Published online Apr 26, 2022. doi: 10.12998/wjcc.v10.i12.3709
Peer-review started: September 1, 2021
First decision: October 25, 2021
Revised: November 8, 2021
Accepted: March 6, 2022
Article in press: March 6, 2022
Published online: April 26, 2022
Processing time: 232 Days and 0.2 Hours
Lymph node metastasis (LNM) of papillary thyroid carcinoma (PTC) has a certain regularity and occurs first to the central lymph node and then to the lateral lymph node. The pathway of PTC LNM can guide surgical prophylactic lymph node dissection (LND) for clinical surgeons.
To investigate the relationship between subgroups of central LNM and lateral LNM in unilateral clinically node-negative PTC (cN0-PTC).
Data were collected for 1089 PTC patients who underwent surgical treatment at the Department of Endocrine and Breast Surgery of the First Hospital of Chongqing Medical University from January 2016 to December 2017. A total of 388 unilateral cN0-PTC patients met the inclusion criteria and were enrolled in this study. The clinical and pathological data for these 388 patients who underwent total thyroidectomy + central LND + lateral LND were retrospectively analyzed. The relationship between the central LNM and lateral LNM subgroups was investigated.
The coincidence rate of cN0-PTC was only 30.0%.Optimal scaling regression analysis showed that sex (57.1% vs 42.9%, P = 0.026), primary tumor size (68.8% vs 31.2%, P = 0.008), tumor location (59.7% vs 40.3%, P = 0.007), extrathyroid extension (ETE) (50.6% vs 49.9%, P = 0.046), and prelaryngeal LNM (57.1% vs 42.9%, P = 0.004) were significantly associated with ipsilateral level-II LNM. Their importance levels were 0.122, 0.213, 0.172, 0.110, and 0.227, respectively. Primary tumor size (74.6% vs 30.2%, P = 0.016), pretracheal LNM (67.5% vs 32.5%, P < 0.001), and paratracheal LNM (71.4% vs 28.6%, P < 0.001) were significantly associated with ipsilateral level-III LNM. Their importance levels were 0.120, 0.408, and 0.351, respectively. Primary tumor size (72.1% vs 27.9%, P = 0.003), ETE (70.4% vs 29.6%, P = 0.016), pretracheal LNM (68.3% vs 31.7%, P=0.001), and paratracheal LNM (80.8% vs 19.2%, P < 0.001) were significantly associated with ipsilateral level-IV LNM. Their importance levels were 0.164, 0.146, 0.216, and 0.472, respectively.
The LNM pathway of thyroid cancer has a certain regularity. For unilateral cN0-PTC patients with a tumor diameter > 2 cm and pretracheal or ipsilateral paratracheal LNM, LND at ipsilateral level III and level IV must be considered. When there is a tumor in the upper third of the thyroid with prelaryngeal LNM, LND at level II, level III and level IV must be considered.
Core Tip: Lymph node metastasis (LNM) of papillary thyroid carcinoma (PTC) has a certain regularity. The pathway of PTC LNM can guide selective lymph node dissection (LND) for clinical surgeons, thereby overcoming the drawbacks of prophylactic LND; accurate surgery can be used to not only radically dissect lymph nodes but also reduce the incidence of complications. The results of our retrospective study found that the LNM pathway of PTC has a certain regularity. Prelaryngeal LNM is a predictor of ipsilateral level-II LNM. Pretracheal and ipsilateral paratracheal LNM are predictors of ipsilateral level-III and level-IV LNM. Therefore, for unilateral cN0-PTC patients with a tumor diameter > 2 cm and pretracheal or ipsilateral paratracheal LNM, LND at ipsilateral level III and level IV must be considered. When there is a tumor in the upper third of the thyroid with prelaryngeal LNM, LND at level II, level III and level IV must be considered.
- Citation: Zhou J, Li DX, Gao H, Su XL. Relationship between subgroups of central and lateral lymph node metastasis in clinically node-negative papillary thyroid carcinoma. World J Clin Cases 2022; 10(12): 3709-3719
- URL: https://www.wjgnet.com/2307-8960/full/v10/i12/3709.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i12.3709
Papillary thyroid carcinoma (PTC) is the most common pathological type of thyroid malignant tumor, and its incidence is increasing every year[1-3]. Most patients with PTC have a good prognosis, but cervical lymph node metastasis (LNM) may still occur in the early stage[4]. The LNM rate of PTC has been reported to be 20%-90%, which may be related to the different stages of the disease, occult LNM and different lymph node dissections (LNDs) in the first operation[4-7]. At present, many scholars recommend cervical LND for PTC with clinically involved neck nodes (cN1-PTC), but there is great controversy about clinically node-negative PTC (cN0-PTC) LND, and most scholars favor central LND (CLND) on at least one side[8,9]. Studies have reported a high rate of occult LNM in cN0-PTC patients for whom prophylactic lateral LND (LLND) is not recommended. This occurs in the central area and the lateral area mainly due to the low accuracy of the preoperative diagnosis of cN0 tumors[10,11]. A study by Kim et al[12] showed that the accuracy of ultrasonography in the diagnosis of lateral LNM (LLNM) of PTC was 51% and that the accuracy of computed tomography was 62%[13,14]. Intraoperative freezing is helpful for the diagnosis of LNM in the central area of cN0-PTC and changes the surgical method of total thyroidectomy in many cN0-PTC patients[15,16]. Previous studies have suggested that central LNM (CLNM) has a predictive effect on cervical LLNM, which is helpful to guide LLND[9,11,17], but the relationship between the subgroup of CLNM and ipsilateral LLNM is not yet clear. The purpose of this research was to analyze the relationship between LNM in subgroups of central and ipsilateral lateral areas in unilateral cN0-PTC and to provide a basis for prophylactic LLND.
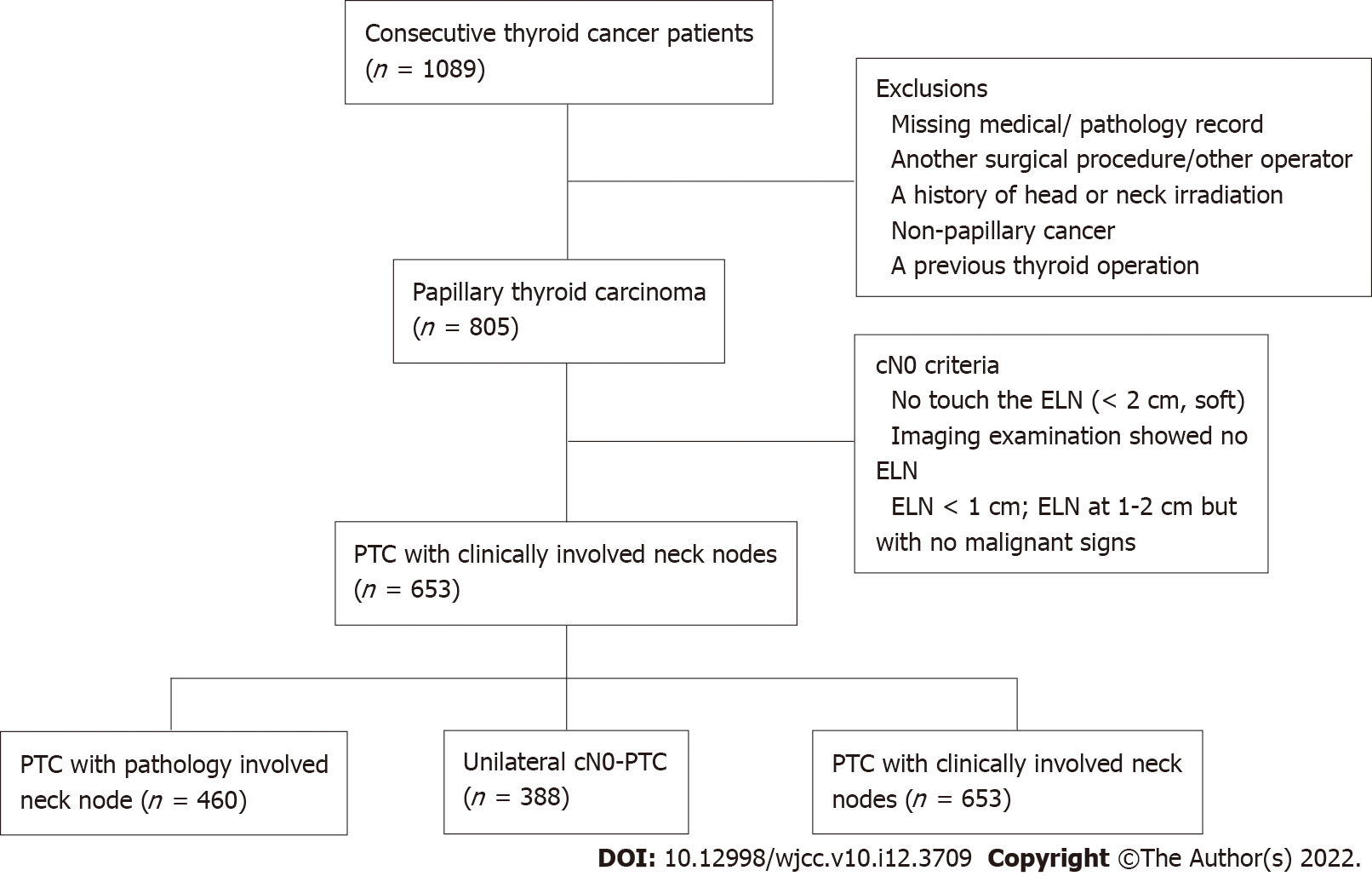
Data were collected for 1089 PTC patients who underwent surgical treatment at the Department of Endocrine and Breast Surgery of the First Hospital of Chongqing Medical University. The criteria for inclusion were as follows: (1) Having complete medical records that fully provided the clinical and pathological features of the patient; (2) Meeting the cN0 criteria based on the clinical diagnostic criteria of cervical lymph nodes proposed by Kouvaraki et al[18] , which are that the clinical examination did not touch the enlarged lymph nodes or that the maximum diameter of the enlarged lymph nodes was < 2 cm and that the enlarged lymph nodes were texturally soft; and the imaging examination showed no enlarged lymph nodes or enlarged lymph nodes with a maximum diameter < 1 cm or enlarged lymph nodes with a maximum diameter of 1-2 cm but an aspect ratio > 2 and having a regular shape, clear boundary of dermis and medulla, no fine calcification of sand particles, central liquefaction necrosis, peripheral enhancement and disappearance of adjacent fat space; (3) The operations were performed by the same operator using the method of total thyroidectomy + CLND + LLND; (4) The tumor was confined to one side of the glandular lobe; and (5) The resected lymph nodes of the patients with PTC confirmed by postoperative pathological examination were strictly zoned and examined, and the pathological examination report data were complete (Figure 1).
The lymph node zoning criteria were as follows. As recommended by the American Academy of Otolaryngology–Head and Neck Surgery Foundation in 1991, cervical lymph nodes were divided into 6 levels: Level I, submandibular area and submental area; Level II, superior jugular vein lymphatic chain group; Level III, middle jugular vein lymphatic chain group; Level IV, inferior jugular vein lymphatic chain group; level V, posterior trigonometric area; and Level VI, anterior jugular area. In 2002, the American Joint Committee on Cancer updated the zoning by adding the anterior superior mediastinal lymph nodes as level VII. In clinical work, central lymph nodes are classified as level VI, and lateral lymph nodes of the neck are classified as levels II-V[19]. Central lymph nodes (level VI) are divided into four subgroups, namely, prelaryngeal, pretracheal, ipsilateral paratracheal and contralateral paratracheal, as recommended by the American Academy of Otolaryngology–Head and Neck Surgery Foundation in 2009[20]. The range of prelaryngeal LND is the area below the hyoid bone and above the cricoid cartilage, that of pretracheal LND is the tracheal surface up to the thyroid gland and down to the innominate artery, and that of paratracheal LND is the area from the internal carotid artery to the paratracheal area.
Regarding neck LND, all of the patients with unilateral cN0-PTC were first treated with unilateral lobectomy plus ipsilateral CLND. The specimens of the central lymph node subgroups of the affected side were strictly divided and frozen during the operation. All the specimens were diagnosed by two pathologists with more than 10 years of work experience, with each lymph node reported separately by area. If intraoperative freezing indicated CLNM and the tumor was located in the upper part of the thyroid gland, then ipsilateral LLND was performed (levels II, III and IV).
Grouping was performed based on whether there was LNM in the central area and its subgroups of the affected side according to the pathological results, e.g., the central area (+) and (-) of the affected side. The other subgroups were categorized in the same way.
SPSS 22.0 software was used for the statistical analysis. In the univariate analysis, the measurement data were analyzed by an independent sample t test, and the count data were analyzed by the chi-square test (Pearson correlation analysis) with a test level of P < 0.05. Variables with statistical significance in the univariate analysis were subjected to optimal scaling regression analysis.
Of 1089 PTC patients, 388 unilateral cN0-PTC patients were enrolled in this study; 653 were evaluated as cN0 before the operation, 193 were pathologically confirmed to be cN0, and 460 had pathologically involved neck nodes (pN1) after the operation. The rate of meeting the cN0 criterion was 30.0% (Figure 1).
The female to male ratio of the 388 unilateral cN0-PTC patients enrolled in this study was 2.22:1; the average age of the patients was 43.5 ± 13.6 years old. The mean tumor size was 14.2 ± 8.9 mm. Hashimoto’s thyroid (HT) was present in 80 patients (20.6%), tumors involving the superior aspect of the thyroid lobe were present in 102 patients (26.3%), extrathyroid extension (ETE) was present in 136 patients (35.1%), and multiple lesions were present in 48 patients (12.4%). The metastasis rate of LLNM was 46.6% in the 388 patients with unilateral cN0-PTC. The LNM rate was 19.8% for level II, 32.5% for level III and 26.8% for level IV lymph nodes of the affected side in patients with unilateral cN0-PTC. The LNM rate was 24.5% in prelaryngeal LNM, 48.7% in pretracheal LNM and 57.0% in ipsilateral paratracheal LNM of the affected side in patients with unilateral cN0-PTC (Table 1).
| Clinicopathological factor | Lateral lymph node metastasis | Ipsilateral lymph node metastasis level II | Ipsilateral lymph node metastasis level III | Ipsilateral lymph node metastasis level IV | |||||||||
| Positive, n = 181 | Negative, n = 207 | P value | Positive, n = 77 | Negative, n = 311 | P value | Positive, n = 126 | Negative, n = 262 | P value | Positive, n = 104 | Negative, n = 284 | P value | ||
| Age (yr) | < 55 | 147 (81.2) | 169 (81.6) | 0.914 | 59 (76.6) | 257 (82.6) | 0.224 | 101 (80.2) | 215 (82.1) | 0.652 | 87 (87.3) | 229 (80.6) | 0.498 |
| ≥ 55 | 34 (18.8) | 38 (18.4) | 18 (23.4) | 54 (17.4) | 25 (19.8) | 47 (17.9) | 17 (16.3) | 55 (19.4) | |||||
| Sex | Female | 118 (65.2) | 154 (74.4) | 0.048 | 44 (57.1) | 228 (73.3) | 0.006 | 85 (67.5) | 187 (71.4) | 0.430 | 70 (67.3) | 202 (71.1) | 0.467 |
| Male | 63 (34.8) | 53 (25.6) | 33 (42.9) | 83 (26.7) | 41 (32.5) | 75 (28.6) | 34 (32.7) | 82 (28.9) | |||||
| Primary tumor size | > 2 cm | 44 (24.3) | 21 (10.1) | < 0.001 | 24 (31.2) | 41 (13.2) | < 0.001 | 32 (25.4) | 33 (12.6) | 0.002 | 29 (27.9) | 36 (12.7) | < 0.001 |
| ≤ 2 cm | 137 (75.7) | 186 (89.9) | 53 (68.8) | 270 (86.8) | 94 (74.6) | 229 (87.4) | 75 (72.1) | 248 (873) | |||||
| Tumor location | UP | 57 (31.5) | 45 (21.7) | 0.029 | 31 (40.3) | 71 (22.8) | 0.002 | 38 (30.2) | 64 (24.4) | 0.230 | 28 (26.9) | 74 (26.1) | 0.864 |
| Non-UP | 124 (68.5) | 162 (78.3) | 46 (59.7) | 240 (77.2) | 88 (69.8) | 198 (75.6) | 76 (73.1) | 210 (73.9) | |||||
| Multifocality | Yes | 27 (14.9) | 21 (10.1) | 0.154 | 15 (19.5) | 33 (10.6) | 0.034 | 19 (15.1) | 29 (11.1) | 0.261 | 17 (16.3) | 31 (10.9) | 0.150 |
| No | 154 (85.1) | 186 (89.9) | 62 (80.5) | 278 (89.4) | 107 (84.9) | 233 (88.91) | 87 (83.7) | 253 (89.1) | |||||
| ETE | Yes | 78 (43.1) | 58 (28.0) | 0.002 | 38 (49.4) | 98 (31.5) | 0.003 | 54 (42.9) | 82 (31.3) | 0.025 | 52 (50.01) | 84 (29.6) | < 0.001 |
| No | 103 (56.9) | 149 (72.0) | 39 (50.6) | 213 (68.5) | 72 (57.1) | 180 (68.7) | 52 (50.0) | 200 (70.4) | |||||
| HT | Yes | 36 (19.9) | 44 (21.3) | 0.740 | 11 (14.3) | 69 (22.2) | 0.125 | 28 (22.2) | 52 (19.8) | 0.344 | 21 (20.2) | 59 (20.8) | 0.900 |
| No | 145 (80.1) | 163 (78.7) | 66 (85.7) | 242 (77.8) | 88 (77.8) | 210 (80.2) | 83 (79.8) | 225 (79.2) | |||||
| CLNM | 0 | 17 (9.4) | 86 (41.5) | < 0.001 | 10 (13.0) | 93 (29.9) | < 0.001 | 14 (11.1) | 89 (34.0) | < 0.001 | 6 (5.8) | 97 (34.2) | < 0.001 |
| 1-2 | 45 (24.9) | 65 (31.4) | 18 (23.4) | 92 (29.6) | 30 (23.8) | 80 (30.5) | 22 (21.2) | 88 (31.0) | |||||
| ≥ 3 | 119 (65.7) | 56 (27.1) | 49 (63.6) | 126 (40.5) | 82 (65.1) | 93 (35.5) | 76 (73.1) | 99 (34.9) | |||||
| Prelaryngeal LNM | 0 | 113 (62.4) | 180 (87.0) | < 0.001 | 44 (57.1) | 249 (80.1) | < 0.001 | 82 (65.1) | 21 (80.5) | 0.001 | 66 (63.5) | 37 (79.9) | < 0.001 |
| 1-2 | 62 (34.3) | 25 (12.1) | 29 (37.7) | 58 (18.6) | 38 (30.2) | 49 (18.7) | 36 (34.6) | 51 (18.0) | |||||
| ≥ 3 | 6 (3.30) | 2 (1.00) | 4 (5.2) | 4 (1.3) | 6 (4.8) | 2 (0.8) | 2 (1.9) | 6 (2.1) | |||||
| Pretracheal LNM | 0 | 65 (35.9) | 134 (64.7) | < 0.001 | 32 (41.6) | 167 (53.7) | 0.121 | 41 (32.5) | 158 (60.3) | < 0.001 | 33 (31.7) | 166 (58.5) | 0.002 |
| 1-2 | 60 (29.0) | 74 (40.9) | 30 (39.0) | 104 (33.4) | 51 (40.5) | 83 (31.7) | 47 (45.2) | 87 (30.6) | |||||
| ≥ 3 | 42 (23.2) | 13 (6.3) | 15 (19.5) | 40 (12.9) | 34 (27.0) | 21 (8.0) | 24 (23.1) | 31 (10.9) | |||||
| Ipsilateral paratracheal LNM | 0 | 46 (25.4) | 121 (58.5) | < 0.001 | 20 (26.0) | 147 (47.3) | < 0.001 | 36 (28.6) | 131 (50.0) | < 0.001 | 20 (19.2) | 147 (51.8) | < 0.001 |
| 1-2 | 73 (40.3) | 64 (30.9) | 26 (33.8) | 111 (35.7) | 42 (33.3) | 95 (36.3) | 45 (43.3) | 92 (32.4) | |||||
| ≥ 3 | 62 (34.3) | 22 (10.6) | 31 (40.3) | 53 (17.0) | 48 (38.1) | 36 (13.7) | 39 (37.5) | 45 (15.8) | |||||
A total of 181 patients (46.6%) had ipsilateral LLNM, and the univariate analysis showed that there was statistical significance in the sex (P = 0.048), primary tumor size (P < 0.001), tumor location (P = 0.029), ETE (P = 0.002), CLNM (P < 0.001), prelaryngeal LNM (P < 0.001), pretracheal LNM (P < 0.001), and paratracheal LNM (P < 0.001). Optimal scaling regression analysis showed that the regression models were statistically significant (adjusted R-squared = 0.223, F = 13.317, P < 0.001). Primary tumor size, tumor location, prelaryngeal LNM, pretracheal LNM, and paratracheal LNM were significantly associated with ipsilateral LLNM. Their importance levels were 0.104, 0.055, 0.168, 0.260 and 0.362, respectively. The total importance levels of prelaryngeal LNM, pretracheal LNM, and paratracheal LNM was 0.790 (Table 2).
| Variable | Beta | Standardized coefficient std. error | Df | F | Sig | Tolerance | Importance | |
| After transformation | Before transformation | |||||||
| Sex | 0.007 | 0.028 | 1 | 0.058 | 0.809 | 0.911 | 0.911 | 0.003 |
| Primary tumor size | 0.132 | 0.045 | 1 | 8.477 | 0.004 | 0.958 | 0.956 | 0.104 |
| Tumor location | 0.119 | 0.044 | 1 | 7.449 | 0.007 | 0.974 | 0.975 | 0.055 |
| ETE | 0.076 | 0.043 | 1 | 3.153 | 0.077 | 0.959 | 0.959 | 0.049 |
| Prelaryngeal LNM | 0.142 | 0.049 | 1 | 8.338 | 0.004 | 0.879 | 0.871 | 0.168 |
| Pretracheal LNM | 0.197 | 0.051 | 1 | 14.773 | 0.000 | 0.836 | 0.831 | 0.260 |
| Ipsilateral paratracheal LNM | 0.239 | 0.049 | 1 | 23.330 | 0.000 | 0.794 | 0.798 | 0.382 |
A total of 77 patients (26.8%) had level-II LNM, and a univariate analysis showed that there was statistical significance in the sex (P = 0.006), primary tumor size (P = 0.002), tumor location (P = 0.002), ETE (P = 0.034) multifocality (P = 0.003), prelaryngeal LNM (P < 0.001), and paratracheal LNM (P < 0.001). Optimal scaling regression analysis showed that the regression models were statistically significant (adjusted R-squared = 0.119, F = 7.563, P < 0.001). Sex, primary tumor size, tumor location, ETE, and prelaryngeal LNM were significantly associated with ipsilateral level-II LNM. Their importance levels were 0.122, 0.213, 0.172, 0.110 and 0.227, respectively (Table 3).
| Variable | Beta | Standardized coefficient std. error | Df | F | Sig | Tolerance | Importance | |
| After transformation | Before transformation | |||||||
| Sex | 0.119 | 0.053 | 1 | 5.029 | 0.026 | 0.913 | 0.913 | 0.122 |
| Primary tumor size | 0.153 | 0.058 | 1 | 7.033 | 0.008 | 0.954 | 0.954 | 0.213 |
| Tumor location | 0.150 | 0.055 | 1 | 7.298 | 0.007 | 0.984 | 0.984 | 0.172 |
| Multifocality | 0.048 | 0.042 | 1 | 1.321 | 0.251 | 0.963 | 0.963 | 0.038 |
| ETE | 0.101 | 0.051 | 1 | 4.005 | 0.046 | 0.964 | 0.964 | 0.110 |
| Prelaryngeal LNM | 0.142 | 0.060 | 1 | 5.668 | 0.004 | 0.907 | 0.906 | 0.227 |
| Ipsilateral paratracheal LNM | 0.239 | 0.047 | 1 | 3.319 | 0.069 | 0.859 | 0.859 | 0.119 |
A total of 126 patients (32.5%) had level-III LNM, and a univariate analysis showed that there was statistical significance in the primary tumor size (P = 0.002), ETE (P = 0.025), CLNM (P < 0.001), prelaryngeal LNM (P < 0.001), pretracheal LNM (P < 0.001), and paratracheal LNM (P < 0.001). Optimal scaling regression analysis showed that the regression models were statistically significant (adjusted R-squared = 0.144, F = 9.108, P < 0.001). Primary tumor size, pretracheal LNM, and paratracheal LNM were significantly associated with ipsilateral level-III LNM. Their importance levels were 0.120, 0.408 and 0.351, respectively. The total importance level of pretracheal LNM and paratracheal LNM was 0.759 (Table 4).
| Variable | Beta | Standardized coefficient std. error | Df | F | Sig | Tolerance | Importance | |
| After transformation | Before transformation | |||||||
| Primary tumor size | 0.121 | 0.050 | 1 | 5.833 | 0.016 | 0.968 | 0.961 | 0.120 |
| ETE | 0.051 | 0.042 | 1 | 1.528 | 0.217 | 0.965 | 0.963 | 0.036 |
| Prelaryngeal LNM | 0.079 | 0.049 | 1 | 2.569 | 0.078 | 0.935 | 0.876 | 0.084 |
| Pretracheal LNM | 0.216 | 0.054 | 1 | 16.140 | 0.000 | 0.874 | 0.849 | 0.408 |
| Ipsilateral paratracheal LNM | 0.200 | 0.056 | 1 | 12.686 | 0.000 | 0.905 | 0.836 | 0.351 |
A total of 104 patients (32.5%) had level- IV LNM, and a univariate analysis showed that there was statistical significance in the primary tumor size (P < 0.001) , ETE (P = 0.002), CLNM (P < 0.001), prelaryngeal LNM (P < 0.001), and pretracheal LNM (P < 0.001), and paratracheal LNM (P < 0.001). Optimal scaling regression analysis showed that the regression models were statistically significant (adjusted R-squared = 0.150, F =10.773, P < 0.001). Primary tumor size, ETE, pretracheal LNM, and paratracheal LNM were significantly associated with ipsilateral level-IV LNM. Their importance levels were 0.164, 0.146, 0.216 and 0.472, respectively. The total importance level of pretracheal LNM and paratracheal LNM was 0.688 (Table 5).
| Variable | Beta | Standardized coefficient std. error | Df | F | Sig | Tolerance | Importance | |
| After transformation | Before transformation | |||||||
| Primary tumor size | 0.150 | 0.051 | 1 | 8.730 | 0.003 | 0.975 | 0.961 | 0.164 |
| ETE | -0.127 | 0.052 | 1 | 5.884 | 0.016 | 0.962 | 0.963 | 0.146 |
| Prelaryngeal LNM | -0.071 | 0.046 | 1 | 2.357 | 0.126 | 0.973 | 0.876 | 0.003 |
| Pretracheal LNM | 0.149 | 0.055 | 1 | 7.415 | 0.001 | 0.884 | 0.849 | 0.216 |
| Ipsilateral paratracheal LNM | 0.251 | 0.049 | 1 | 26.079 | 0.000 | 0.889 | 0.846 | 0.472 |
To date, whether LND should be performed in cN0-PTC patients is still controversial[10]. Most scholars support prophylactic CLND, while prophylactic LLND is controversial[8]. Some articles argue that prophylactic LLND is beneficial for accurate postoperative staging and reduces the chances of local recurrence and distant metastasis, while others disapprove of prophylactic LLND[16]. Since cervical LLNM does not affect the overall survival of PTC patients and prophylactic LLND is extensive and difficult, the incidence of chylous leakage, pain, cervical dysfunction, edema, sensory abnormalities and other surgical complications has increased[21]. At present, the cN0 criterion for preoperative evaluation of the lymph node condition in PTC patients is not very accurate. In this study, the coincidence rate of cN0 was only 30.0%, which is low, similar to results in previous studies. Therefore, exploring the regularity of PTC LNM to determine the range of LND is helpful for identifying and addressing occult metastasis and for reducing local recurrence and distant metastasis.
Some scholars have suggested that lymphatic metastasis of thyroid carcinoma is usually predictable, occurring first to the central area of the affected side and then to the lateral lymph node, and that leaping metastasis is rare[11,22,23]. Lim et al[24] studied 246 patients with PTC with metastasis in the central area and found that CLNM was an independent risk factor for LLNM (OR = 38.82, P < 0.001). Liu et al[22] reported that CLNM was a predictor of LLNM in 966 patients who underwent total thyroidectomy + CLND + selective LLND. Isaacs et al[23] suggested that LNM in the prelaryngeal subgroup of the central area of the affected side had predictive value for the LLNM. The results of this study reveal that there is LNM in the central area and that the rate of LLNM is 46.6%. CLNM was positively associated with the risk of LLNM (P < 0.001). Prelaryngeal, pretracheal and ipsilateral paratracheal LNM had a substantial influence on ipsilateral LLNM. According to the optimal scaling regression models, prelaryngeal, pretracheal and ipsilateral paratracheal LNM were positively correlated with ipsilateral LLNM and with a relatively high importance level, which suggests that the subgroup of the central area of the affected side is the main factor affecting ipsilateral LLNM. The subgroup of the central LNM accounted for 84.3% of the factors affecting LLNM; these results indicated that under other conditions, with an increase in the number of positive LNMs, the possibility of LNM was obvious, with approximately 84.3% of LLNM determined by the CLNM subgroup, which is consistent with the above results. Thus, the CLNM subgroup has predictive value for LNM on the affected side. LLND should be considered when intraoperative freezing indicates LNM in a subgroup of the central area.
This study first investigated the relationship between LNM in subgroups of the central area and ipsilateral LLNM. The optimal scaling regression models showed that prelaryngeal LNM was associated with ipsilateral level-II LNM, and with a relatively high importance level, which suggests that prelaryngeal LNM is a main factor affecting ipsilateral level-II LNM, indicating that under other conditions, with an increase in positive numbers of prelaryngeal LNMs, the possibility of ipsilateral level-II LNM was obvious, and prelaryngeal LNM was the most important influencing factor of ipsilateral level-II LNM. According to the importance of the model, approximately 22.7% of ipsilateral level-II LNM was determined by prelaryngeal LNM, which may be related to the following factors. Anatomically, the prelaryngeal lymph nodes are located between the thyroid cartilage and hyoid bone to drain the lymph of the thyroid and larynx, which is close to the location of lymph nodes in level II. Some scholars have suggested that the lymphatic vessels accompanying the superior thyroid artery mainly collect the drained lymph from the upper part of the thyroid gland into the venous system through the lateral lymph node[23,25-27]. Our research indicated that the probability of ipsilateral level-II LNM also increased with primary tumor size and tumor location, and the importance level was relatively high. The optimal scaling regression models showed that given a tumor in the upper third of the thyroid with a larger diameter and the increase in positive numbers of prelaryngeal LNMs, the possibility of ipsilateral level-II LNM was obvious. Many studies have shown that the location of a PTC affects the probability of LLNM. Hunt et al[28] suggested that tumors involving the superior aspect of the thyroid lobe were more likely to be associated with metastasis to the lateral cervical lymph nodes (P < 0.01), and 76.9% of patients with lateral cervical lymph node disease had involvement of the superior aspect of the lobe. Kwak et al[29] suggested that a tumor in the upper third of the thyroid was associated with a 4.7-fold increased risk of LLNM. Dou et al[30] suggested that a tumor on the upper third of the thyroid could be used to assist in the evaluation of LLNM in PTC patients. In this study, dissection of the lymph node at level II of the affected side of unilateral cN0-PTC patients with prelaryngeal LNM must be considered.
Our univariate and multivariate analyses indicated that the risk of ipsilateral level-III LNM and level-III LNM increased with primary tumor size, ETE and CLNM subgroup. The optimal scaling regression models showed that pretracheal and ipsilateral paratracheal LNM were associated with ipsilateral level-III LNM and ipsilateral level-IV LNM, which indicated that under other conditions, with the increase in positive numbers of pretracheal and ipsilateral paratracheal LNMs, the possibility of ipsilateral level-III LNM and ipsilateral level-IV LNM were obvious. According to the importance of the model, the sum important influencing factor of pretracheal and ipsilateral paratracheal LNM that affected ipsilateral level-III LNM was 75.9%; that is, approximately 75.9% of ipsilateral level-III LNM was determined by the above two factors. Similarly, approximately 68.8% of ipsilateral level-IV LNM was determined by pretracheal and ipsilateral paratracheal LNM, and approximately 47.2% of ipsilateral level-IV LNM was determined by ipsilateral paratracheal LNM. This may be related to the lymphatic drainage of the thyroid. Some studies have suggested that the lymphatic vessels accompanying the inferior thyroid artery mainly collect the middle and lower parts of the thyroid gland into the lateral lymph node through the pretracheal and ipsilateral paratracheal lymph nodes and finally into the internal jugular vein[23,25-27]. This study indicated that the probability of ipsilateral level-III LNM and ipsilateral level-IV LNM also increased with primary tumor size, which was consistent with a previous study. Feng et al[31] suggested that a tumor size > 1 cm was associated with a 3.474-fold increased risk of LLNM. Ma et al[32] showed that a greater tumor size was significantly associated with LNM. In this study, it was found that dissection of the lymph node at levels III and IV of the affected side of unilateral cN0-PTC patients must be considered when the tumor diameter is > 2 cm with pretracheal or ipsilateral paratracheal LNM.
In summary, lymphatic metastasis of PTC is traceable. Prelaryngeal LNM is related to LNM in ipsilateral level-II LNM and pretracheal and ipsilateral paratracheal LNM to LNM in ipsilateral level-III and level-IV LNM; these findings reveal the lymphatic drainage pathway around the thyroid gland, provide a basis for regional dissection and accurate dissection of lateral lymph nodes of unilateral cN0-PTC patients and help determine the extent of LLND. However, further in-depth studies are needed to prove these findings in consideration of the small sample size of this study, lack of basic research support and insufficient follow-up testing.
For unilateral cN0-PTC patients, an understanding of LNM in each subgroup of the central area is helpful to determine the range of prophylactic lateral cervical LND. For unilateral cN0-PTC patients with a tumor diameter > 2 cm and pretracheal or ipsilateral paratracheal LNM, LND at ipsilateral level III and level IV can be considered. When there is a tumor in the upper third of the thyroid with prelaryngeal LNM, LND at level II, level III and level IV can be considered.
Prophylactic lateral lymph node dissection (LLND) is controversial for clinically node-negative papillary thyroid carcinoma (cN0-PTC), mainly due to the low accuracy of the preoperative diagnosis of cN0. CN0 is not equal to pathological node negative, and many researchers are working to identify risk factors for lateral lymph node metastasis (LNM) to realize selective prophylactic LLND.
The relationship between the central LNM and lateral LNM subgroups demonstrates a regularity in LNM in PTC. It is the basis of accurate surgical decisions by clinical surgeons.
To investigate the relationship between subgroups of central LNM and lateral LNM of unilateral cN0-PTC.
The clinical and pathological data of 388 patients with unilateral cN0-PTC from the Endocrine Mammary Surgery Department of the First Affiliated Hospital of Chongqing Medical University from January 2016 to December 2017 were retrospectively analyzed. Optimal scaling regression analysis explained the relationship between subgroups of central LNM and lateral LNM of unilateral cN0-PTC.
The coincidence rate of cN0 was only 30.0%, and sex (57.1% vs 42.9%, P = 0.026), primary tumor size (68.8% vs 31.2%, P = 0.008), tumor location (59.7% vs 40.3%, P = 0.007), extrathyroid extension (ETE) (50.6% vs 49.9%, P =0.046), and prelaryngeal LNM (57.1% vs 42.9%, P = 0.004) were independent risk factors for ipsilateral level-II LNM. Primary tumor size (74.6% vs 30.2%, P = 0.016), pretracheal LNM (67.5% vs 32.5%, P < 0.001), and paratracheal LNM (71.4% vs 28.6%, P < 0.001) were independent risk factors for ipsilateral level-III LNM. Primary tumor size (72.1% vs 27.9%, P = 0.003), ETE (70.4% vs 29.6%, P = 0.016), pretracheal LNM (68.3% vs 31.7%, P = 0.001), and paratracheal LNM (80.8% vs 19.2%, P < 0.001) were independent risk factors for ipsilateral level-IV LNM.
For unilateral cN0-PTC patients, different central LNM subgroups predicted different subgroups of lateral LNM, which is helpful for determining the extent of prophylactic lateral LND.
For unilateral cN0-PTC patients with prelaryngeal LNM, LNM may occur at ipsilateral level-II, and prophylactic level-II LND can be considered. When pretracheal or ipsilateral paratracheal LNM occurs, LNM may occur at ipsilateral level III and level IV, and prophylactic level-III and level-IV LND can be considered.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Elkady N, Egypt; Kadriyan H, Indonesia S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10769] [Cited by in RCA: 9637] [Article Influence: 1070.8] [Reference Citation Analysis (1)] |
| 2. | Deng Y, Li H, Wang M, Li N, Tian T, Wu Y, Xu P, Yang S, Zhai Z, Zhou L, Hao Q, Song D, Jin T, Lyu J, Dai Z. Global Burden of Thyroid Cancer From 1990 to 2017. JAMA Netw Open. 2020;3:e208759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 204] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 3. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55742] [Article Influence: 7963.1] [Reference Citation Analysis (132)] |
| 4. | Zhao H, Huang T, Li H. Risk factors for skip metastasis and lateral lymph node metastasis of papillary thyroid cancer. Surgery. 2019;166:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 5. | Yang Z, Heng Y, Lin J, Lu C, Yu D, Tao L, Cai W. Nomogram for Predicting Central Lymph Node Metastasis in Papillary Thyroid Cancer: A Retrospective Cohort Study of Two Clinical Centers. Cancer Res Treat. 2020;52:1010-1018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 6. | Liu H, Li Y, Mao Y. Local lymph node recurrence after central neck dissection in papillary thyroid cancers: A meta analysis. Eur Ann Otorhinolaryngol Head Neck Dis. 2019;136:481-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Huang H, Wu L, Liu W, Liu J, Liu Y, Xu Z. Long-term outcomes of patients with papillary thyroid cancer who did not undergo prophylactic central neck dissection. J Cancer Res Ther. 2020;16:1077-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Wang Y, Guan Q, Xiang J. Nomogram for predicting central lymph node metastasis in papillary thyroid microcarcinoma: A retrospective cohort study of 8668 patients. Int J Surg. 2018;55:98-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Hu D, Zhou J, He W, Peng J, Cao Y, Ren H, Mao Y, Dou Y, Xiong W, Xiao Q, Su X. Risk factors of lateral lymph node metastasis in cN0 papillary thyroid carcinoma. World J Surg Oncol. 2018;16:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Lee YC, Na SY, Park GC, Han JH, Kim SW, Eun YG. Occult lymph node metastasis and risk of regional recurrence in papillary thyroid cancer after bilateral prophylactic central neck dissection: A multi-institutional study. Surgery. 2017;161:465-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Eltelety AM, Terris DJ. Neck Dissection in the Surgical Treatment of Thyroid Cancer. Endocrinol Metab Clin North Am. 2019;48:143-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Kim E, Park JS, Son KR, Kim JH, Jeon SJ, Na DG. Preoperative diagnosis of cervical metastatic lymph nodes in papillary thyroid carcinoma: comparison of ultrasound, computed tomography, and combined ultrasound with computed tomography. Thyroid. 2008;18:411-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 295] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 13. | Suh CH, Baek JH, Choi YJ, Lee JH. Performance of CT in the Preoperative Diagnosis of Cervical Lymph Node Metastasis in Patients with Papillary Thyroid Cancer: A Systematic Review and Meta-Analysis. AJNR Am J Neuroradiol. 2017;38:154-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 14. | Zhao H, Li H. Meta-analysis of ultrasound for cervical lymph nodes in papillary thyroid cancer: Diagnosis of central and lateral compartment nodal metastases. Eur J Radiol. 2019;112:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 148] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 15. | Raffaelli M, De Crea C, Sessa L, Giustacchini P, Bellantone R, Lombardi CP. Can intraoperative frozen section influence the extension of central neck dissection in cN0 papillary thyroid carcinoma? Langenbecks Arch Surg. 2013;398:383-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Lee CW, Gong G, Roh JL. Intraoperative diagnosis of central compartment lymph node metastasis predicts recurrence of patients with papillary thyroid carcinoma and clinically node-negative lateral neck and may guide extent of initial surgery. World J Surg. 2015;39:194-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Zhan S, Luo D, Ge W, Zhang B, Wang T. Clinicopathological predictors of occult lateral neck lymph node metastasis in papillary thyroid cancer: A meta-analysis. Head Neck. 2019;41:2441-2449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Kouvaraki MA, Shapiro SE, Fornage BD, Edeiken-Monro BS, Sherman SI, Vassilopoulou-Sellin R, Lee JE, Evans DB. Role of preoperative ultrasonography in the surgical management of patients with thyroid cancer. Surgery. 2003;134:946-54; discussion 954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 336] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 19. | Robbins KT, Clayman G, Levine PA, Medina J, Sessions R, Shaha A, Som P, Wolf GT; American Head and Neck Society; American Academy of Otolaryngology--Head and Neck Surgery. Neck dissection classification update: revisions proposed by the American Head and Neck Society and the American Academy of Otolaryngology-Head and Neck Surgery. Arch Otolaryngol Head Neck Surg. 2002;128:751-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 630] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 20. | American Thyroid Association Surgery Working Group; American Association of Endocrine Surgeons; American Academy of Otolaryngology-Head and Neck Surgery; American Head and Neck Society, Carty SE, Cooper DS, Doherty GM, Duh QY, Kloos RT, Mandel SJ, Randolph GW, Stack BC Jr, Steward DL, Terris DJ, Thompson GB, Tufano RP, Tuttle RM, Udelsman R. Consensus statement on the terminology and classification of central neck dissection for thyroid cancer. Thyroid. 2009;19:1153-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 405] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 21. | Jo YJ, Choi HR, Park SH, Jeong YJ. Extent of thyroid surgery for clinically node-negative papillary thyroid carcinoma with confirmed nodal metastases after prophylactic central neck dissection: a 15-year experience in a single center. Ann Surg Treat Res. 2020;99:197-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Liu C, Xiao C, Chen J, Li X, Feng Z, Gao Q, Liu Z. Risk factor analysis for predicting cervical lymph node metastasis in papillary thyroid carcinoma: a study of 966 patients. BMC Cancer. 2019;19:622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 158] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 23. | Isaacs JD, McMullen TP, Sidhu SB, Sywak MS, Robinson BG, Delbridge LW. Predictive value of the Delphian and level VI nodes in papillary thyroid cancer. ANZ J Surg. 2010;80:834-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Lim YC, Liu L, Chang JW, Koo BS. Lateral lymph node recurrence after total thyroidectomy and central neck dissection in patients with papillary thyroid cancer without clinical evidence of lateral neck metastasis. Oral Oncol. 2016;62:109-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Fisch UP, Sigel ME. Cervical lymphatic system as visualized by lymphography. Ann Otol Rhinol Laryngol. 1964;73:870-882. [PubMed] |
| 26. | Andrade M, Jacomo A. Anatomy of the human lymphatic system. Cancer Treat Res. 2007;135:55-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Zhu J, Huang R, Yu P, Hu D, Ren H, Huang C, Su X. Clinical implications of Delphian lymph node metastasis in papillary thyroid carcinoma. Gland Surg. 2021;10:73-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Hunt JP, Buchmann LO, Wang L, Abraham D. An analysis of factors predicting lateral cervical nodal metastases in papillary carcinoma of the thyroid. Arch Otolaryngol Head Neck Surg. 2011;137:1141-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Kwak JY, Kim EK, Kim MJ, Son EJ, Chung WY, Park CS, Nam KH. Papillary microcarcinoma of the thyroid: predicting factors of lateral neck node metastasis. Ann Surg Oncol. 2009;16:1348-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Dou Y, Hu D, Chen Y, Xiong W, Xiao Q, Su X. PTC located in the upper pole is more prone to lateral lymph node metastasis and skip metastasis. World J Surg Oncol. 2020;18:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Feng JW, Yang XH, Wu BQ, Sun DL, Jiang Y, Qu Z. Predictive factors for central lymph node and lateral cervical lymph node metastases in papillary thyroid carcinoma. Clin Transl Oncol. 2019;21:1482-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 32. | Ma B, Wang Y, Yang S, Ji Q. Predictive factors for central lymph node metastasis in patients with cN0 papillary thyroid carcinoma: A systematic review and meta-analysis. Int J Surg. 2016;28:153-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |