Published online Apr 6, 2022. doi: 10.12998/wjcc.v10.i10.3251
Peer-review started: October 18, 2021
First decision: December 17, 2021
Revised: December 31, 2021
Accepted: February 23, 2022
Article in press: February 23, 2022
Published online: April 6, 2022
Processing time: 161 Days and 21.3 Hours
Scedosporium apiospermum (S. apiospermum) is a clinically rare and aggressive fungus mainly found in contaminated water, wetlands, decaying plants, stagnant water, and potted plants in hospitals. The lung, bone, joint, eye, brain, skin, and other sites are easily infected, and there is a marked risk of misdiagnosis. There have been few case reports of infection by S. apiospermum of the lumbar vertebrae; most reports have focused on infection of the lung.
An otherwise healthy 60-year-old man presented with a 4-mo history of lumbosacral pain, stooping, and limited walking. The symptoms were significantly aggravated 10 d prior to hospitalization, and radiating pain in the back of his left lower leg developed, which was so severe that he could not walk. Movement of the lumbar spine was significantly limited, anterior flexion was about 30°; backward extension, right and left lateral curvature, and rotational mobility were about 10°; tenderness of the spinous processes of the lumbar 3-5 vertebrae was evident, and the muscle strength of both lower limbs was grade IV. Imaging suggested bony destruction of the lumbar 3, 4, and 5 vertebrae and sacral 1 vertebra; in addition, the corresponding intervertebral spaces were narrowed and the lumbar 5 vertebra was posteriorly displaced and unstable. Lumbar vertebral infection was also noted, and the possibility of lumbar tuberculosis was considered. We first performed surgical intervention on the lesioned lumbar vertebrae, cleared the infected lesion, and performed stable fixation of the lesioned vertebral body using a lumbar internal fixation device, which restored the stability of the lumbar vertebrae. Cytological and pathological examination of the lesioned tissue removed during surgery confirmed S. apiospermum infection of the lumbar vertebrae; on this basis, the patient was administered voriconazole. At the 6-mo follow-up, efficacy was significant, no drug-related side effects were observed, and imaging examination showed no evidence of recurrence.
S. apiospermum infection can occur in immunocompetent individuals with no history of near drowning. Voriconazole is effective for the treatment of S. apiospermum infection of the lumbar vertebrae for which it is suitable as the first-line therapy.
Core Tip: Scedosporium apiospermum (S. apiospermum) infection can occur in immunocompetent individuals with no history of a near drowning event. S. apiospermum infection of the lumbar vertebrae is rare, leading to risks of misdiagnosis and mistreatment. Cytology and pathology of lesion tissue play a decisive role in diagnosis. Further cases would expand our understanding of this rare fungal infection.
- Citation: Shi XW, Li ST, Lou JP, Xu B, Wang J, Wang X, Liu H, Li SK, Zhen P, Zhang T. Scedosporium apiospermum infection of the lumbar vertebrae: A case report. World J Clin Cases 2022; 10(10): 3251-3260
- URL: https://www.wjgnet.com/2307-8960/full/v10/i10/3251.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i10.3251
Scedosporium apiospermum (S. apiospermum) is a rare and aggressive filamentous fungus widely distributed in contaminated water, wetlands, decaying plants, stagnant water, and potted plants in hospitals[1,2]. Immunocompetent individuals become vulnerable to this fungus following near drowning events[3]. In the case reported here, the patient had not experienced a recent near drowning event and had not been exposed to contaminated water or decaying plants. S. apiospermum infection occurs in patients with organ transplantation, acquired immunodeficiency syndrome (AIDS), long-term use of immunosuppressants, immune dysfunction of other causes, and invasion of the lung, bone, joints, eyes, brain, skin, and other organs[4-7] (Table 1). There have been no previous case reports of S. apiospermum infection of lumbar vertebrae; most reports have focused on infection of the lung.
| Ref. | Age (yr), Sex | Pathogenesis | Site of infection | Symptoms | Diagnostic method | Characteristics of S. apiospermum | Therapeutic method | Therapeutic drugs | Duration of medication | Outcome |
| Agarwal et al[3], 2021 | 62, Male | Not reported | Right index finger | Swelling of the right index finger | Microscopy, culture, and identification of the pathogen | The surface of the colonies was brownish gray to black, the back surface was black, and microscopically, a single, oval, colorless, basal truncated ring spore could be seen germinating from the ring | Not reported | Not reported | Not reported | Not reported |
| Agarwal et al[4], 2021 | 62, Male | Not reported | Left eye | Redness and sudden loss of vision in the left eye | Bacterial culture and identification | Vitreous sample and the explanted intraocular lens inoculated onto BA, CA, and SDA showed colonies with a clear outer pale zone and central brownish growth with mycelial tufts suggestive of S. apiospermum | Medication | Voriconazole | 6 mo | Recovery |
| Chen et al[5], 2016 | 62, Male | Near drowning | Brain and lungs | Persistent headache and urinary incontinence | Cerebrospinal fluid culture and strain identification | Not reported | Medication | Voriconazole and terbinafine | 6 mo | Recovery |
| Todokoro et al [12], 2018 | 75, Male | Hypertension, colon cancer, and metastatic hepatic tumor | Left eye | Decreased visual acuity in the left eye | DNA sequencing, PCR | Microscopic features: septate hyphae 2 μm in diameter and branching irregularly, along with the production of lateral and terminal conidia, which were round or oval (3-5 by 5-10 μm) | Medication | Voriconazole | 5 mo | Partial recovery |
| Oliveira et al[17], 2013 | 58, Female | Prior injury to foot while handling a dairy cow | Left foot | Swelling and pain in the left foot | Tissue culture | Not reported | Medication | Itraconazole | Not reported | Recovery |
| Girmenia et al[20], 1998 | 25, Male | Acute myeloid leukemia | Face | Pain in the face, multiple papular skin lesions | Bacterial culture and identification | Microscopic examination showed septate hyaline hyphae with conidia 9 by 5 mm in diameter borne terminally, singly, or in small groups on elongated simple or branched conidiophores or laterally on hyphae | Medication | Voriconazole | 1 mo | Death |
An otherwise healthy 60-year-old man presented with a 4-mo history of lumbosacral pain, stooped back, and restricted walking with no obvious cause. The symptoms had become significantly aggravated 10 d prior to hospitalization.
This patient had a 4-mo history of lumbosacral pain with no obvious cause, along with stooped and restricted walking. He had visited a local hospital and received Chinese medicine. The patient’s lumbosacral pain worsened, and oral pain medication was not effective. 10 d before admission, the symptoms were further aggravated and radiating pain developed in the back of his left lower leg, which was so strong he could not walk. The patient again visited a local hospital, and lumbar vertebrae computed tomography (CT) showed lumbar vertebral infection. The patient was referred to our hospital due to lumbar vertebral infection.
The patient had no relevant past medical history.
No special personal and family history.
The patient was able to stand using crutches and did not have scoliosis. Movement of the lumbar spine was significantly limited, with approximately 30° anterior flexion, kyphosis, left-right scoliosis, and rotational mobility of about 10° and tenderness of the spinous processes of the lumbar 3-5 vertebrae bilaterally. Cutaneous sensation of both lower extremities was unremarkable, muscle tone was normal, and muscle strength was class IV. Range of motion was essentially normal at the hips, knees, and ankles bilaterally, although the movements were slowed by pain. Knee and Achilles tendon reflexes were normal bilaterally and the Babinski sign was negative.
At admission, his laboratory data were as follows: Erythrocyte sedimentation rate (ESR) 120 mm/h (normal range 0-15 mm/h), C-reactive protein (CRP) 8.33 mg/dL (< 0.8 mg/dL), fibrinogen (FIB) 8.26 g/L (1.8-3.5 g/L), fibrinogen degradation products (FDP) 9.2 µg/mL (0-5 µg/mL), and D-dimer 3.5 mg/L (0-0.55 mg/L). However, tumor markers, brucellosis agglutination, tuberculosis cell immunoassay, and sputum acid-fast bacilli smear were negative.
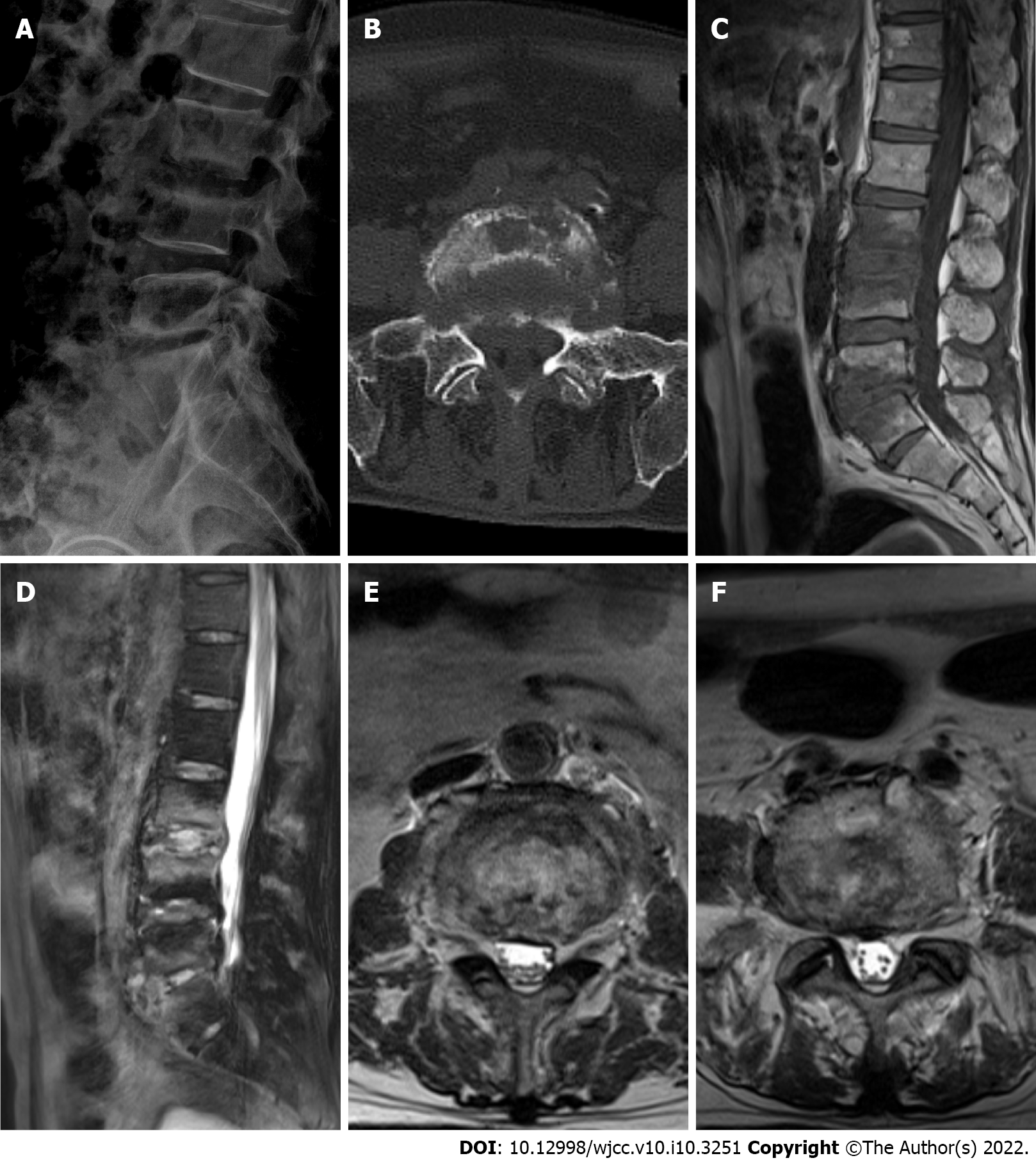
X-ray analysis showed hyperostosis, sclerosis, and tapering of the fifth lumbar (L5) vertebra and the first sacral (S1) vertebral margins. The third lumbar (L3) vertebra to S1 vertebral margins showed local nonunion with slightly rough margins. The posterior border of L5 and S1 vertebra was incomplete, and L5 vertebra was displaced slightly posteriorly, not exceeding one quarter of the anterior posterior diameter of the S1 vertebra; the L5/S1 intervertebral space was narrowed (Figure 1A).
CT revealed bone destruction and hyperplasia at the relative margins of the L3, L4, L5 and S1 vertebra, a small amount of low-density shadow encircling the paravertebral spaces, and bone destruction at the right sacroiliac joint surface (Figure 1B).
On magnetic resonance imaging (MRI), the bone signals of L3, L4, L5 and S1 vertebra were abnormal, showing long T1 and mixed long T2 signals. A small amount of equal-T2 signals surrounded the paravertebral space. The T2 weighted imaging signal of each intervertebral disc was weak. Annular soft tissue shadows were seen at the edges of the L3/L4, L4/L5 and L5/S1 vertebral discs. A soft tissue shadow with prominent limitations was evident at the posterior margin, and the corresponding spinal canal was slightly narrowed (Figure 1C-F).
S. apiospermum infection of the lumbar vertebrae.
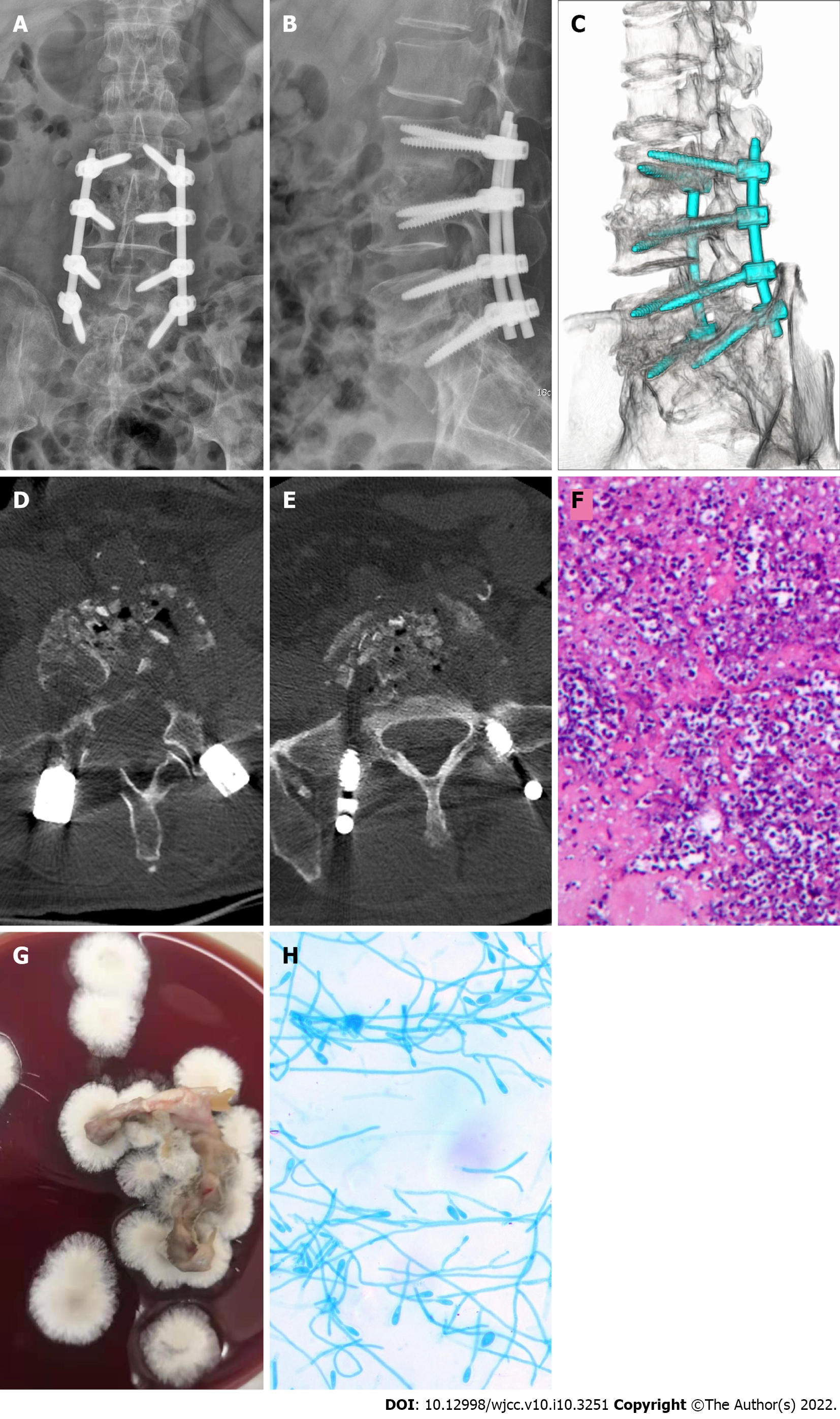
After 1 wk of hospitalization, the patient underwent surgical intervention. After induction of general anesthesia, a surgical incision was made at the median of the lower back, starting at the spinous process of the L3 vertebra and ending at the spinous process of the S1 vertebra (length about 15 cm). The right vertebral lamina and zygapophysial joint of the L3 to S1 vertebrae were first revealed. Next, the left L3 to S1 vertebral zygapophysial joint was exposed (Wiltze approach). After positioning via X-ray fluoroscopy, appropriately sized pedicle screws were implanted on both sides of the L3 to S1 vertebrae. Fluoroscopy showed that the individual pedicle screws were suitably positioned, and the L3/L4 and L5/S1 right hemivertebrae were sequentially decompressed and the articular processes of the lower right parts of the L3 and L5 vertebrae were resected. This showed destruction of the L3/L4 and L5/S1 discs and soft granulation-like tissue formation at the L3 and L5 vertebrae. In addition, destruction of endplates at the upper border of the lower, L4 and S1 vertebral bodies was evident. The lesion tissues in the intervertebral space were cleaned and sent for bacterial culture and pathological examination. This created a good bone graft surface, and medical gel foam mixed with isoniazid, rifamycin, and vancomycin was implanted into the intervertebral space. An appropriate amount of left iliac bone was removed through the incision and cut into bone blocks of appropriate size and mixed with allogeneic bone and bone induction material (recombinant human bone morphogenetic protein-2). During the operation, X-ray imaging showed sufficient bone grafting in the L3/L4 and L5/S1 intervertebral spaces. Next, we connected the bilateral posterior longitudinal connecting rod and fixed the lumbar vertebrae. Postoperative lumbar X-ray and CT imaging indicated good positioning of the lumbar internal fixation, and that bone graft placement was adequate in the L3/L4 and L5/S1 intervertebral spaces (Figure 2A-E). Pathological examination results showed a large number of inflammatory cells in the tissues examined, and staining revealed PAS (+), and acid resistance (-) (Figure 2F). Tissue culture on blood agar medium was performed twice (30°C, 7 d). The resultant colonies were cashmere-like and the back was gray-black (Figure 2G). Under the microscope (× 400), lactic acid phenol cotton blue staining showed that most of the hyphae were irregularly branched, producing round or oval lateral and terminal conidia (Figure 2H) (Table 1). Three microbiologists in our hospital confirmed the culture and microscopic examination results, and all agreed on the identification as S. apiospermum. The patient was given voriconazole (Pfizer, United States) 200 mg ivgtt every 12 h for antifungal treatment and cefoperazone sodium sulbactam (Pfizer, United States) 3 g ivgtt every 8 h to prevent postoperative infection. After 10 d, there were no abnormalities found during routine blood and biochemical analyses. The ESR was 34 mm/h (normal range 0–15 mm/h), CRP was 1.09 mg/dL (< 0.8 mg/dL), and PCT and interleukin-6 (IL-6) were within the respective normal ranges. The patient continued to take voriconazole for 6 mo.
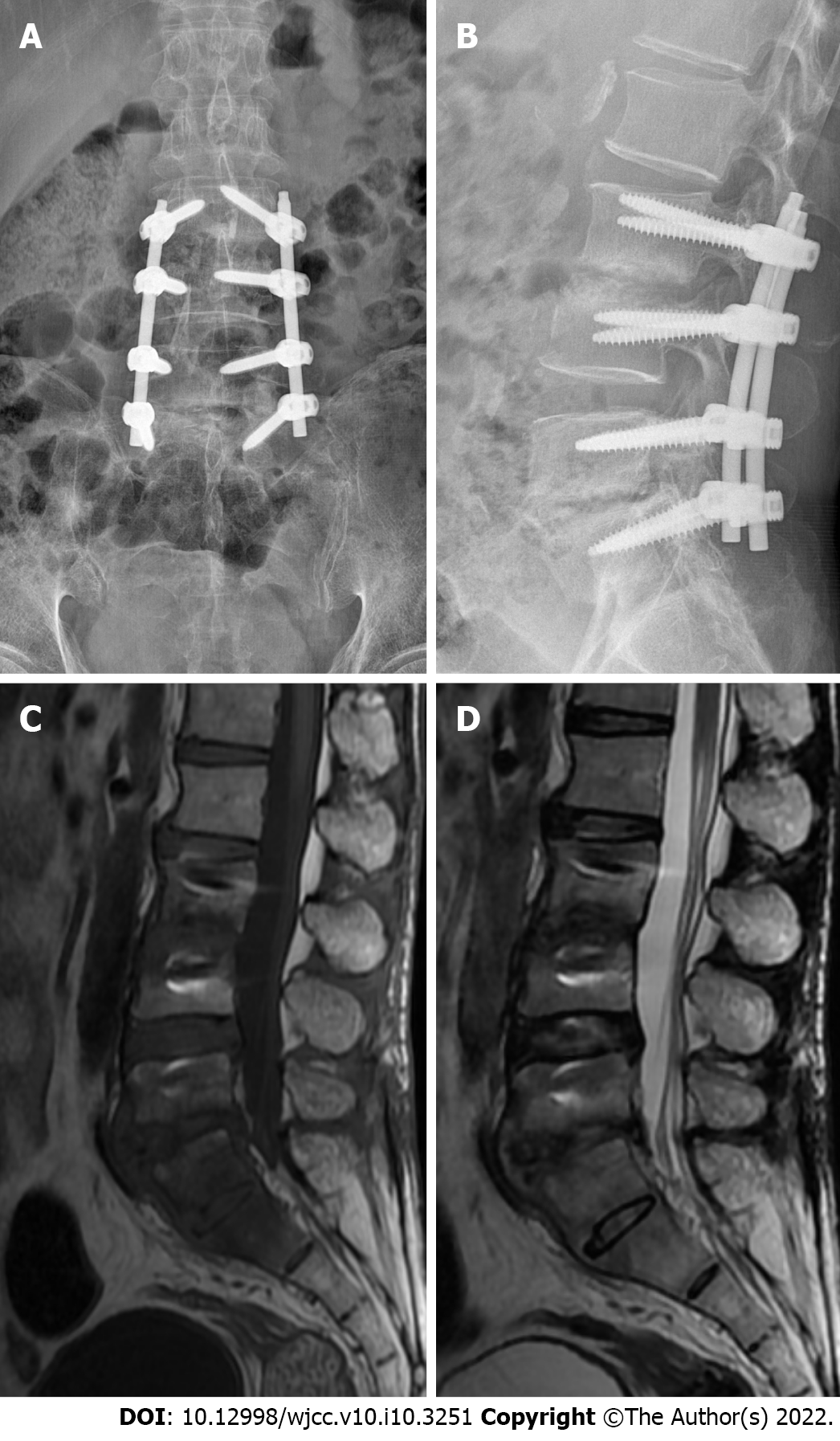
At the 6-mo follow-up, the ESR, CRP, PCT, and IL-6 were all within the respective normal ranges. X-ray and MRI of the lumbar vertebrae showed that the fixation position of the L3-S1 vertebral body was good, and the density in the L3/L4 and L5/S1 intervertebral spaces was increased, showing a short T1 signal on MRI (Figure 3). The patient was able to move with the assistance of a lumbar brace. However, the activity of the lumbar spine was limited, with anterior flexion of approximately 50°, posterior extension of about 15°, left–right scoliosis, and rotational activities of about 20°; nonetheless, walking and daily life were unaffected. The patient was satisfied with the outcome, but unfortunately refused further follow-up despite being informed of the risk of recurrence.
The S. apiospermum complex consists of S. apiospermum, S. aurantiacum, S. boydii, S. minutisporum, and S. dehoogii, of which the former three cause human infection[8]. S. apiospermum is distributed worldwide, but infections are rare. The clinical manifestations and imaging results differ according to the site of infection. Most doctors are unfamiliar with S. apiospermum infection, resulting in a risk of misdiagnosis. X-ray, CT, MRI, and other imaging examinations have limited diagnostic utility, typically showing only abscess formation; these modalities are mainly used for surgical planning and follow-up evaluation. Before surgery, histopathological and pathological examination, the patient was mistakenly believed to have Mycobacterium tuberculosis infection based on the imaging findings. The diagnosis of S. apiospermum infection was made by histocytology and pathology, and by isolation of the fungus in culture. Its pathological manifestations are similar to Aspergillus and Fusarium infection[9,10]. S. apiospermum colonies grow rapidly, and are cashmere like, white at first and subsequently gray black. Production of pigment or brown conidia, leads to gray/brown or black mature colonies. Microscopically, most S. apiospermum hyphae are irregularly branched, producing round or oval lateral and terminal conidia[11,12]. We analyzed lesional tissue removed at surgery via culture and microscopy, and the results were similar to those of previous reports. In immunocompetent patients, the diagnosis of S. apiospermum infection is delayed by almost 6 mo; significantly longer than in near drowning patients[13,14]. If hospital facilities are inadequate or the physician has insufficient experience, the probability of misdiagnosis increases significantly. S. apiospermum can form a fungal ball in human tissue due to fungal invasion and intravascular thrombosis, resulting in tissue necrosis[15,16].
The sites of S. apiospermum infection vary and the treatment modality differs according to site. In cases of limited infective focus and feasible surgical intervention, surgical removal of the infective focus improves the prognosis. We performed surgical intervention on the diseased lumbar vertebrae of this patient, cleared the infected lesion, and performed stable fixation of the diseased vertebral body using a lumbar internal fixation device, which restored spinal stability. S. apiospermum is resistant to most antifungal drugs, which makes treatment difficult and the prognosis poor. It is most sensitive to voriconazole, followed by posaconazole, itraconazole, and amphotericin B[17,18]; voriconazole is the first-line therapy[19-21] (Table 1). Due to the lack of prospective clinical studies, the dosage and duration of voriconazole for the treatment of S. apiospermum infection are unclear. We typically decide on treatments by following the drug manufacturer’s instructions and the advice of clinical pharmacists. Our patient was discharged and took voriconazole tablets (200 mg po q12h) for 6 mo, and no drug-related adverse effects were observed. In addition, imaging revealed no evidence of recurrence. Micafungin is the second-line agent for the treatment of S. apiospermum. The combination of micafungin and voriconazole in vitro has a significant effect on S. apiospermum[22,23]. Our patient did not receive the micafungin and voriconazole combination but achieved a satisfactory outcome.
S. apiospermum infection can occur in immunocompetent individuals with no history of near drowning. Infection involving multiple vertebral bodies, intervertebral spaces, and paravertebral tissues cannot be diagnosed by imaging alone, and surgical intervention is the first-line treatment. Lesion tissues should be removed for cytological and pathological examination, and an accurate diagnosis is needed to prevent mistreatment. Multidisciplinary treatment promotes rehabilitation. Voriconazole is effective for the treatment of lumbar vertebrae infection by S. apiospermum.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Galgoczy L, Katip W S-Editor: Chen YL L-Editor: Webster JR P-Editor: Chen YL
| 1. | McKelvie PA, Wong EY, Chow LP, Hall AJ. Scedosporium endophthalmitis: two fatal disseminated cases of Scedosporium infection presenting with endophthalmitis. Clin Exp Ophthalmol. 2001;29:330-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Summerbell RC, Krajden S, Kane J. Potted plants in hospitals as reservoirs of pathogenic fungi. Mycopathologia. 1989;106:13-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 85] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Agarwal R, Singh M, Chawla R, Kumar GD, Mishra A, Jain S. Localized cutaneous infection caused by Scedosporium apiospermum: Report of a case diagnosed on cytology. Diagn Cytopathol. 2021;49:E187-E189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Agarwal M, Garg A, Pegu J, Singh S, Shah S, Gandhi A. Postoperative endophthalmitis by Scedosporium apiospermum – A case report. Indian J Ophthalmol. 2021;1. |
| 5. | Chen TC, Ho MW, Chien WC, Lin HH. Disseminated Scedosporium apiospermum infection in a near-drowning patient. J Formos Med Assoc. 2016;115:213-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Cruz R, Barros M, Reyes M. [Pulmonary non invasive infection by Scedosporium apiospermum]. Rev Chilena Infectol. 2015;32:472-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Solé A. [Scedosporium apiospermum disseminated infection in a single lung transplant recipient]. Rev Iberoam Micol. 2011;28:139-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Tóth EJ, Nagy GR, Homa M, Ábrók M, Kiss IÉ, Nagy G, Bata-Csörgő Z, Kemény L, Urbán E, Vágvölgyi C, Papp T. Recurrent Scedosporium apiospermum mycetoma successfully treated by surgical excision and terbinafine treatment: a case report and review of the literature. Ann Clin Microbiol Antimicrob. 2017;16:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Cimon B, Carrère J, Vinatier JF, Chazalette JP, Chabasse D, Bouchara JP. Clinical significance of Scedosporium apiospermum in patients with cystic fibrosis. Eur J Clin Microbiol Infect Dis. 2000;19:53-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 209] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 10. | Johnson LS, Shields RK, Clancy CJ. Epidemiology, clinical manifestations, and outcomes of Scedosporium infections among solid organ transplant recipients. Transpl Infect Dis. 2014;16:578-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Guarro J, Kantarcioglu AS, Horré R, Rodriguez-Tudela JL, Cuenca Estrella M, Berenguer J, de Hoog GS. Scedosporium apiospermum: changing clinical spectrum of a therapy-refractory opportunist. Med Mycol. 2006;44:295-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 230] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 12. | Todokoro D, Hoshino J, Yo A, Makimura K, Hirato J, Akiyama H. Scedosporium apiospermum infectious scleritis following posterior subtenon triamcinolone acetonide injection: a case report and literature review. BMC Ophthalmol. 2018;18:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Katragkou A, Dotis J, Kotsiou M, Tamiolaki M, Roilides E. Scedosporium apiospermum infection after near-drowning. Mycoses. 2007;50:412-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Liu W, Feng RZ, Jiang HL. Scedosporium spp lung infection in immunocompetent patients: A systematic review and MOOSE-compliant meta-analysis. Medicine (Baltimore). 2019;98:e17535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Liu W, Feng R, Jiang H. Management of pulmonary Scedosporium apiospermum infection by thoracoscopic surgery in an immunocompetent woman. J Int Med Res. 2020;48:300060520931620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Schwartz DA. Organ-specific variation in the morphology of the fungomas (fungus balls) of Pseudallescheria boydii. Development within necrotic host tissue. Arch Pathol Lab Med. 1989;113:476-480. [PubMed] |
| 17. | Oliveira Fde M, Unis G, Hochhegger B, Severo LC. Scedosporium apiospermum eumycetoma successfully treated with oral voriconazole: report of a case and review of the Brazilian reports on scedosporiosis. Rev Inst Med Trop Sao Paulo. 2013;55:121-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Wang H, Wan Z, Li R, Lu Q, Yu J. Molecular identification and susceptibility of clinically relevant Scedosporium spp. in China. Biomed Res Int. 2015;2015:109656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Cuenca-Estrella M, Ruiz-Díez B, Martínez-Suárez JV, Monzón A, Rodríguez-Tudela JL. Comparative in-vitro activity of voriconazole (UK-109,496) and six other antifungal agents against clinical isolates of Scedosporium prolificans and Scedosporium apiospermum. J Antimicrob Chemother. 1999;43:149-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 145] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 20. | Girmenia C, Luzi G, Monaco M, Martino P. Use of voriconazole in treatment of Scedosporium apiospermum infection: case report. J Clin Microbiol. 1998;36:1436-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 92] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Radford SA, Johnson EM, Warnock DW. In vitro studies of activity of voriconazole (UK-109,496), a new triazole antifungal agent, against emerging and less-common mold pathogens. Antimicrob Agents Chemother. 1997;41:841-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 133] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Heyn K, Tredup A, Salvenmoser S, Müller FM. Effect of voriconazole combined with micafungin against Candida, Aspergillus, and Scedosporium spp. and Fusarium solani. Antimicrob Agents Chemother. 2005;49:5157-5159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Lackner M, de Hoog GS, Verweij PE, Najafzadeh MJ, Curfs-Breuker I, Klaassen CH, Meis JF. Species-specific antifungal susceptibility patterns of Scedosporium and Pseudallescheria species. Antimicrob Agents Chemother. 2012;56:2635-2642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 224] [Article Influence: 17.2] [Reference Citation Analysis (0)] |