Published online Apr 6, 2022. doi: 10.12998/wjcc.v10.i10.3313
Peer-review started: November 30, 2021
First decision: January 12, 2022
Revised: January 26, 2022
Accepted: February 20, 2022
Article in press: February 20, 2022
Published online: April 6, 2022
Processing time: 119 Days and 10.7 Hours
Pneumocystis jiroveci pneumonia (PJP) is a serious opportunistic infection that occurs mostly in patients with immunodeficiency and long-term immunosuppressive therapy. In non-human immunodeficiency virus-infected patients, the most important risk factor for PJP is the use of glucocorticoids in combination with other immunosuppressive treatments. The management of glucocorticoids during the perioperative period in patients with dermatomyositis requires special care.
We report a case of PJP in the perioperative period. A 61-year-old woman with a history of anti-melanoma differentiation-associated gene 5 (MDA5)-positive dermatomyositis and interstitial pneumonia was administered with long-term oral methylprednisolone and cyclosporine. The patient underwent right total hip arthroplasty in the orthopaedic department for bilateral osteonecrosis of the femoral head. She was given intravenous drip hydrocortisone before anesthesia and on the first day after surgery and resumed oral methylprednisolone on the second postoperative day. On the fifth day after surgery, the patient suddenly developed dyspnea. The computed tomography scan showed diffuse grid shadows and ground glass shadows in both lungs. Polymerase chain reaction testing of bronchoalveolar lavage fluid was positive for Pneumocystis jiroveci. The patient was eventually diagnosed with PJP and was administered with oral trimethoprim-sulfamethoxazole. At the 6-mo review, there was no recurrence or progression.
Continued perioperative glucocorticoid use in patients with anti-MDA5-positive dermatomyositis may increase the risk of PJP.
Core Tip: In non-human immunodeficiency virus infected patients, the most important risk factor for Pneumocystis jiroveci pneumonia (PJP) is the use of glucocorticoids in combination with other immunosuppressive therapies. For patients with PJP risk factors, pneumonia, and suggestive radiographic findings, the possibility of PJP should be considered. Balancing the benefits and risks of glucocorticoids in the treatment of autoimmune diseases in the perioperative period remains a difficult question. The use of glucocorticoids should be determined based on the possibility of hypothalamic-pituitary-adrenal axis inhibition and the degree of surgical stress.
- Citation: Hong M, Zhang ZY, Sun XW, Wang WG, Zhang QD, Guo WS. Pneumocystis jiroveci pneumonia after total hip arthroplasty in a dermatomyositis patient: A case report . World J Clin Cases 2022; 10(10): 3313-3320
- URL: https://www.wjgnet.com/2307-8960/full/v10/i10/3313.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i10.3313
Pneumocystis jiroveci pneumonia (PJP) is more likely to occur in patients with immunodeficiency or long-term immunosuppressive therapy[1]. Early onset is insidious, progresses rapidly, and may even be life-threatening. Approximately 1%-2% of patients with rheumatic diseases develop PJP, especially when glucocorticoids are combined with immunosuppressive therapy[2]. Some rheumatologists believe that patients with dermatomyositis have a higher risk of PJP when the same intensity of immunosuppression is used[3]. A study included 293 cases of PJP from 1990 to 2010, of which inflammatory diseases accounted for 15%[3]. The population with the highest risk (incidence > 45/100000) included patients with dermatomyositis. In non-human immunodeficiency virus (HIV)-infected patients, the most important risk factor for PJP is the use of glucocorticoids[4]. A 12-year follow-up study from Norway included 297 non-HIV-infected patients with a first episode of PJP, 72.1% of whom had used glucocorticoids before diagnosis. The perioperative management of patients with dermatomyositis treated with long-term glucocorticoids needs to be carefully managed. The occurrence of PJP in the perioperative period is rare after kidney transplantation[5], but it has not been reported after arthroplasty.
A 61-year-old woman was admitted to the hospital for "bilateral osteonecrosis of the femoral head (ONFH) for 1 year after long-term use of glucocorticoids".
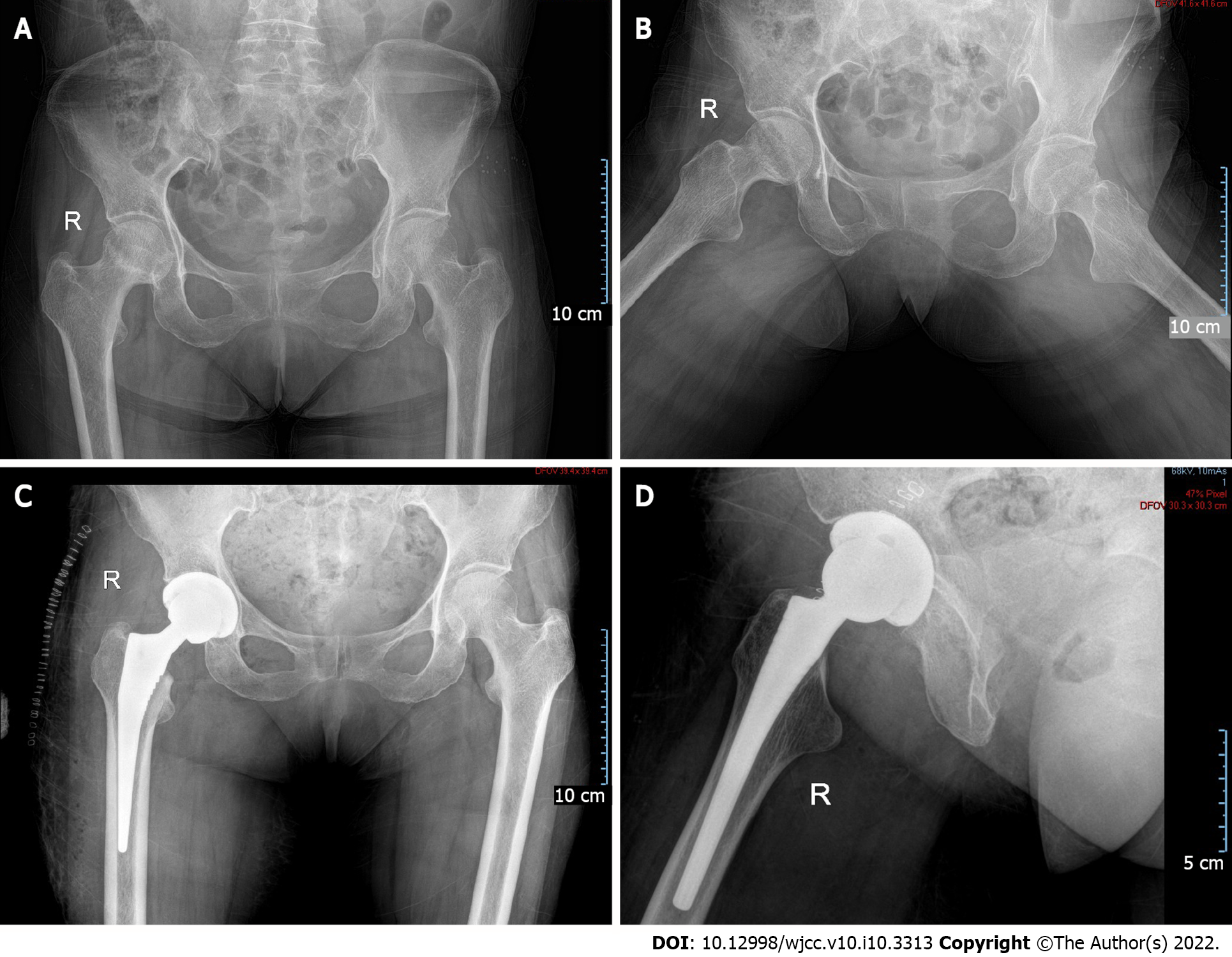
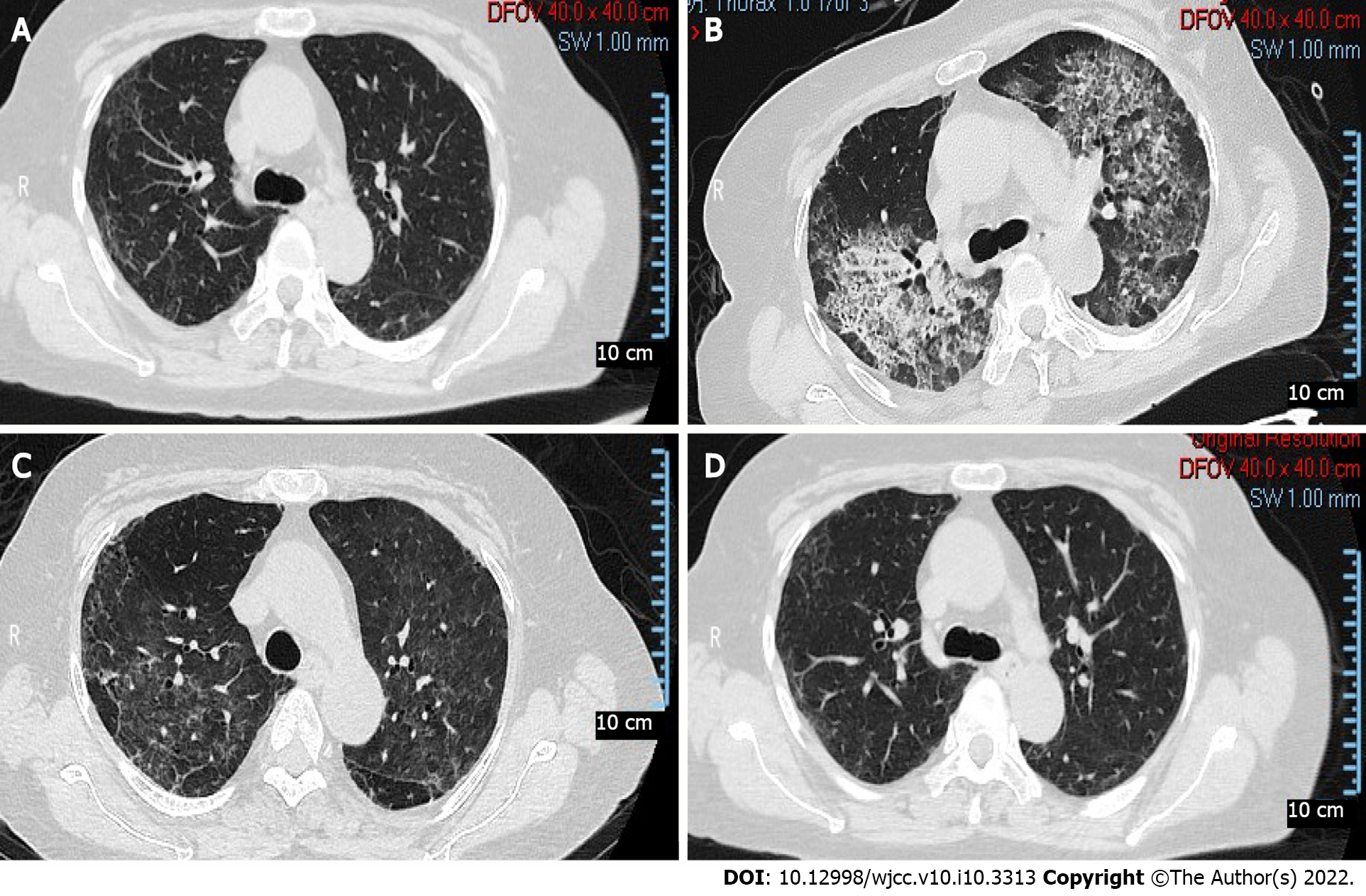
The patient was diagnosed with bilateral ONFH (Figure 1A and B) one year prior because of the long-term use of glucocorticoids. When the patient was admitted to the hospital, the control of the primary disease was stable (Figure 2A). She underwent right total hip arthroplasty (Figure 1C and D) in the orthopaedic department. Hydrocortisone (50 mg) was administered through intravenous drip 1 d before, during, immediately after, and 1 d after the operation. The patient resumed 8 mg of oral methylprednisolone on the second postoperative day. On the fifth day after surgery, the patient suddenly developed dyspnea when walking, accompanied by chest tightness, palpitation, and blood in the sputum.
The patient had a history of anti-melanoma differentiation-associated gene 5 (MDA5)-positive dermatomyositis and interstitial pneumonia for 3 years. She had was administered 8 mg of methylprednisolone daily and 50 mg of cyclosporine every 12 h to control her condition.
She was born in Shenyang and has lived there almost all her life. The patient’s living conditions were good, and she has no bad personal habits or customs. Her parents have both died.
The patient's temperature was 38.3 °C. She had tachypnea (respiratory rate: 30 breaths/min) and tachycardia (heart rate: 115 beats/min). Her blood pressure was 126/78 mmHg, and her blood oxygen saturation was 86%. She was conscious and able to answer questions correctly. Heart sounds and abdominal examinations were normal, and there was no increase in lower extremity edema and facial rash.
Laboratory tests showed a high white blood counts (WBC) count of 16.14 × 109/L, a high absolute lymphocyte count (ALC) of 0.81 × 109/L, a high C-reactive protein (CRP) level of 7.54 mg/dL, a high erythrocyte sedimentation rate of 75 mm/h, and a normal creatinine level of 85.9 μmol/L. The results of arterial blood gas analysis showed the following: pH 7.49, PaCO2 of 32 mmHg, PaO2 of 47 mmHg, HCO3- of 26 mmol/L, BE of 2.1 mmol/L, and Lac of 1.5 mmol/L.
The computed tomography (CT) scan (Figure 2B) showed diffuse grid shadows and ground glass shadows in both lungs, and left pleural effusion was distributed throughout the upper lobe of both lungs.
Polymerase chain reaction (PCR) testing of bronchoalveolar lavage fluid (BALF) was positive for Pneumocystis jiroveci.
The patient was eventually diagnosed with PJP.
Considering severe pneumonia, the patient was transferred to the respiratory intensive care units for further treatment. Noninvasive mechanical ventilation and high-flow oxygen inhalation were administered, and SpO2 was maintained at 98%-100%. She was administered 1.2 g of oral trimethoprim-sulfamethoxazole (TMP-SMX) every 6 h (total of 5 d), 8 mg of methylprednisolone every day, and 50 mg of cyclosporine every 12 h. On the ninth day after surgery, the mask was replaced with oxygen inhalation. The results of arterial blood gas analysis showed the following: FiO2 of 0.31, pH 7.465, PaCO2 of 36.0 mmHg, PaO2 of 145 mmHg, HCO3- of 26.5 mmol/L, BE of 2.1 mmol/L, Lac of 0.8 mmol/L, SaO2 of 98.0%, and an oxygenation index of 467 mmHg. Ambroxol hydrochloride (60 mg) was administered intravenously every day for 3 d. On the 11th day after surgery, oxygen inhalation was changed to a nasal cannula. The WBC count and ALC were normal, the CRP level was 1.72 mg/dL, and the creatinine level was 99.6 μmol/L. The CT scan (Figure 2C) showed ground glass and mesh shadows in both lungs, which were significantly reduced compared with the onset. TMP-SMX was adjusted to 0.8 g every 6 h (total of 5 d), and cyclosporine was stopped.
On the 16th day after surgery, the WBC, CRP, ALC, and creatinine levels were normal. The results of arterial blood gas analysis without oxygen inhalation showed the following: pH 7.438, PaCO2 of 33.1 mmHg, PaO2 of 74.5 mmHg, HCO3- of 23.4 mmol/L, BE of 1.5 mmol/L, Lac of 1.4 mmol/L, SaO2 of 95.6%, and an oxygenation index of 354 mmHg. The patient was discharged from the hospital and continued to take 8 mg of methylprednisolone every day and 0.8 g of TMP-SMX every 8 h. One month after surgery, cyclosporine was gradually increased to 50 mg every 12 h, and the administration of methylprednisolone (8 mg/day) and TMP-SMX (0.8 g/day) were maintained. There was no recurrence 6 mo after surgery. The CT scan (Figure 2D) showed a significant improvement compared to the images at the time of discharge.
This case posed a major challenge to the perioperative treatment and prevention of patients with autoimmune diseases and left important lessons for practice and research. First, the continued use of glucocorticoids in anti-MDA5-positive dermatomyositis patients may lead to immunosuppressive opportunistic infections. Second, surgery increases the additional conditions of immune stress and susceptibility to infection. Finally, the best management strategy for perioperative immunosuppressive therapy remains inconclusive due to a lack of evidence. Our treatment is consistent with the guidelines proposed by the American College of Rheumatology/American Association of Hip and Knee Surgeons Guideline for the Perioperative Management of Antirheumatic Medication in Patients with Rheumatic Diseases Undergoing Elective Total Hip or Total Knee Arthroplasty[6].
The severe respiratory complications of dermatomyositis pose a considerable challenge for diagnosis and treatment, and the prognosis is poor[7]. Differential diagnoses include cardiac complications, respiratory muscle weakness, drug allergy, rapid progressive interstitial lung disease (RP-ILD), and severe opportunistic infections[8]. In many cases, it is difficult to make a definitive diagnosis. There are several explanations for acute respiratory disease in this case. First, for patients with PJP risk factors, pneumonia, and suggestive radiographic findings, PJP should be considered. Typical radiographic features include bilateral diffuse interstitial infiltration[9]. If the chest radiograph results are normal, a high-resolution CT scan may show extensive ground-glass opaque areas or cystic lesions[9]. Our diagnostic method involves microbiological identification of pathogenic microorganisms when conditions permit. PCR testing of the BALF or induced sputum is necessary. Compared with HIV-infected people, the number of Pneumocystis jiroveci in non-HIV-infected people is significantly lower[10]. When respiratory samples cannot be obtained safely, treatment can be initiated based on the patient’s risk, clinical manifestations, and serum β-D-glucan testing[11]. Second, the progression of this pneumonia does not exclude RP-ILD. The risk of RP-ILD in anti-MDA5-positive patients is more than 20 times higher than that in anti-MDA5-negative patients[12]. Approximately 42%-100% of patients develop RP-ILD soon after the onset of disease and rapidly develop respiratory failure[13]. Despite active respiratory support and intensive immunosuppressive therapy, the effect is poor, and the prognosis is extremely poor, which is the main cause[14]. This patient had no exacerbation of the rash, and the serum ferritin level was not high, which is different from the RP-ILD symptoms caused by typical dermatomyositis. Finally, anti-MDA5-positive dermatomyositis patients appear to be predisposed to developing PJP from the outset, not just in relation to the immunosuppressive regimen they are receiving[15]. Li et al[16] reported that the 7 of 8 patients with dermatomyositis who developed PJP infection were anti-MDA5-positive. Aymonier et al[17] reported two anti-MDA5-positive dermatomyositis patients who developed RPILD due to PJP and eventually died after receiving immunosuppressive therapy.
For non-HIV-infected patients with PJP, the first choice is TMP-SMX treatment for 21 d[18]. Non-HIV-infected immunocompromised patients treated for PJP usually have worse outcomes than HIV-infected patients. The mortality rate of PJP in HIV-infected patients is 10%-20%, while that in non-HIV-infected patients is 35%-50%[19]. There is no strong immunosuppressive therapy that can severely suppress the human immune system. Lowering the counts of lymphocytes and CD4 lymphocytes will increase opportunistic infections such as Pneumocystis jiroveci, fungi, and cytomegalovirus[19]. TMP-SMX treatment is recommended for the following high-risk populations to prevent the occurrence of PJP: Patients who have received ≥ 20 mg/d prednisone (or equivalent dose) for ≥ 1 mo and have other causes of immune function[18].
The management method of stress-dose glucocorticoids for autoimmune diseases in the perioperative period is mainly based on low-quality evidence and expert opinions. Existing research data are relatively limited, and the practice of clinicians is not the same. The use of glucocorticoids should be based on the possibility of hypothalamic-pituitary-adrenal axis (HPA) axis inhibition and the degree of surgical stress. Patients who use any dose of glucocorticoids for no more than 3 wk or < 5 mg/d of prednisone (or equivalent doses) in the morning will not have HPA axis suppression and do not require additional glucocorticoids in the perioperative period[20]. For patients who are taking prednisone > 20 mg/d (or equivalent doses) or are complicated with Cushing’s syndrome, glucocorticoids should be supplemented according to the degree of stress during the perioperative period[21]. For patients who use 5-20 mg/d of prednisone (or equivalent dose) > 3 wk, an evaluation is recommended before surgery because of the substantial difference in HPA axis inhibition[20].
For patients with autoimmune diseases who require glucocorticoid supplementation during the perioperative period, it is necessary to use low-dose glucocorticoids to minimize other risks, such as infection. If the patient needs > 10 mg/day of prednisone (or equivalent dose), it means that the disease has not been adequately controlled, and elective surgery should be postponed[22]. A propensity score matching study analyzed data from 9911 rheumatoid arthritis (RA) patients who underwent elective total knee or hip replacement surgery and found that glucocorticoids were dose-dependently associated with postoperative infection, hospitalization, and increased risk of artificial joint infection[23]. Compared with patients who did not receive glucocorticoid therapy within 90 d of knee or hip arthroplasty, patients who used > 10 mg/day of prednisone (or equivalent doses) were expected to have a higher risk of hospitalization for infection (13.25 % vs 6.78%)[23]. The cumulative incidence of periprosthetic joint infections is expected to be higher in one year (3.83% vs 2.09%)[23]. Another population-based study included data from 381 knee or hip replacement surgeries in 259 patients with RA. The risk of infection after replacement can increase by up to 20 times[24].
Balancing the benefits and risks of glucocorticoids in the treatment of autoimmune diseases in the perioperative period remains a difficult question. Continued use of glucocorticoids in patients with anti-MDA5-positive dermatomyositis may lead to PJP. The use of glucocorticoids should be determined based on the possibility of HPA axis inhibition and the degree of surgical stress. For patients with autoimmune diseases who require glucocorticoid supplementation during the perioperative period, low-dose glucocorticoids should be used as much as possible to minimize the risk of PJP.
We are grateful to the family of the patient for their consent to publish this case report.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Moshref L, Selva-O'Callaghan A S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Fishman JA. Pneumocystis jiroveci. Semin Respir Crit Care Med. 2020;41:141-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 2. | Sepkowitz KA. Opportunistic infections in patients with and patients without Acquired Immunodeficiency Syndrome. Clin Infect Dis. 2002;34:1098-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 351] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 3. | Fillatre P, Decaux O, Jouneau S, Revest M, Gacouin A, Robert-Gangneux F, Fresnel A, Guiguen C, Le Tulzo Y, Jégo P, Tattevin P. Incidence of Pneumocystis jiroveci pneumonia among groups at risk in HIV-negative patients. Am J Med. 2014;127:1242.e11-1242.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 4. | Fillâtre P, Revest M, Belaz S, Robert-Gangneux F, Zahar JR, Roblot F, Tattevin P. [Pneumocystosis in non-HIV-infected immunocompromised patients]. Rev Med Interne. 2016;37:327-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Marinaki S, Vallianou K, Melexopoulou C, Lionaki S, Darema M, Lambrou P, Boletis I. The Changing Landscape of Pneumocystis Jiroveci Infection in Kidney Transplant Recipients: Single-Center Experience of Late-Onset Pneumocystis Pneumonia. Transplant Proc. 2021;53:1576-1582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Goodman SM, Springer B, Guyatt G, Abdel MP, Dasa V, George M, Gewurz-Singer O, Giles JT, Johnson B, Lee S, Mandl LA, Mont MA, Sculco P, Sporer S, Stryker L, Turgunbaev M, Brause B, Chen AF, Gililland J, Goodman M, Hurley-Rosenblatt A, Kirou K, Losina E, MacKenzie R, Michaud K, Mikuls T, Russell L, Sah A, Miller AS, Singh JA, Yates A. 2017 American College of Rheumatology/American Association of Hip and Knee Surgeons Guideline for the Perioperative Management of Antirheumatic Medication in Patients With Rheumatic Diseases Undergoing Elective Total Hip or Total Knee Arthroplasty. J Arthroplasty. 2017;32:2628-2638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 7. | Bai Z, Shen G, Dong L. Analysis of risk factors of interstitial lung disease and mortality rates in Chinese patients with idiopathic inflammatory myopathy. Int J Rheum Dis. 2021;24:815-827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Murray SG, Schmajuk G, Trupin L, Lawson E, Cascino M, Barton J, Margaretten M, Katz PP, Yelin EH, Yazdany J. A population-based study of infection-related hospital mortality in patients with dermatomyositis/polymyositis. Arthritis Care Res (Hoboken). 2015;67:673-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Obmann VC, Bickel F, Hosek N, Ebner L, Huber AT, Damonti L, Zimmerli S, Christe A. Radiological CT Patterns and Distribution of Invasive Pulmonary Aspergillus, Non-Aspergillus, Cryptococcus and Pneumocystis Jirovecii Mold Infections - A Multicenter Study. Rofo. 2021;193:1304-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Sarasombath PT, Thongpiya J, Chulanetra M, Wijit S, Chinabut P, Ongrotchanakun J, Jitmuang A, Wanachiwanawin D. Quantitative PCR to Discriminate Between Pneumocystis Pneumonia and Colonization in HIV and Non-HIV Immunocompromised Patients. Front Microbiol. 2021;12:729193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Corsi-Vasquez G, Ostrosky-Zeichner L, Pilkington EF 3rd, Sax PE. Point-Counterpoint: Should Serum β-d-Glucan Testing Be Used for the Diagnosis of Pneumocystis jirovecii Pneumonia? J Clin Microbiol. 2019;58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Kishaba T, McGill R, Nei Y, Ibuki S, Momose M, Nishiyama K, Nagano H, Yamashiro S. Clinical characteristics of dermatomyosits/polymyositis associated interstitial lung disease according to the autoantibody. J Med Invest. 2018;65:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | González-Moreno J, Raya-Cruz M, Losada-Lopez I, Cacheda AP, Oliver C, Colom B. Rapidly progressive interstitial lung disease due to anti-MDA5 antibodies without skin involvement: a case report and literature review. Rheumatol Int. 2018;38:1293-1296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Cobo-Ibáñez T, López-Longo FJ, Joven B, Carreira PE, Muñoz-Fernández S, Maldonado-Romero V, Larena-Grijalba C, Cubas IL, Muriel ET, Mateos CB, de la Peña Lefebvre PG, Gomez-Gomez A, Nogal LB, Pérez A, Almodovar R, Lojo L, Ruiz-Gutiérrez L, López-Robledillo JC, García de Yébenes MJ, Nuño-Nuño L. Long-term pulmonary outcomes and mortality in idiopathic inflammatory myopathies associated with interstitial lung disease. Clin Rheumatol. 2019;38:803-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Tseng CW, Wang KL, Fu PK, Huang CY, Hsieh TY, Hsieh CW, Lai KL, Hung WT, Lin CT, Tang KT, Chen YM, Huang WN, Chen YH. GAP Score and CA-153 Associated with One-Year Mortality in Anti-MDA-5 Antibody-Positive Patients: A Real-World Experience. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Li J, Li J, Yu Y, Wang R, Zhou M, Lu L. Pneumocystis pneumonia and rheumatic disease: diagnostic potential of circulating microbial cell-free DNA sequencing. Rheumatol Adv Pract. 2022;6:rkab105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Aymonier M, Abed S, Boyé T, Barazzutti H, Fournier B, Morand JJ. [Dermatomyositis associated with anti-MDA5 antibodies and pneumocystis pneumonia: Two lethal cases]. Ann Dermatol Venereol. 2017;144:279-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Mecoli CA, Saylor D, Gelber AC, Christopher-Stine L. Pneumocystis jiroveci pneumonia in rheumatic disease: a 20-year single-centre experience. Clin Exp Rheumatol. 2017;35:671-673. [PubMed] |
| 19. | Huang L, Fu Q, Ye Y, Lin Y, Yan Q, Chen S. High incidence and mortality of Pneumocystis jirovecii infection in anti-MDA5-antibody-positive dermatomyositis: experience from a single center. Arthritis Res Ther. 2021;23:232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 20. | Seo KH. Perioperative glucocorticoid management based on current evidence. Anesth Pain Med (Seoul). 2021;16:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Ramesh AV, Pufulete M, Reeves BC, Fletcher S, Tomlinson JW, Gibbison B. Peri-operative corticosteroid supplementation for patients on therapeutic glucocorticoids: a national survey. Anaesthesia. 2020;75:1396-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Strehl C, Bijlsma JW, de Wit M, Boers M, Caeyers N, Cutolo M, Dasgupta B, Dixon WG, Geenen R, Huizinga TW, Kent A, de Thurah AL, Listing J, Mariette X, Ray DW, Scherer HU, Seror R, Spies CM, Tarp S, Wiek D, Winthrop KL, Buttgereit F. Defining conditions where long-term glucocorticoid treatment has an acceptably low level of harm to facilitate implementation of existing recommendations: viewpoints from an EULAR task force. Ann Rheum Dis. 2016;75:952-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 255] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 23. | George MD, Baker JF, Winthrop K, Alemao E, Chen L, Connolly S, Hsu JY, Simon TA, Wu Q, Xie F, Yang S, Curtis JR. Risk of Biologics and Glucocorticoids in Patients With Rheumatoid Arthritis Undergoing Arthroplasty: A Cohort Study. Ann Intern Med. 2019;170:825-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 24. | Somayaji R, Barnabe C, Martin L. Risk factors for infection following total joint arthroplasty in rheumatoid arthritis. Open Rheumatol J. 2013;7:119-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |