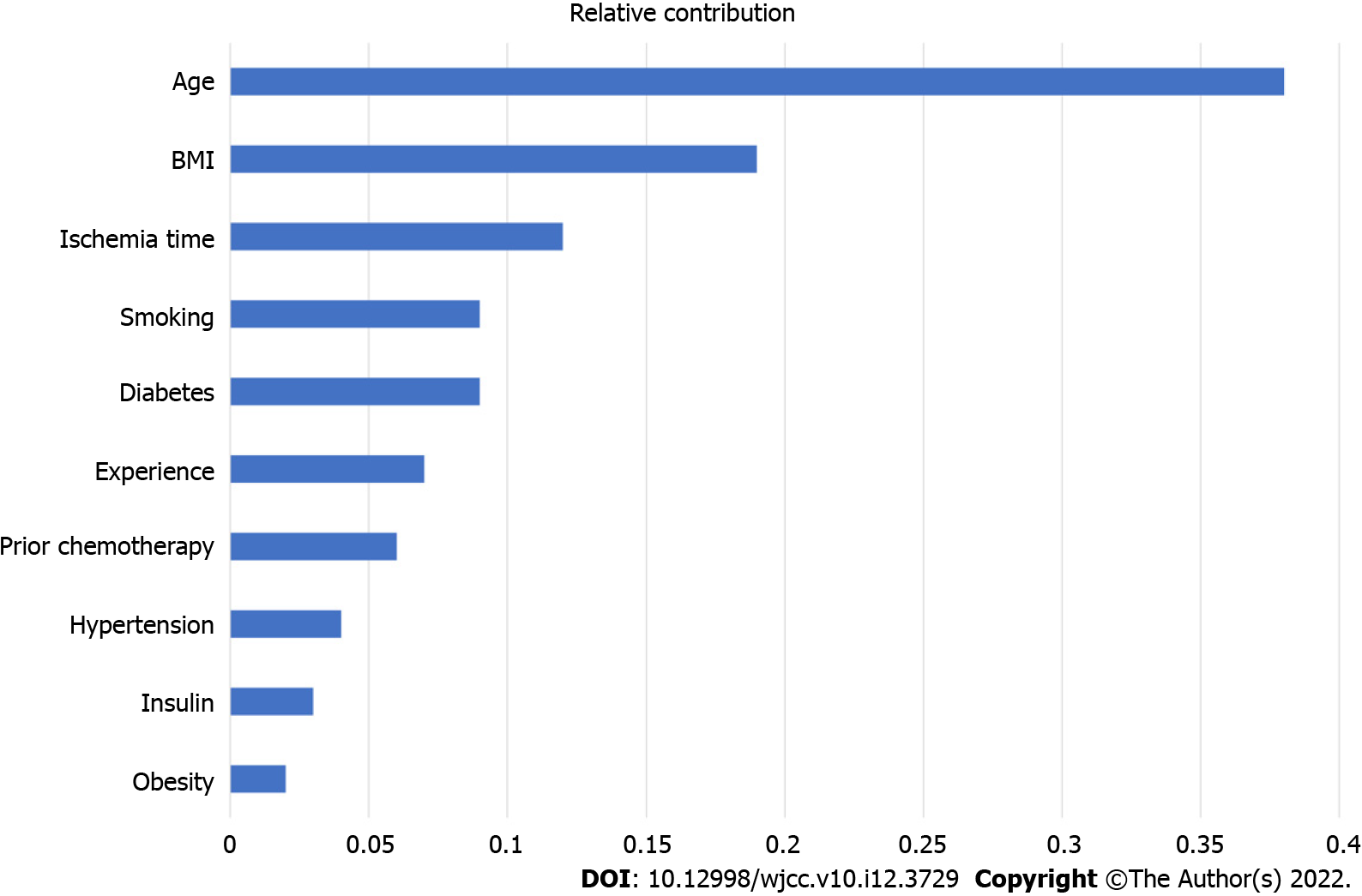
Post-surgery, the flap failure event occurred in 34 patients (3.6%). The machine learning models based on various preoperative and intraoperative variables were successfully developed. Among them, the random forest classifier reached the best performance in receiver operating characteristic curve, with an area under the curve score of 0.770 in the test set. The top 10 variables in the random forest were age, body mass index, ischemia time, smoking, diabetes, experience, prior chemotherapy, hypertension, insulin, and obesity. Interestingly, only age, body mass index, and ischemic time were statistically associated with the outcomes.
Machine learning-based algorithms, especially the random forest classifier, were very important in categorizing patients at high risk of flap failure. The occurrence of flap failure was a multifactor-driven event and was identified with numerous factors that warrant further investigation. Importantly, the successful application of machine learning models may help the clinician in decision-making, understanding the underlying pathologic mechanisms of the disease, and improving the long-term outcome of patients.
Core Tip: Flap failure is a rare but severe event in microvascular tissue reconstruction. It is generally associated with the additional economic burden and mental stress to the patients. Therefore, identifying the risk factors and screening high-risk patients carries a significant value in the clinical practice. Machine learning is an artificial intelligence based on the computer learning to learn from data and thus automatically make decisions. This retrospective study applied machine learning for the risk factor analysis of flap failure during microvascular tissue reconstruction.
- Citation: Shi YC, Li J, Li SJ, Li ZP, Zhang HJ, Wu ZY, Wu ZY. Flap failure prediction in microvascular tissue reconstruction using machine learning algorithms. World J Clin Cases 2022; 10(12): 3729-3738
- URL: https://www.wjgnet.com/2307-8960/full/v10/i12/3729.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i12.3729
Microvascular reconstruction surgery is a commonly used technique to treat various defects, including remodeling after mastectomy[1], head and neck trauma repair[2], and profound burn tissue remodeling[3]. However, even though this technique is quite robust, many adverse complications arise after the reconstruction procedure, such as postoperative incision infection and reoperation[4,5]. Among these complications, the failure of flap transplantation after microvascular tissue reconstruction is the most important event, as it is associated with the arterial blood supply, ischemia-reperfusion, and venous return of the flap[6,7]. Despite the rare occurrence of flap failure, it can result in devastating consequences for patients, such as permanent scarring of the face and breast. Moreover, it increases complication in postoperative care, length of hospital stays, financial burden, and mental stress to the patients[8,9]. Therefore, it is important to identify the relevant factors and screen out high-risk patients before surgery, which might result in flap failure.
Previous studies have analyzed the multifactorial aspect of flap failure[10,11]. Associated preoperative risk factors include, but are not limited to age, smoking, diabetes, hypertension, and obesity[12,13]. Related intraoperative factors included the surgeon’s lack of experience and the choice of free flaps[14]. However, these studies were based on traditional logistic regression methods and were limited to nonlinearity and variable set[15]. Of note, precise, logistic regression analysis assumes that variables are linearly correlated, and therefore potential nonlinear interactions can compromise the outcome[15,16]. Moreover, only a small number of variables could be included in the analysis, overlooking the many potentially relevant factors[16]. These deficiencies in the analytical methods needs to be addressed using an advanced algorithm. Therefore, the recently emerging algorithms of machine learning might be a better option for the data analysis.
Machine learning, a branch of artificial intelligence (AI), literally meaning where machines can understand and learn from data to make decisions like humans[17,18]. In 2017, an AI called AlphaGo won worldwide attention by beating the international GO master Li Shiming. Due to its advantages in computational capacity and problem-solving techniques, machine learning has been widely used in medicine for many purposes, including the interpretation of test results[19], diagnosis of skin diseases[20], pathology[21], prediction of adverse complications[22], and the prognosis of cancer patients[23]. However, in plastic surgery the use of clinical application of machine learning is still rare[24]. Therefore, this study aimed to apply AI in the field of plastic surgery, assessing the factors associated with the prognosis of microvascular tissue reconstruction for identifying high-risk patients with flap failure.
A total of 946 consecutive patients were recruited in the study from January 1, 2006 to December 12, 2020. These recruited patients underwent microvascular tissue reconstruction of free flap for head and neck, breast, back, and extremity at the Department of Plastic Surgery of Affiliated Hospital in Guangdong Medical University. Exclusion criteria included: patients with more than 30% data loss and who refused surgical treatment. Inclusion criteria/variables included: (1) Preoperative variables such as sex, age, body mass index (BMI), smoking, alcohol use, blood pressure, medication history, complications, laboratory findings, preoperative chemotherapy, preoperative radiotherapy, free flap location, and recipient surgical site; and (2) Intraoperative variables like duration of operation, duration of anesthesia, hypotensive events, use of vasoactive agents, duration of flap ischemia, number of vascular anastomoses, use of venous grafts, and surgeon’s experience measured as the time since the flap procedures.
Open-source software Python (version 3.6) and Scikit-learn package (https://scikit-learn.org/) were used for the data processing and analysis. Univariate analyses were done using the χ2 and Fisher’s exact tests for categorical variables, whereas the t-test and Mann-Whitney U tests were used for the continuous variables. A subset of data was usually selected from the entire database for model training to train a suitable algorithm. The rest of the subset was used for the performance test of the model. Conceptually, the whole data set was divided into a training and testing subset according to the ratio of 5:5. Then, GridSearch was performed with the 5-fold cross-validation, where the training data set was further split into five parts and five repetitions. At each repetition, there were four random parts that served as the training set, whereas the remaining part served as the testing set. Multivariable regression was performed for the most critical variables in the random forest model to identify the risk factors in the traditional logistic regression model. A P value less than 0.05 was considered statistically significant.
We employed the following machine learning methods:
Random forest: The random forest classifier, one of the most used techniques in the data mining or automatic learning, was developed from the training data set using the python programming software. Random forest, introduced by Ishwaran, was used as decision tool based on a binary tree. It uses a branching structure like a binary tree to form a decision model and analyze possible results. Each node in these binary tree structures represents a decision (based on selected variables), whereas the two branches of the node represent the two kinds of classification results. Each branch produces two leaf nodes and other subtrees, depending on the classification when the variable is analyzed. For assessing the variables importance, variables in the random forest are determined by the average distance of the branching nodes in the tree structure from the roots. Thus, the higher a variable is in an inverted binary tree, the closer it would be to the root, with the higher ranking.
Support vector machine: Support vector machine is an algorithm for creating nonlinear discriminative classifier, governed by an optimal hyperplane that separates examples of different classes (the notable kernel trick).
Gradient boosting machine: Gradient boosting is a supervised machine learning technique for solving regression and classification problems that yield predictive models in the form of an ensemble of weak predictive models (e.g., decision trees). Through pooling weak predictive models into a more powerful and reliable prediction model, the gradient tree boosting technique incorporated in the eXtreme Gradient Boosting system becomes a robust machine learning classifier.
The model evaluation used performance indicators used in the machine learning. The primary evaluation method was the receiver operating characteristic curve and the area under the curve (AUC) score. Other relevant indicators included accuracy, precision, recall, and F1 score. The higher value indicators represented the better predictive performance of the model.
A total of 946 patients who underwent free flap transplantation for head and neck (40.2%), breast (38.3%), and extremity reconstruction (21.5%) were recruited. Overall, 58.3% of the recruited population was female, with an average age of 42 years (range: 13-65 years). The average BMI of the studied population was 24.9 ± 6.3 (mean ± standard deviation). Other potential factors for flaps failure were obesity (23.4%), smoking (30.3%), diabetes (6.3%), insulin (1.3%), hypertension (16.2%), preoperative tumor chemotherapy (25.3%), and preoperative tumor radiotherapy (19.2%). Table 1 showed the clinical characteristics of the patients in the training and the test sets. However, no significant statistical difference was observed between the two subsets.
| Variables | Training set | Test set | P value |
| Patient population, n | 473 | 473 | |
| Age (yr) | 41 (13-64) | 43 (15-65) | 0.115 |
| Male, n (%) | 274 (57.9) | 278 (58.8) | 0.258 |
| BMI (kg/m2) | 25.3(16.9-32.8) | 25.9 (16.7-35.5) | 0.079 |
| Systolic blood pressure | 119 (87-165) | 121(85-177) | 0.658 |
| Smoking, n (%) | 142 (30.0) | 145 (30.7) | 0.583 |
| Alcohol, n (%) | 163 (34.5) | 172 (36.4) | 0.158 |
| Diabetes, n (%) | 34 (7.2) | 26 (5.5) | 0.098 |
| Insulin, n (%) | 8 (1.7) | 4 (0.8) | 0.059 |
| Hypertension, n (%) | 73 (15.4) | 80 (16.9) | 0.113 |
| Preoperative chemotherapy, n (%) | 117 (24.7) | 122 (25.8) | 0.358 |
| Preoperative radiotherapy, n (%) | 100 (21.1) | 82 (17.3) | 0.663 |
| Obesity, n (%) | 112 (23.7) | 109 (23.0) | 0.487 |
| WBC (× 103/µL) | 7.5 (3.2-14.3) | 7.2 (3.1-15.9) | 0.226 |
| Hemoglobin (mg/dL) | 12.6 (9.8-16.6) | 12.9 (10.1-16.9) | 0.460 |
| PLT (× 103/µL) | 156 (102-253) | 165 (113-267) | 0.115 |
| Creatinine (mg/dL) | 0.89 (0.69-1.20) | 0.83 (0.65-1.15) | 0.328 |
| Glucose (mg/dL) | 10.5(5.1-16.5) | 11.3 (4.4-18.8) | 0.085 |
| Cholesterol (mg/dL) | 159.2 (137.3-195.3) | 144.0 (127.4-199.8) | 0.075 |
| Beta blockers, n (%) | 51 (10.8) | 55 (11.6) | 0.165 |
| Aspirin, n (%) | 43 (9.1) | 47 (9.9) | 0.392 |
| Flap ischemia time (min) | 123 (108-145) | 117 (101-153) | 0.558 |
| Hypotensive events, n (%) | 11 (2.3) | 15 (3.2) | 0.663 |
Major complications after flap transplantation were hematoma in 69 cases (7.3%), infection in 49 cases (5.2%), and damaged flap circulation in 65 cases (6.9%). Salvage measures were implemented for 95 cases (10%), where 61 cases were successfully saved, with a success rate of 64.2%. Finally, 34 patients (3.6%) had flap failure, with the most common cause of postoperative infection followed by hematoma formation.
We developed three machine learning-based models based on the various preoperative and intraoperative data for analyzing the potential risk factors associated with the flap failure after microvascular tissue reconstruction. A total of 473 patients and 16 events were included in the training set, while a total of 473 patients and 18 events were included in the test set. The receiver operating characteristic graph was drawn based on model sensitivity and specificity, whereas the random forest model yielded the highest AUC score in the test set (AUC = 0.770, 95% confidence interval: 0.726-0.854) (Figure 1). The random forest model maintained a very high predictive ability for predicting the flap failure events, indicating that the classification model based on the binary tree could accurately divide the samples into with and without flap failure events. Other model indicators in the random forest were: (1) The value of precision based on the true positive divided by the sum of true positive and false positive was 0.82; (2) The values of recall obtained by dividing true positive by the sum of the true positive and false negative was 0.69; and (3) The values of the F1 score obtained by the precision-recall curve was 0.75 (Table 2).
| Accuracy | Precision | Recall | F1 score | AUC | |
| Random forest | 0.78 | 0.82 | 0.69 | 0.75 | 0.770 |
| Support vector machine | 0.71 | 0.79 | 0.58 | 0.67 | 0.720 |
| Gradient boosting | 0.68 | 0.76 | 0.53 | 0.65 | 0.707 |
Figure 2 represents the importance ranking of tested variables in the random forest model to predict flap failure. The variables were ranked based on the average distance from the split branch to the tree root in the binary tree. The line length measured the variable importance in the random forest model (Figure 2). The top ten variables in the random forest model were age, BMI, ischemia time, smoking, diabetes, experience, prior chemotherapy, hypertension, insulin, and obesity.
Table 3 outlines the statistical analysis results of the top ten variables of the random forest model in the traditional logistic regression analysis. Of note, among the top ten variables, only age, BMI, and ischemic time were significantly associated with the outcomes of the multivariate analysis. For the remaining seven variables, P values for diabetes and prior chemotherapy were 0.06 and 0.07, respectively. Interestingly, surgeon’s experience was not found to be statistically significant in the multivariate analysis.
| Variables | Odds ratio (95%CI) | P value |
| Age | 1.56 (0.57-5.87) | 0.04 |
| Body mass index | 2.83 (0.68-5.54) | 0.02 |
| Ischemia time | 1.98 (0.53-3.24) | 0.001 |
| Smoking | 1.13 (0.28-2.89) | 0.87 |
| Diabetes | 1.15 (0.53-3.28) | 0.06 |
| Experience | 0.86 (0.18-4.87) | 0.79 |
| Prior chemotherapy | 1.15 (0.56-2.68) | 0.07 |
| Hypertension | 1.08 (0.25-2.64) | 0.28 |
| Insulin | 1.27 (0.64-3.21) | 0.54 |
| Obesity | 1.09 (0.57-2.95) | 0.13 |
Free flap transplantation is a robust technique, ensuring the success of tissue reconstruction even with various postoperative complications through timely rescue attempts[25]. However, though the incidence of flap failure is relatively low, once it occurs it is generally detrimental for the patient, as it results in the permanent scarring of the graft area, especially at the region of the face and breast[26]. Therefore, in this study a random forest model based on machine learning was used for a series of preoperative and intraoperative variables, aiming to assess and analyze the risk factors associated with the flap failure after microvascular tissue reconstruction and to screen out the high-risk groups in clinical practice. To best of our knowledge, this is the first report about the application of the random forest model for flap failure after microvascular tissue reconstruction.
When the event-to-variable ratio was greater than 10, an ideal prediction model in multivariate logistic regression analysis was successfully constructed[27]. However, owing to the low incidence of flap failure, the event-to-variable ratio in this study was approximately 1. Therefore, even reducing the variables of the analysis could not achieve the ideal ratio value. Moreover, the traditional logistic regression could not consider the nonlinear relationship between the variables[15]. Therefore, in this study, due to the potential overfitting phenomenon, the utility of the prediction model based on the traditional multivariate analysis might be compromised. The phenomenon partially explains that only three factors, including age, BMI, and ischemia time, were considered statistically significant for flap failure using the multivariate analysis.
Other research has widely explored the comparison between the traditional logistic regression and emerging machine learning for the data analysis. In 2018, Lee et al[15] published a study for predicting acute kidney injury after liver transplantation. Their research indicated that the AUC score of the prediction model based on machine learning could reach up to 0.90, while that of the logistic regression model was only 0.61[15]. In 2020, Arkin et al[28] analyzed 30-d survival prediction of cancer patients by comparing the machine learning and logistic regression to understand the better statistical methods for the relatively accurate prediction of survival. Their results showed that the machine learning-based artificial neural network yielded a higher AUC score of 0.86 than the AUC score of 0.76 in the logistic regression model[28]. Considering the abovementioned pitfall of event-to-variable ratio, several techniques, such as GridSearch, 5-fold cross-validation, and oversampling to avoid potential overfitting defects were used in the current study. We found the adopted machine learning models achieved the highest AUC score of 0.772 in the random forest classifier, as to the interesting outcome of flap failure. The effectiveness of our machine learning model was similar to that of the predictive models demonstrated in other studies. Formeister et al[29] yielded a decision tree model that could correctly classify outcomes with an accuracy ranging from 65% to 75%. O’Neill et al[30] achieved an AUC of 0.95 in the training set and 0.67 in the testing set for 2012 patients within microvascular breast reconstruction.
The potential risk factors involved in the development of flap failure and the contribution ranking in a random forest classifier is shown in Figure 2. These observations are suggestive that the occurrence of flap failure is a multifactor-driven event with the identified numerous factors. Reported preoperative risk factors included BMI, ischemia time, and limited surgical experience[31-33]. Specifically, it is widely accepted that there was an increase in the postoperative complications for the free flap transplantation in the obese patients[34]. At the same time, Chang et al[35] recommended that the microvascular tissue reconstruction should be performed with full discretion of patients with high BMI. Additionally, prolonged ischemia time of the free flap and subsequent ischemia-reperfusion injury can also increase the risk of postoperative complications and eventually flap failure[36].
There were some limitations for our study. First, this was a single-center retrospective study. Thus, although the model achieved high accuracy, relatively few factors and limited cases were included. Second, we did not perform the external validation of the samples from other institutions, so there might be differences that occur in the results obtained from other institutions and larger data sets. Third, we only developed one machine learning model of the random forest classifier, which may become more efficient if we would have used more algorithms during the data analysis. Finally, although we showed the importance of ranking variables in the random forest models, the black-box effect of the predictive models and the analytical decision on the samples remain ambiguous.
In our study, the random forest machine learning technique predicted the flap failure in patients following the microvascular tissue reconstruction for head and neck, breast, and extremities. We also identified the relevant risk factors of the outcome and further analysis in the traditional multivariate logistic regression. The findings of our study will help plastic surgeons to identify the potential risk factors associated with the flap failure and in screening high-risk events. These observations will eventually assist the clinician in decision-making by understanding the underlying pathologic mechanisms of the disease and improving the long-term outcome of the patients. Future multicentric research is required to develop an AI-based, big-data-driven clinical decision support system with a larger sample size.
Microvascular tissue reconstruction is a well-established technique used for the wide variety of tissue defects. However, still a risk of experiencing flap failure exist that eventually results in additional hospital stays, financial burden, and mental stress of the patients.
The application of the machine learning technique in flap failure events remains an underestimated area.
The objective of the current study was to develop machine learning-based predictive models for the flap failure to identify potential factors and screening the high-risk patients.
To establish machine learning classifiers, we used a data set with 945 consecutive patients who underwent microvascular tissue reconstruction. Model performances were evaluated by the indicators including area under the receiver operating characteristic curve, accuracy, precision, recall, and F1 score. A multivariable regression analysis was also performed for the essential variables in the random forest model.
The flap failure event occurred in 152 patients (1.9%) after the operation. The random forest classifier based on various preoperative and intraoperative variables performed the best, with an area under the curve score of 0.770 in the test set. The top variables in the random forest were age, body mass index, ischemia time, smoking, diabetes, experience, prior chemotherapy, hypertension, insulin, and obesity.
Machine learning models were successfully developed for identifying the potential factors and screening out the high-risk patients for the interesting outcome of flap failure.
In our study, the machine learning technique correctly predicted flap failure in the patients who followed microvascular tissue reconstruction. Results from our research will help the clinician in decision-making by better understanding the underlying pathologic mechanisms of the disease and improving the long-term outcome of patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Anemona L, Italy; Gilmore N, United States; Mohan S, India S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Gong ZM
| 1. | Parikh RP, Sharma K, Guffey R, Myckatyn TM. Preoperative Paravertebral Block Improves Postoperative Pain Control and Reduces Hospital Length of Stay in Patients Undergoing Autologous Breast Reconstruction after Mastectomy for Breast Cancer. Ann Surg Oncol. 2016;23:4262-4269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Usubuchi M, Matsuura K, Goto T, Asada Y, Imai T, Ogawa T, Kato K, Saijo S. Professional Oral Health Care at General Dental Clinic Reduces Postoperative Complications of Head and Neck Free-Flap Reconstruction Surgery. J Cancer. 2019;10:205-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Jabir S, Frew Q, Magdum A, El-Muttardi N, Philp B, Dziewulski P. Microvascular free tissue transfer in acute and secondary burn reconstruction. Injury. 2015;46:1821-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Chung SW, Byun IH, Lee WJ. Sequential reconstruction for recurrent head and neck cancer: A 10-year experience. Arch Plast Surg. 2019;46:449-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Kim S, Lee DH, Ahn KM. Microvascular reconstruction for maxillofacial defects: a retrospective analysis of outcomes and complications in 121 consecutive cases. Maxillofac Plast Reconstr Surg. 2020;42:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Wang B, Zhang C, Chu D, Ma X, Yu T, Liu X, Hu C. Astragaloside IV improves angiogenesis under hypoxic conditions by enhancing hypoxia-inducible factor-1α SUMOylation. Mol Med Rep. 2021;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Xu PF, Fang MJ, Jin YZ, Wang LS, Lin DS. Effect of oxytocin on the survival of random skin flaps. Oncotarget. 2017;8:92955-92965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Kanuri A, O'Kelly ND, Shuck J, Kim P, Evans KK, Attinger CE. The Effect of Positive Postdebridement Cultures on Local Muscle Flap Reconstruction of the Lower Extremity. Plast Reconstr Surg Glob Open. 2018;6:e1864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Lassig AAD, Bechtold JE, Lindgren BR, Pisansky A, Itabiyi A, Yueh B, Joseph AM. Tobacco exposure and wound healing in head and neck surgical wounds. Laryngoscope. 2018;128:618-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 10. | Kwok AC, Agarwal JP. An analysis of free flap failure using the ACS NSQIP database. Does flap site and flap type matter? Microsurgery. 2017;37:531-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Yu J, Hong JP, Suh HP, Park JY, Kim DH, Ha S, Lee J, Hwang JH, Kim YK. Prognostic Nutritional Index is a Predictor of Free Flap Failure in Extremity Reconstruction. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Garip M, Van Dessel J, Grosjean L, Politis C, Bila M. The impact of smoking on surgical complications after head and neck reconstructive surgery with a free vascularised tissue flap: a systematic review and meta-analysis. Br J Oral Maxillofac Surg. 2021;59:e79-e98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 13. | Shin JY, Roh SG, Lee NH, Yang KM. Is obesity a predisposing factor for free flap failure and complications? Medicine (Baltimore). 2016;95:e4072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Lin Y, He JF, Zhang X, Wang HM. Intraoperative factors associated with free flap failure in the head and neck region: a four-year retrospective study of 216 patients and review of the literature. Int J Oral Maxillofac Surg. 2019;48:447-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Lee HC, Yoon SB, Yang SM, Kim WH, Ryu HG, Jung CW, Suh KS, Lee KH. Prediction of Acute Kidney Injury after Liver Transplantation: Machine Learning Approaches vs. Logistic Regression Model. J Clin Med. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 124] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 16. | Lee HC, Yoon HK, Nam K, Cho YJ, Kim TK, Kim WH, Bahk JH. Derivation and Validation of Machine Learning Approaches to Predict Acute Kidney Injury after Cardiac Surgery. J Clin Med. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 17. | Ajani TS, Imoize AL, Atayero AA. An Overview of Machine Learning within Embedded and Mobile Devices-Optimizations and Applications. Sensors (Basel). 2021;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 18. | Yoo S, Gujrathi I, Haider MA, Khalvati F. Prostate Cancer Detection using Deep Convolutional Neural Networks. Sci Rep. 2019;9:19518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 19. | Gunčar G, Kukar M, Notar M, Brvar M, Černelč P. An application of machine learning to haematological diagnosis. Sci Rep. 2018;8:411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 20. | Rinner C, Kittler H, Rosendahl C, Tschandl P. Analysis of Collective Human Intelligence for Diagnosis of Pigmented Skin Lesions Harnessed by Gamification Via a Web-Based Training Platform: Simulation Reader Study. J Med Internet Res. 2020;22:e15597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Cui M, Zhang DY. Artificial intelligence and computational pathology. Lab Invest. 2021;101:412-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 223] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 22. | Ravaut M, Sadeghi H, Leung KK, Volkovs M, Kornas K, Harish V, Watson T, Lewis GF, Weisman A, Poutanen T, Rosella L. Predicting adverse outcomes due to diabetes complications with machine learning using administrative health data. NPJ Digit Med. 2021;4:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 23. | van den Bosch T, Warps AK, de Nerée Tot Babberich MPM, Stamm C, Geerts BF, Vermeulen L, Wouters MWJM, Dekker JWT, Tollenaar RAEM, Tanis PJ, Miedema DM; Dutch ColoRectal Audit. Predictors of 30-Day Mortality Among Dutch Patients Undergoing Colorectal Cancer Surgery, 2011-2016. JAMA Netw Open. 2021;4:e217737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 24. | Liang X, Yang X, Yin S, Malay S, Chung KC, Ma J, Wang K. Artificial Intelligence in Plastic Surgery: Applications and Challenges. Aesthetic Plast Surg. 2021;45:784-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 25. | Kim HS, Chung CH, Chang YJ. Free-flap reconstruction in recurrent head and neck cancer: A retrospective review of 124 cases. Arch Craniofac Surg. 2020;21:27-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Zhu G, Li C, Chen J, Cai Y, Li L, Wang Z. Modified free latissimus dorsi musculocutaneous flap in the reconstruction of extensive postoncologic defects in the head and neck region. J Craniofac Surg. 2015;26:572-576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Abdollahpour I, Aguilar-Palacio I, Gonzalez-Garcia J, Vaseghi G, Otroj Z, Manteghinejad A, Mosayebi A, Salimi Y, Haghjooy Javanmard S. Model Prediction for In-Hospital Mortality in Patients with COVID-19: A Case-Control Study in Isfahan, Iran. Am J Trop Med Hyg. 2021;104:1476-1483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Arkin FS, Aras G, Dogu E. Comparison of Artificial Neural Networks and Logistic Regression for 30-days Survival Prediction of Cancer Patients. Acta Inform Med. 2020;28:108-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Formeister EJ, Baum R, Knott PD, Seth R, Ha P, Ryan W, El-Sayed I, George J, Larson A, Plonowska K, Heaton C. Machine Learning for Predicting Complications in Head and Neck Microvascular Free Tissue Transfer. Laryngoscope. 2020;130:E843-E849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 30. | O'Neill AC, Yang D, Roy M, Sebastiampillai S, Hofer SOP, Xu W. Development and Evaluation of a Machine Learning Prediction Model for Flap Failure in Microvascular Breast Reconstruction. Ann Surg Oncol. 2020;27:3466-3475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 31. | Fischer JP, Nelson JA, Kovach SJ, Serletti JM, Wu LC, Kanchwala S. Impact of obesity on outcomes in breast reconstruction: analysis of 15,937 patients from the ACS-NSQIP datasets. J Am Coll Surg. 2013;217:656-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 161] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 32. | Las DE, de Jong T, Zuidam JM, Verweij NM, Hovius SE, Mureau MA. Identification of independent risk factors for flap failure: A retrospective analysis of 1530 free flaps for breast, head and neck and extremity reconstruction. J Plast Reconstr Aesthet Surg. 2016;69:894-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 33. | Mahmoudi E, Lu Y, Chang SC, Lin CY, Wang YC, Chang CJ, Cheng MH, Chung KC. Associations of Surgeon and Hospital Volumes with Outcome for Free Tissue Transfer by Using the National Taiwan Population Health Care Data from 2001 to 2012. Plast Reconstr Surg. 2017;140:455e-465e. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | O'Neill AC, Sebastiampillai S, Zhong T, Hofer SOP. Increasing body mass index increases complications but not failure rates in microvascular breast reconstruction: A retrospective cohort study. J Plast Reconstr Aesthet Surg. 2019;72:1518-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Chang DW, Wang B, Robb GL, Reece GP, Miller MJ, Evans GR, Langstein HN, Kroll SS. Effect of obesity on flap and donor-site complications in free transverse rectus abdominis myocutaneous flap breast reconstruction. Plast Reconstr Surg. 2000;105:1640-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 275] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 36. | Seyid M, Tiftikcioglu Y, Erdem M, Akdemir O, Tatar BE, Uyanıkgil Y, Ercan G. The Effect of Ceruloplasmin Against Ischemia-Reperfusion Injury in Epigastric Island Flap in Rats. J Surg Res. 2021;267:627-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |