Published online Apr 6, 2022. doi: 10.12998/wjcc.v10.i10.3206
Peer-review started: October 1, 2021
First decision: January 11, 2022
Revised: January 20, 2022
Accepted: February 23, 2022
Article in press: February 23, 2022
Published online: April 6, 2022
Processing time: 179 Days and 8.2 Hours
Uterine myoma is the most common benign tumor among women and is often accompanied by anemia. Here, we report the case of a patient with a very large leiomyoma but with a hemoglobin level as high as 197 g/L. After undergoing hysterectomy, all her hematological parameters returned to normal. Immunohistochemical staining of her myoma for erythropoietin showed strong positivity, which suggested that erythropoietin may be the cause of her erythrocytosis. A multidisciplinary team played a significant role in treating the disease.
A 47-year-old woman visited our department complaining that her abdomen had been continuously growing for the past 2 years. After careful examinations, she was suspected of having a very large leiomyoma. She was also diagnosed with erythrocytosis because her RBC count was 6.49 × 1012/L, hemoglobin was 197 g/L. Following a multidisciplinary team consultation, bilateral ureteral stents were placed, and 800 mL blood was removed by phlebotomy. The patient then underwent hysterectomy and bilateral salpingectomy. She recovered well from the operation, and her hemoglobin level decreased sharply following the surgery. Low-molecular-weight heparin was administered daily to prevent postoperative thrombosis. She was discharged from the hospital on the fourth postoperative day. Two months later, all her hematological parameters returned to normal. Pathological analysis of the myoma revealed that it was a benign leiomyoma, with partial hyalinization, and strong positivity for erythropoietin in immunohistochemical staining suggested that erythropoietin may be responsible for the erythrocytosis.
Erythropoietin ectopically produced from the myoma was responsible for the erythrocytosis in this patient. A multidisciplinary team is strongly recommended.
Core Tip: Despite chronic lung disease and malignant tumors, uterine myoma can also be the cause of secondary erythrocytosis, the mechanisms of which may be ectopically produced erythropoietin originating from the leiomyoma. A multidisciplinary team is strongly recommended to ensure that the patient has received the optimal treatment and has a good prognosis.
- Citation: Shu XY, Chen N, Chen BY, Yang HX, Bi H. Myomatous erythrocytosis syndrome: A case report. World J Clin Cases 2022; 10(10): 3206-3212
- URL: https://www.wjgnet.com/2307-8960/full/v10/i10/3206.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i10.3206
Myoma is the most common benign tumor in the female reproductive system and is often accompanied by anemia but seldom by erythrocytosis. When a patient presents extraordinarily high levels of RBCs, hemoglobin (Hb), or hematocrit (Hct) in routine blood tests, myomatous erythrocytosis syndrome (MES) is indicated, which is a very rare type of secondary erythrocytosis, the occurrence of which is only 0.02%-0.5%[1]. Since Thomson and Marson[2] reported the first case in 1953, only approximately 50 cases have been published worldwide. The diagnostic criteria include (1) Erythrocytosis; (2) Uterine fibroid myoma; and (3) The normalization of the RBC count after surgical removal of the myoma[3]. Here, we report a patient with a large myoma as well as an Hb level as high as 197 g/L, and all of her hematological parameters decreased immediately after hysterectomy. With the immunochemical staining of her myoma for erythropoietin showing strong positivity, we believe that the ectopically produced erythropoietin (EPO) was responsible for her erythrocytosis. The importance of a multidisciplinary team (MDT) cannot be neglected in treating the disease.
A 47-year-old premenopausal nulliparous woman visited our hospital, complaining that her abdomen had been growing in size over the past 2 years.
The patient had a history of myoma more than 20 years previously. At first presentation, the myoma measured 2-3 cm in diameter, but it continued to grow yearly. Six years ago, the myoma had grown to 8 cm, but the patient did not have any symptoms, including abnormal uterine bleeding or frequent micturition, and she refused to undergo surgery at that time. She claimed that her abdomen had grown over the past 2 years, but she thought that she was just gaining weight, so she decided to diet and exercise and lost more than 5 kg within 2 years. Despite these efforts, her abdomen continued to grow, which raised her family’s concern, so she finally visited our hospital.
Her past medical history was unremarkable, and she denied having any history of systemic diseases or allergies. She claimed to have a normal routine blood test 2 years ago, but she had not been reexamined since.
The patient was gravida 0, para 0, and reported a regular menstrual cycle, with no family history of tumors or chronic diseases. She is an illustrator.
Her height was 163 cm, and her weight was 53 kg (body mass index 19.95 kg/m2). She looked as if she was carrying a full-term fetus. A very large mass was observed rising from the pelvis to the xiphoid, which was hard in texture.
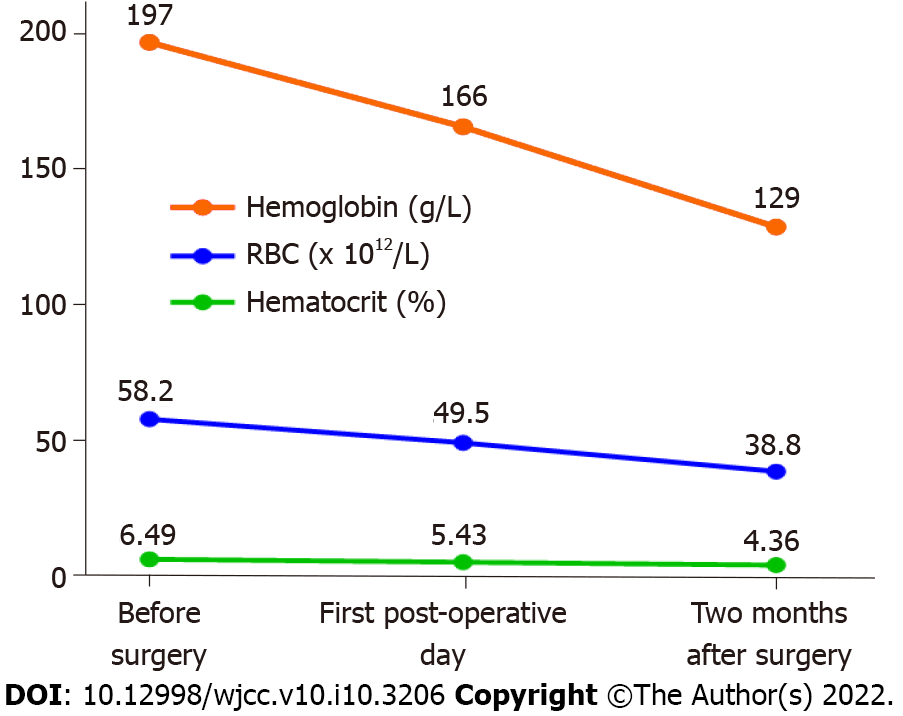
Her routine blood test showed that her Hb was 197 g/L, her RBC count was 6.49 × 1012/L, and her Hct was 58.2%.
Gynecological ultrasound revealed a very large mass originating from the posterior wall of the uterus, which was more than 35 cm in diameter, with only slight venous vascularity on color flow imaging, suggesting a uterine myoma. The enhanced CT showed a similar result. Right-sided hydronephrosis (1.6 cm in width) was also noted on ultrasound.
The patient was diagnosed with a giant uterine leiomyoma and erythrocytosis.
After careful consultation with an MDT consisting of experienced gynecologists, urologists, anesthesiologists, hematologists, and doctors from the blood transfusion department, transabdominal hysterectomy and bilateral salpingectomy were scheduled. Two days before surgery, bilateral ureteral stents were placed through a cystoscope to ensure the safety of the operation. To minimize the risk of thrombosis, 800 mL blood was removed by phlebotomy before the operation, and 2500 mL normal saline and 500 mL colloid were transfused back into her vein. The first pack of blood was dark brown, while the second pack was dark red (Figure 1). The surgery was difficult but successful, with a blood loss of 200 mL, and the operation lasted 112 min.
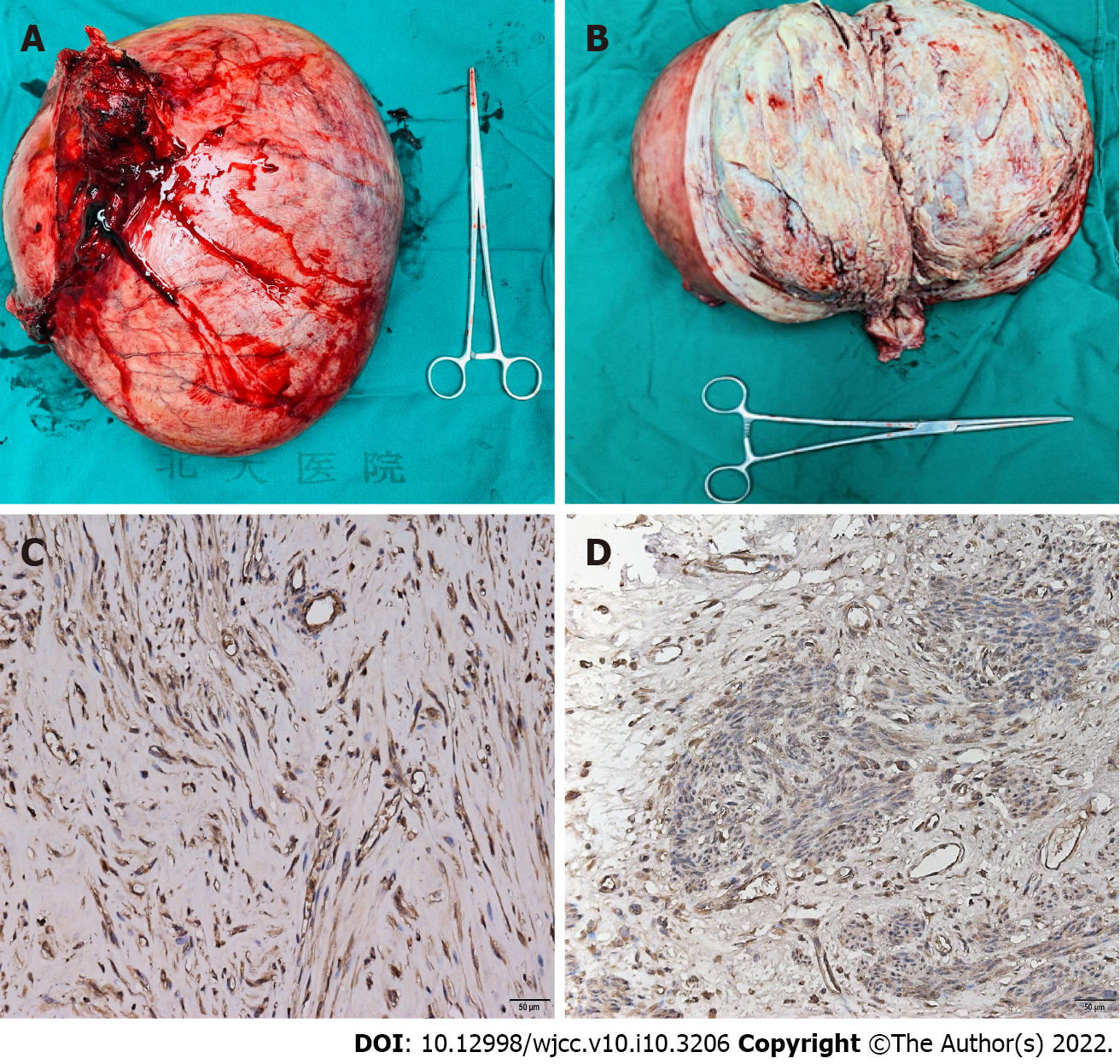
The whole uterus weighed 6500 g, with the largest diameter of 35 cm (Figure 2A and B). The pathology revealed a benign uterine leiomyoma with partial hyalinization that originated from the posterior wall of the uterus, and there were no signs of malignancy. Immunohistochemical staining of the myoma of this patient for EPO (ABclonal Technology, China) was compared with that of 2 other large myomas from patients without erythrocytosis as controls. Although three myomas were positive for EPO, but the myoma of this patient showed a stronger positivity than the others (Figure 2C and D).
On the first day after surgery, her RBC count decreased to 5.43 × 1012/L, her Hb was 166 g/L, and her Hct was 49.5%. She was encouraged to get out of bed and walk on the first day after surgery, and low-molecular-weight heparin (0.4 mL) was administered daily to prevent thrombosis. She was discharged from the hospital 4 d after surgery and was instructed to continue injecting low-molecular-weight heparin for another 10 d. After 2 mo, all of her hematological parameters returned to normal, with an RBC count of 4.36 × 1012/L, an Hb of 129 g/L and an Hct of 38.8%. She was pleased with the outcome and was instructed to undergo routine blood tests regularly. The changes in her hematological parameters are shown in Figure 3.
Secondary erythrocytosis is a condition where excessive production of RBCs occurs outside the bone marrow. Secondary erythrocytosis is defined as an elevated Hb of 165 g/L and above or an Hct of 48% and above in females[4]. Etiology includes low oxygen conditions such as living at high altitude, chronic lung diseases, or malignant tumors such as renal cell carcinoma, hepatocellular carcinoma, or cerebellar hemangioblastoma. Under rare conditions, secondary erythrocytosis can originate from benign tumors, such as uterine myoma[4], which is called myomatous erythrocytosis syndrome. As myoma may cause abnormal uterine bleeding, which leads to fluctuations in hemodynamics such as anemia, the actual occurrence of MES may be higher than reported[5,6].
Recently, Mui et al[5] reported a case of MES and summarized all 55 previous similar cases. In his study, the mean age at presentation was 48.7 ± 12.3 years, and there was no difference in parity or menopausal status among the patients in the reported cases. The most common symptom was abdominopelvic distension or a mass (93%); myomas weighed 4.9 ± 3.6 kg on average and were 22.6 ± 10 cm in length. However, some small myomas can also cause MES; for instance, the uterus can weigh approximately 1000 g[7-9].
The mechanism of MES is complex and has not been elucidated. In 1955, Horwitz and McKelway[10] first showed that an arteriovenous shunt was responsible for excessive RBCs and that deoxygenated arteries may stimulate the marrow to generate more RBCs as compensation. However, this notion was rebuked since peripheral arteriovenous fistulas may only increase focal RBCs rather than affect all hemodynamic parameters[3]. Subsequently, the compression theory was proposed, arguing that either the renal parenchyma or the diaphragm was compressed by the large myoma, which caused additional EPO production[11,12]. However, these theories were rejected, as compression symptoms, such as hydronephrosis or dyspnea, were not always seen[1].
To date, the prevailing view is that uncontrolled ectopically produced EPO is responsible for the overproduction of RBCs[7,13-15]. EPO is a hematopoietic cytokine that is usually produced in the kidneys of adults[4]. EPO acts on progenitor RBCs via the stimulation of cell growth, differentiation, and antiapoptotic factors and interacts with its receptor (EPO-R), which is commonly expressed in erythroid cells, and together they regulate the formation of RBCs during the process of hematopoiesis[16]. Under pathologic conditions, EPO can also be generated ectopically in ischemic tissue, in the retinopathy protection process and in tumor promotion, whereas EPO-R can also be expressed outside RBCs, such as in the endothelial cells of the vascular tissue of embryos and cancer cells, participating in angiogenesis[16,17]. In 2002, Yasuda et al[18] showed that in female reproductive malignant tumors, both the mRNA and protein of EPO are expressed, with EPO-R also expressed in the capillary endothelium of the tumor, suggesting that a paracrine and autocrine loop of EPO and EPO-R may exist and contribute to tumorigenesis. Similar results have been demonstrated in leiomyoma patients. In 1999, Yoshida et al[19] found that the EPO level from the myoma was extremely high. Kohama et al[13] and Suzuki et al[20] later proved that EPO mRNA was also strongly expressed in these patients. However, in myomas without erythrocytosis, the expression of EPO was weaker[8]. This is in accordance with our findings as well as those of Pollio et al[14], who studied a control group of 16 myoma patients without erythrocytosis (half of whom were pregnant women). By comparing the immunohistochemical expression of EPO and EPO-R, Pollio et al[14] found that in addition to strong expression of EPO and EPO-R in the case group (MES in a pregnant woman), EPO was also moderately or weakly expressed in all 8 pregnant control patients, and EPO-R was weakly expressed in 6 patients. In the nonpregnant control patients, although the expression of EPO and EPO-R was also observed, the frequency and intensity were much lower (4/8 with weak EPO expression and 3/8 with weak EPO-R expression). The author then pointed out that EPO was exclusively localized within the cytoplasm of uterine smooth muscles, whereas EPO-R was almost entirely localized within the vascular endothelial cells, in both the case and the control groups[14], proving that this autocrine or paracrine mechanism may exist, stimulating myoma cell growth, stabilizing vascular integrity, increasing the number of epithelial cells, protecting them against ischemia and apoptosis, and thus contributing to the unusual size of the myoma[15,16]. According to our findings, we also believe that the EPO produced by the uterine myoma is the cause of the excessive RBCs in the blood system, and the level of erythrocytosis is related to the amount of EPO. Notably, even with the prevailing theory, the EPO level of the myoma may still be normal in some patients. Macciò et al[21] reported the case of a patient with MES with a uterus weighing 5400 g and a high serum EPO level (45 mIU/mL, normal: 0-29 mIU/mL), but surprisingly, the EPO level in the myoma tissue was similar to that in the control samples (1.5 mIU/mg and 1 mIU/mg). The author attributed this to the physiological obstacle preventing the oxygenation of the blood due to the compression of the large uterus, which suggests that some other mechanisms may also exist in this syndrome.
It is important to differentiate MES from polycythemia vera (PCV), a primary erythrocytosis, which is a myeloproliferative neoplasm. PCV is also characterized by elevated RBC, Hb and Hct levels, 98% of which is due to Janus kinase 2 genetic mutation but more notably presents a lower serum EPO level, unlike in MES[22]. In the case of the patient presented here, although screening for Janus kinase 2 gene mutations was not performed, with the sharp decrease in RBC, Hb, and Hct levels after the removal of the myoma together with the EPO immunohistochemistry results, we have reason to believe that it was MES rather PCV that caused erythrocytosis.
As erythrocytosis is a leading cause of thrombotic diseases[4,23], anticoagulation is essential in the perioperative period. Common treatment includes physical therapy, such as pneumatic compression stockings, and preventive medicine, such as acetylsalicylic acid (75-81 mg/d), hydroxyurea (500 mg/d), and fondaparinux (2.5 mg/d)[5,24,25]. Moreover, phlebotomy is also used, especially when Hct is above 55%, the frequency of which was reported to range from every two days to twice a week, to minimize the risk of thrombosis, embolization, and other cardiovascular complications[5,25,26], and the average amount of blood removed is 1.8 ± 1.2 L[5]. Normovolemic hemodilution is also helpful in preventing massive bleeding during surgery.
Since leiomyomas are often very large and sometimes cause hydronephrosis, bilateral ureteral stents are often used on such occasions to avoid urinary injuries[5]. Gonadotropin-releasing hormone agonists have also been used in several reports, for instance, to induce amenorrhea while waiting for elective surgery[25] or to decrease estradiol levels and to reduce blood loss[13]; however, they do not reduce the size of the myoma.
The strength of our study is that we not only comprehensively presented this rare case but also attempted to discover the underlying mechanisms of MES via immunohistological analysis of the myomas of this patient and other myoma patients. Furthermore, we are the first to stress the importance of an MDT, and with joint efforts, we successfully completed the surgery without any complications. However, the lack of EPO results in the serum test is one limitation of our study, as the findings would be more convincing if we had quantitatively measured and compared the EPO levels of this patient with those of the other reported patients and performed immunohistochemical staining for EPO-R at the same time. The current findings support the prevailing view that EPO overproduction in the myoma is the cause of the excessive RBCs in the blood system.
MES is a rare disease of erythrocytosis secondary to uterine myoma. It is believed that the uncontrolled ectopic production of EPO from the myoma and EPO-R from the vascular endothelium together via an autocrine and paracrine loop contributed to erythrocytosis and resulted in the overgrowth of the myoma. The cooperation of the multidisciplinary team was the key to the thorough evaluation and ensured successful treatment. In the future, EPO/EPO-R inhibitors may be a potential therapy, especially in the preoperative period, or serve as a conservative treatment.
The author thanks Hong-Bo Su for her pathological assistance in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Obstetrics and gynecology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hidaka E, Dai Hai S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | LevGur M, Levie MD. The myomatous erythrocytosis syndrome: a review. Obstet Gynecol. 1995;86:1026-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (2)] |
| 3. | Fleming AR, Markley JC. Polycythemia associated with uterine myomas. Am J Obstet Gynecol. 1957;74:677-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | McMullin MF. Secondary erythrocytosis. Hematology. 2014;19:183-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Mui J, Yang MMH, Cohen T, McDonald DI, Hunt H. More Than a Myoma: A Review of Myomatous Erythrocytosis Syndrome. J Obstet Gynaecol Can. 2020;42:198-203.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Suresh P, Rizk S. Myomatous Erythrocytosis Syndrome: Case Report and Review of the Literature. Cureus. 2020;12:e6892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Ono Y, Hidaka T, Fukuta K, Kouchi K, Yasoshima K, Takagawa K, Arai T. A case of myomatous erythrocytosis syndrome associated with a large uterine leiomyoma. Case Rep Obstet Gynecol. 2014;2014:602139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Yokoyama Y, Shinohara A, Hirokawa M, Maeda N. Erythrocytosis due to an erythropoietin-producing large uterine leiomyoma. Gynecol Obstet Invest. 2003;56:179-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 9. | Vlasveld LT, de Wit CW, Verweij RA, Castel A, Jansen PM, Peters AA. Myomatous erythrocytosis syndrome: further proof for the pathogenic role of erythropoietin. Neth J Med. 2008;66:283-285. [PubMed] |
| 10. | Horwitz A, McKelway WP. Polycythemia associated with uterine myomas. J Am Med Assoc. 1955;158:1360-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Paranjothy D, Vaish SK. Polycythaemia associated with leiomyoma of the uterus. J Obstet Gynaecol Br Commonw. 1967;74:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Menzies DN. Fibromyomata and polycythaemia. J Obstet Gynaecol Br Commonw. 1961;68:505-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Kohama T, Shinohara K, Takahura M, Inoue M. Large uterine myoma with erythropoietin messenger RNA and erythrocytosis. Obstet Gynecol. 2000;96:826-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Pollio F, Staibano S, Mansueto G, De Rosa G, Persico F, De Falco M, Di Lieto A. Erythropoietin and erythropoietin receptor system in a large uterine myoma of a patient with myomatous erythrocytosis syndrome: possible relationship with the pathogenesis of unusual tumor size. Hum Pathol. 2005;36:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 15. | Viva W, Juhi D, Kristin A, Micaela M, Marcus B, Ibrahim A, Dirk B. Massive uterine fibroid: a diagnostic dilemma: a case report and review of the literature. J Med Case Rep. 2021;15:344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Kimáková P, Solár P, Solárová Z, Komel R, Debeljak N. Erythropoietin and Its Angiogenic Activity. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 17. | Kertesz N, Wu J, Chen TH, Sucov HM, Wu H. The role of erythropoietin in regulating angiogenesis. Dev Biol. 2004;276:101-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 168] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Yasuda Y, Fujita Y, Masuda S, Musha T, Ueda K, Tanaka H, Fujita H, Matsuo T, Nagao M, Sasaki R, Nakamura Y. Erythropoietin is involved in growth and angiogenesis in malignant tumours of female reproductive organs. Carcinogenesis. 2002;23:1797-1805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 91] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Yoshida M, Koshiyama M, Fujii H, Konishi M. Erythrocytosis and a fibroid. Lancet. 1999;354:216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Suzuki M, Takamizawa S, Nomaguchi K, Suzu S, Yamada M, Igarashi T, Sato I. Erythropoietin synthesis by tumour tissues in a patient with uterine myoma and erythrocytosis. Br J Haematol. 2001;113:49-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Macciò A, Chiappe G, Lavra F, Sanna E, Nieddu R, Madeddu C. Laparoscopic hysterectomy as optimal approach for 5400 grams uterus with associated polycythemia: A case report. World J Clin Cases. 2019;7:3027-3032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Fox S, Griffin L, Robinson Harris D. Polycythemia Vera: Rapid Evidence Review. Am Fam Physician. 2021;103:680-687. [PubMed] |
| 23. | Unosawa S, Hata M, Sezai A, Niino T, Yoshitake I, Minami K. Pulmonary embolism with myomatous erythrocytosis syndrome and extreme obesity. Thorac Cardiovasc Surg. 2009;57:313-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Kobayashi M, Akatsu M, Fujita Y, Nishikawa K. Successful perioperative management of a patient with erythropoietin-producing uterine myoma. JA Clin Rep. 2018;4:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | de Guzman GS, Manalo EM. Myomatous erythrocytosis syndrome: A case series. Case Rep Womens Health. 2019;24:e00139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Ozsaran AA, Itil IM, Terek C, Kazandi M, Dikmen Y. Giant myoma and erythrocytosis syndrome. Aust N Z J Obstet Gynaecol. 1999;39:384-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |